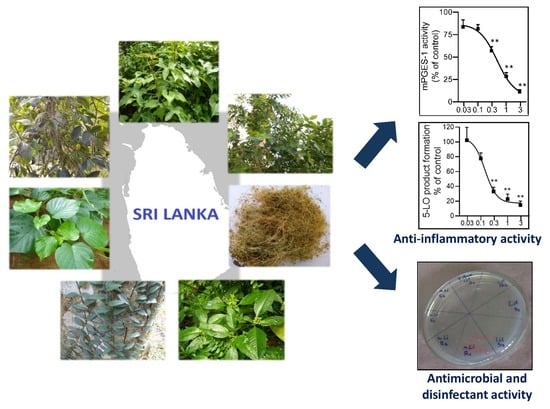
The Anti-Inflammatory and Antimicrobial Potential of Selected Ethnomedicinal Plants from Sri Lanka
Abstract
1. Introduction
2. Results
2.1. Inhibition of 5-LO by the Plant Extracts
2.2. Inhibition of mPGES-1
2.3. NO Scavenging Activity
2.4. Antibacterial Activity
2.5. Disinfectant Activity
2.6. Phytochemical Analysis of the Most Potent Extracts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Crude Extracts
4.3. Evaluation of Bioactivities
4.3.1. 5-LO Activity in Intact Neutrophils
4.3.2. 5-LO Activity in Cell-Free Assays
4.3.3. Determination of mPGES-1 Activity
4.3.4. NO Scavenging Activity
4.3.5. Antibacterial Activity
4.3.6. Disinfectant Potency
4.3.7. Gas Chromatography Coupled to Mass Spectrometric Analysis
4.3.8. Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
4.3.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| COX | Cyclooxygenase |
| DCM | Dichloromethane |
| H(P)ETE | 5-Hydro(pero)xyeicosatetraenoic acid |
| iNOS | Inducible nitric oxide synthase |
| LT | Leukotriene |
| 5-LO | 5-Lipoxygenase |
| mPGES-1 | Microsomal prostaglandin E2 synthase-1 |
| MHA | Muller Hinton Agar |
| MHB | Muller Hinton Broth |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NSAID | Nonsteroidal anti-inflammatory drugs |
| PG | Prostaglandin |
| PLA2 | Phospholipase A2 |
| UPLC-MS/MS | Ultra-high performance liquid chromatography-tandem mass spectrometry |
Appendix
| Plant | Vernacular Name (in Sinhala) | Traditional Usage [4] | Voucher Specimen Number |
|---|---|---|---|
| Argyreia populifolia | Girithilla | Antiseptic, for rabid dog bites, to treat weak and spongy gum | MN_12_008 |
| Garcinia cambogia | Goraka | Antiseptic, to treat skin infections, rheumatism, hypertension | MN_15_013 |
| Hibiscus furcatus | Nabirtitha | Suppuration of boils, eye disease, as a remedy for swellings, for cleansing kidneys | MN_12_006 |
| Mollugo cerviana | Pathpadagum | Antiseptic, cure itch and other skin diseases, to treat gonorrhoea, fever, cough and to reduce body odour | MN_12_005 |
| Nyctanthes arbor-tristis | Sepalika | Carminative, stomachic, purgative, astringent, antibilious, expectorant, diuretic, treatment for piles, various skin diseases, fever, rheumatism | MN_15_009 |
| Ophiorrhiza mungos | Dathkatiya | To treat snake bites, dressing ulcers and external tumors | MN_12_007 |
| Pothos scandens | Potawel | cholagogues, diuretic, to treat wounds, rheumatic fever, chronic malarial fever, small pox, asthma, snake bites | MN_15_010 |
| Test | Comparison of Mean Colony Counts of Different Microorganisms on Different Surfaces | |||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | MRSA | |||||||
| ANOVA Test Result | Smooth Surface p = 0.01975 | Rough Surface p = 0.07249 | Smooth Surface p = 0.000038 | Rough Surface p = 0.044589 | ||||
| Mean Colony Counts | Post Hoc Test vs. Untreated | Mean Colony Counts | Post Hoc Test vs. Untreated | Mean Colony Counts | Post Hoc Test vs. Untreated | Mean Colony Counts | Post Hoc Test vs. Untreated | |
| Untreated surface | 9.3 | 4.0 | 12.2 | 5.0 | ||||
| n-Hexane extract | 0.0 | p = 0.0063 | 0.0 | p = 0.0201 | 1.75 | p = 0.000074 | 0.75 | p = 0.0272 |
| DCM extract | 2.5 | p = 0.0214 | 1.0 | p = 0.0531 | 1.75 | p = 0.000074 | 0.0 | p = 0.0116 |
| Lifebuoy® soap | 1.5 | p = 0.0128 | 1.5 | p = 0.0897 | 0.5 | p = 0.000024 | 2.5 | p = 0.1688 |
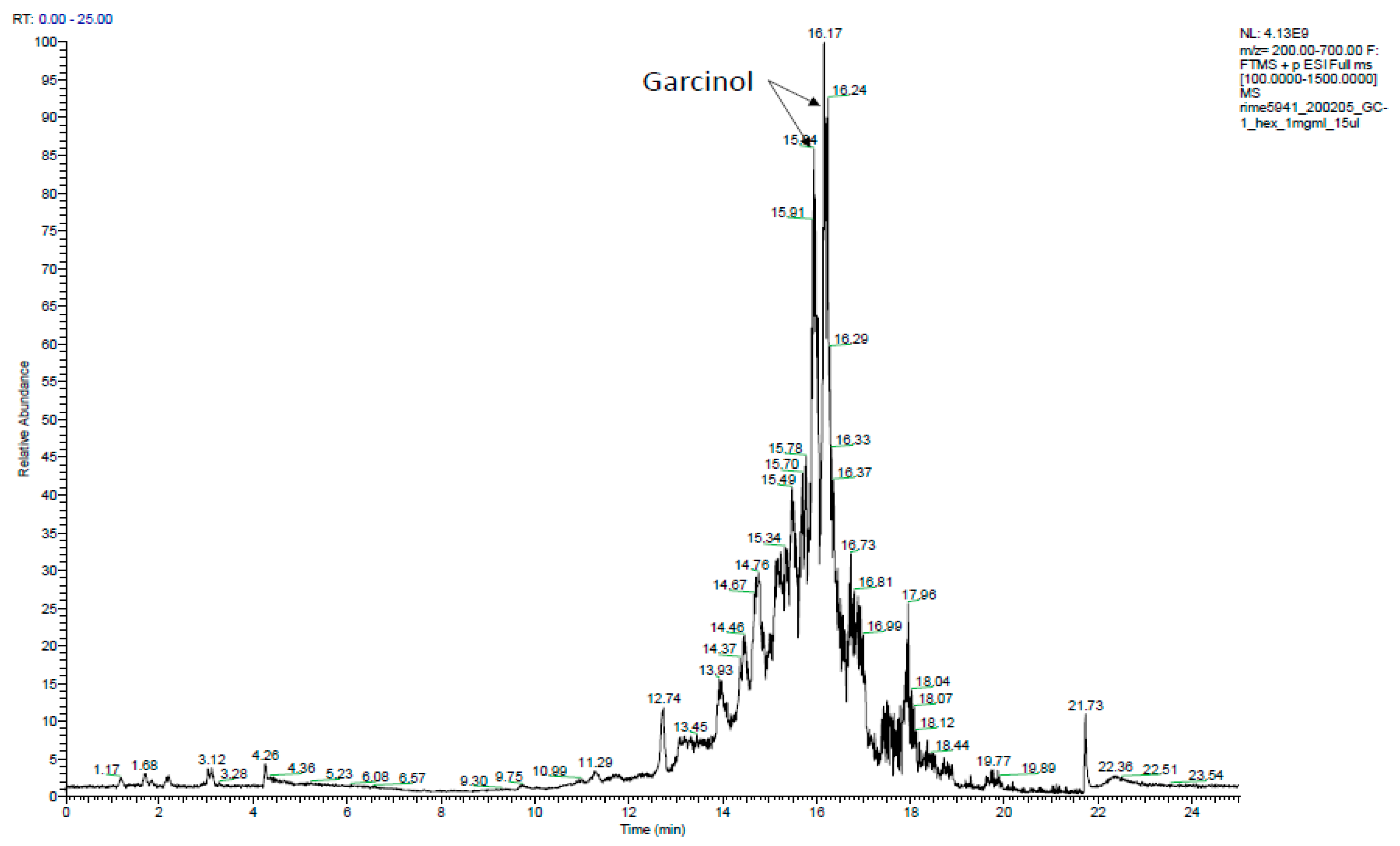
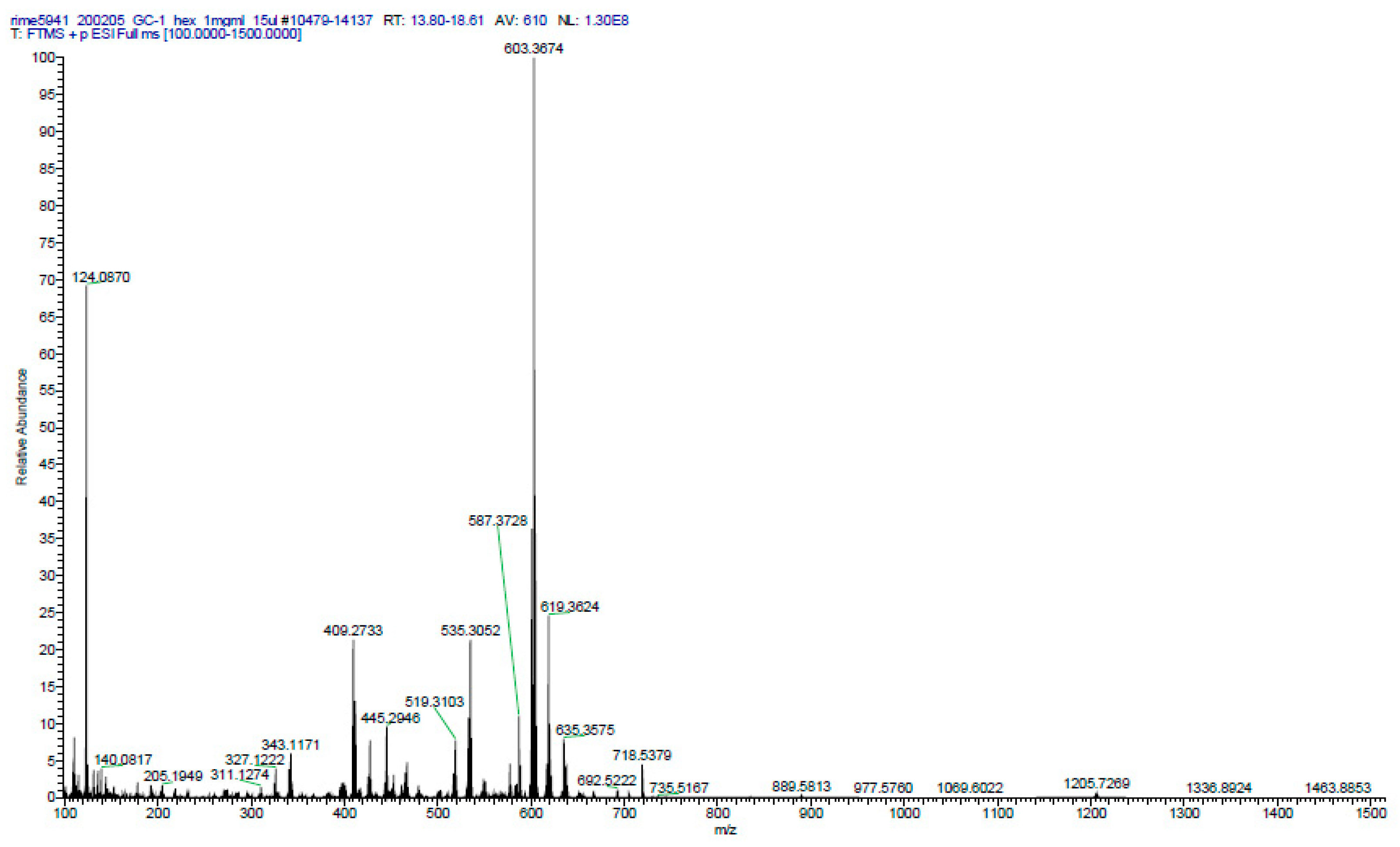
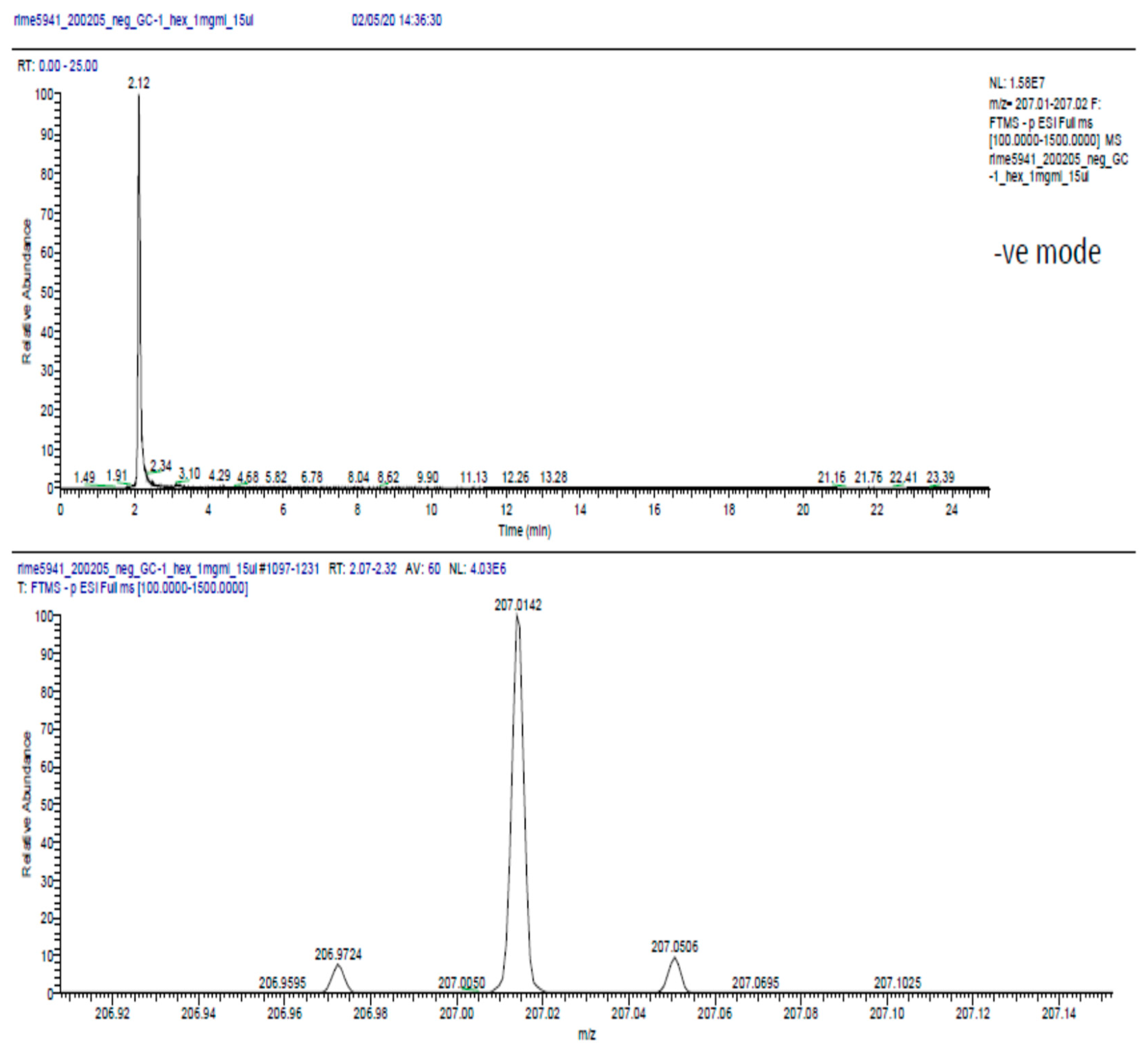

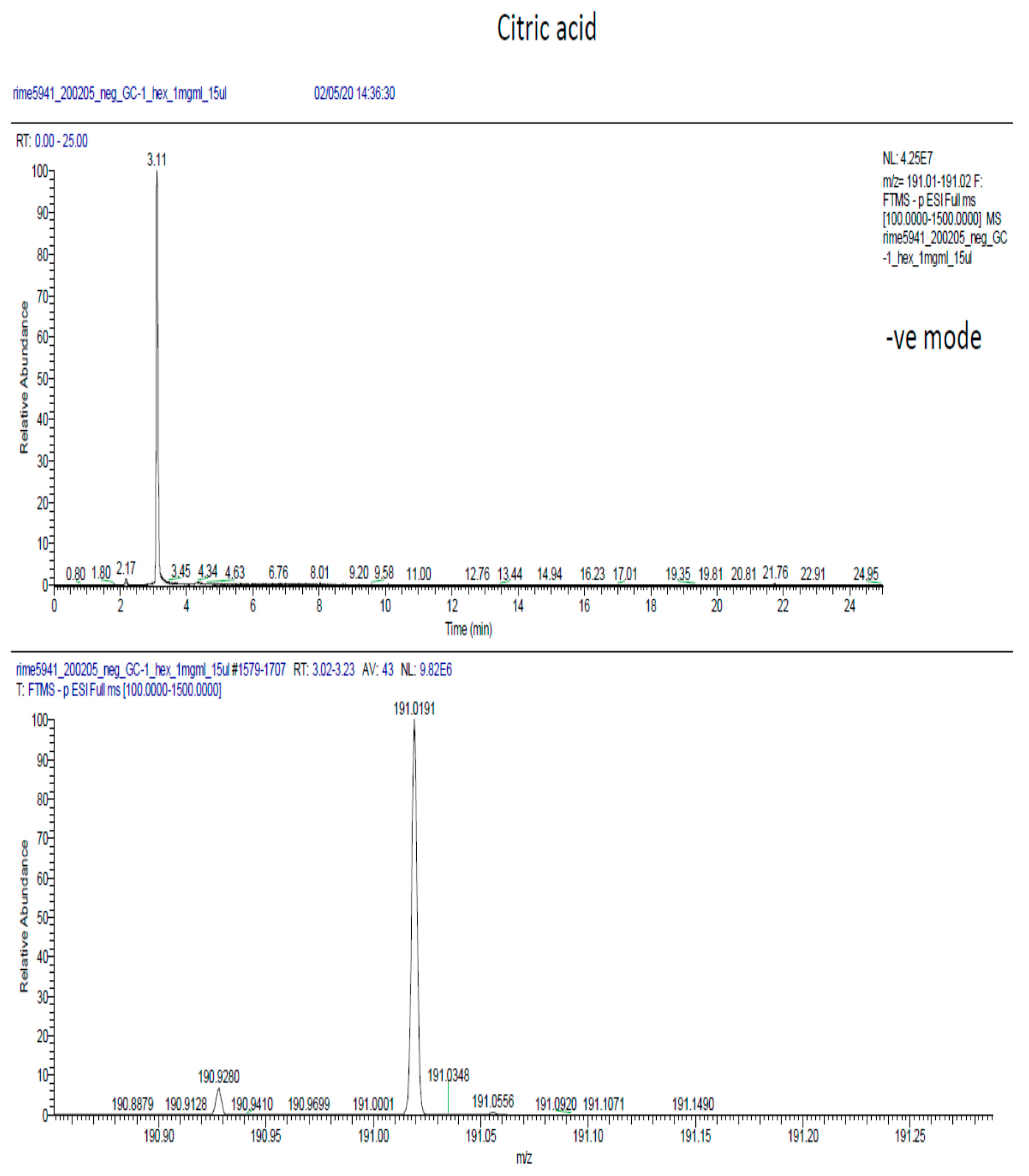
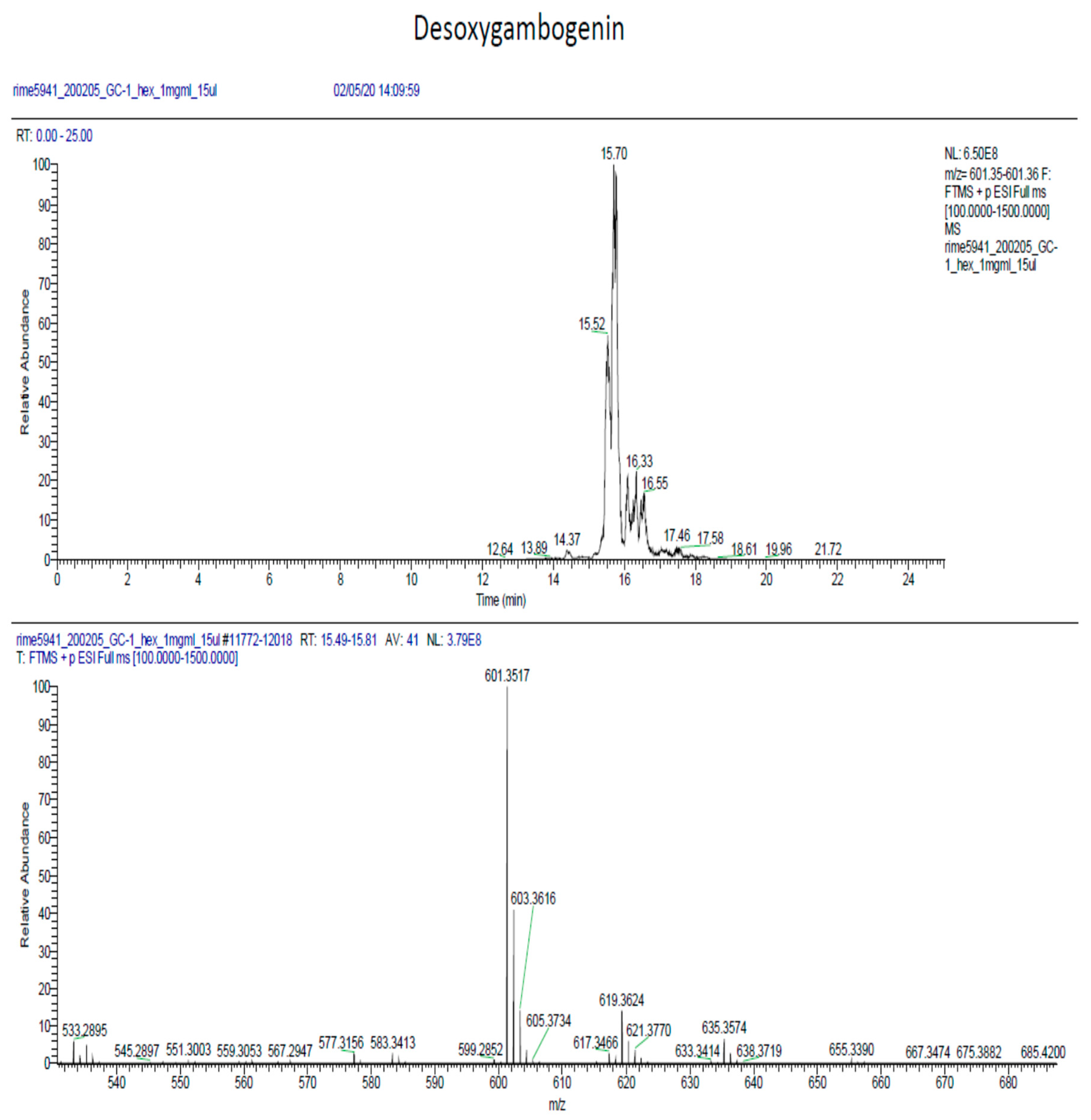
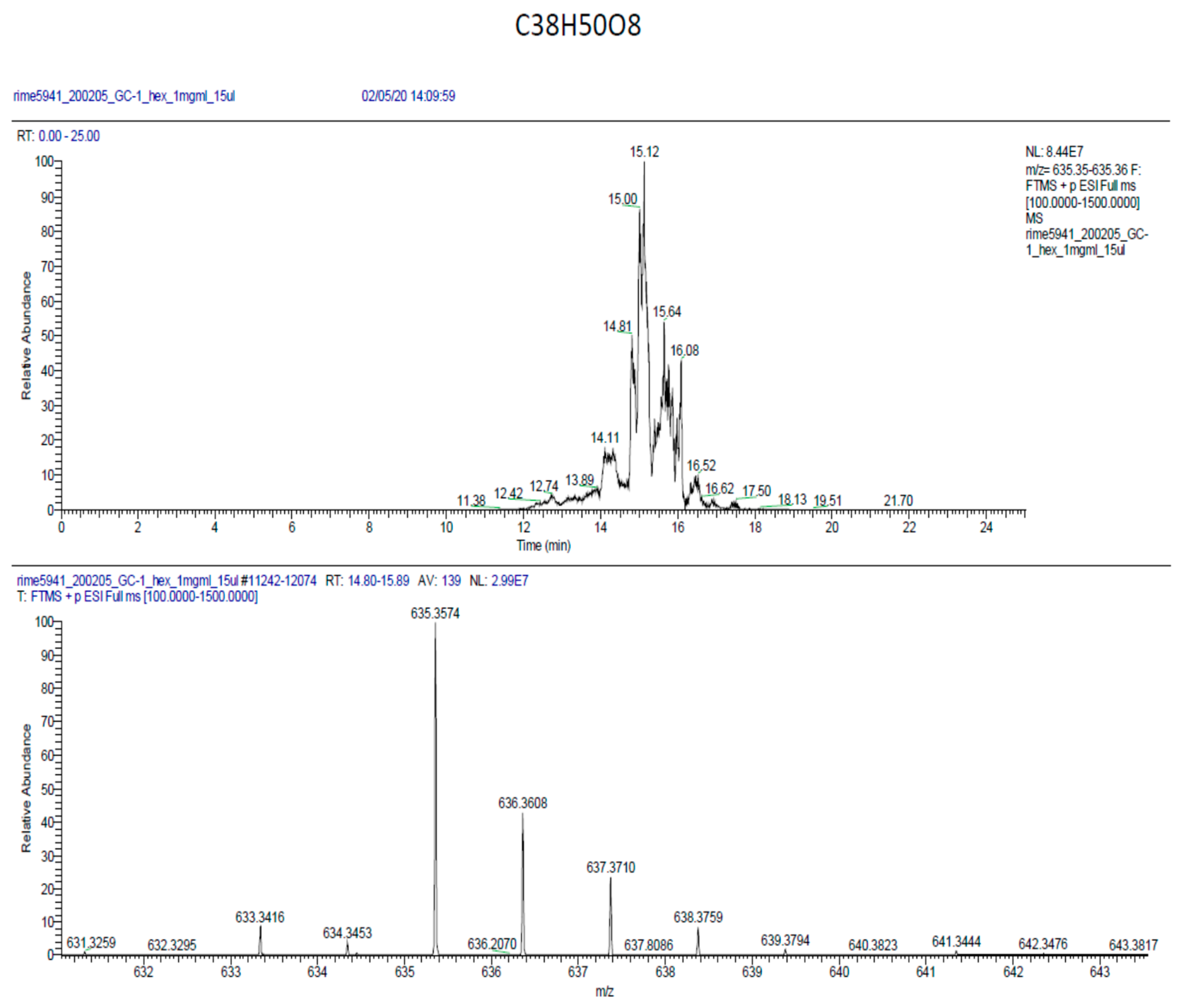
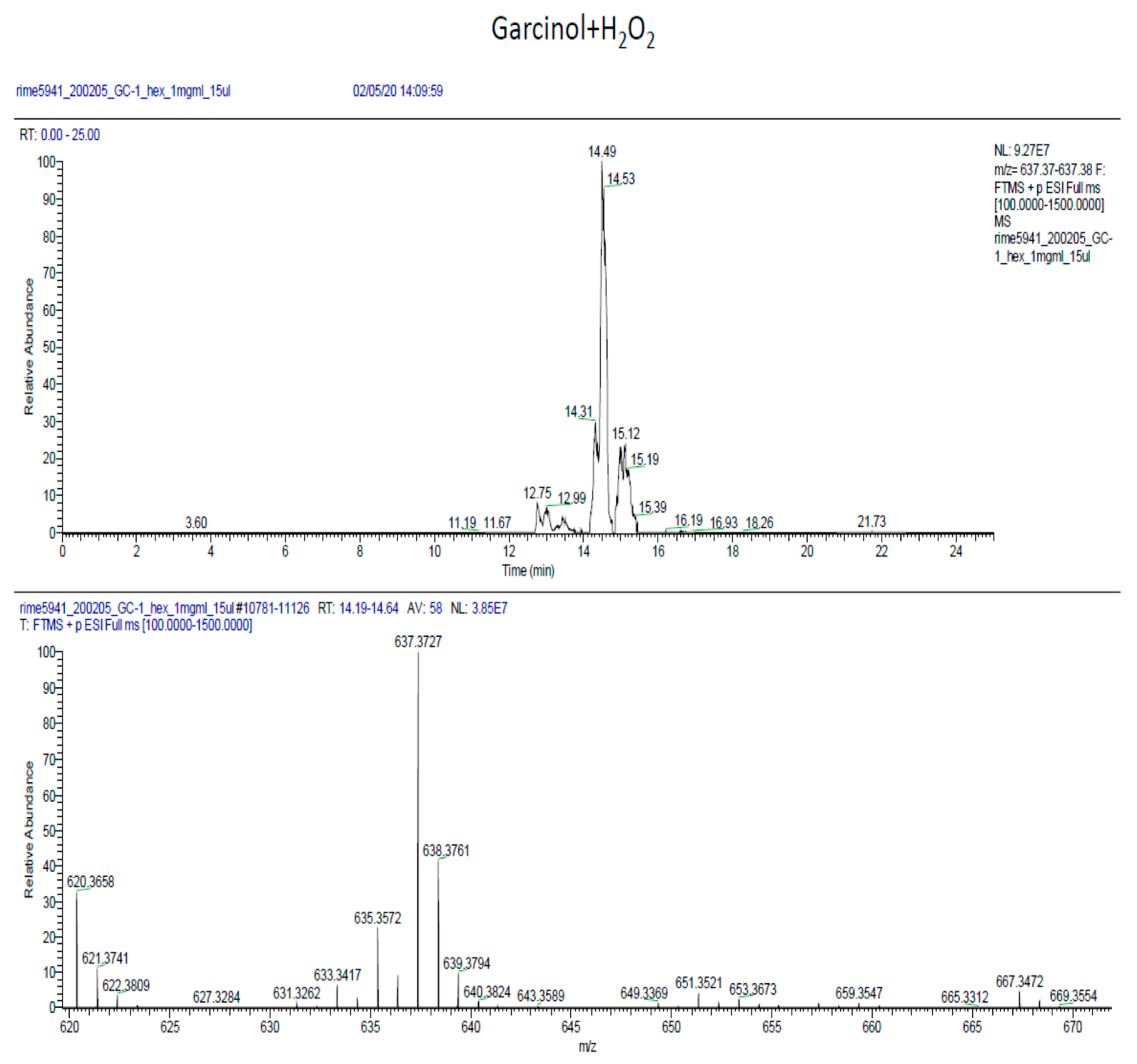
References
- Wijesundera, D.S.A. Inventory, documentation and medicinal plant research in Sri Lanka. In Medicinal Plant Research in Asia Volume 1: The Framework and Project Work Plans; Batugal, P.A., Kanniah, J., Lee, S.Y., Oliver, J.T., Eds.; International Plant Genetic Resources Institute—Regional Office for Asia, the Pacific and Oceania (IPGRI-APO): Serdang, Selangor DE, Malaysia, 2004; Volume 1, pp. 184–195. [Google Scholar]
- Napagoda, M.T.; Sundarapperuma, T.; Fonseka, D.; Amarasiri, S.; Gunaratna, P. Traditional uses of medicinal plants in Polonnaruwa district in North Central Province of Sri Lanka. Scientifica 2019, 2019, 9737302. [Google Scholar] [CrossRef]
- Napagoda, M.T.; Sundarapperuma, T.; Fonseka, D.; Amarasiri, S.; Gunaratna, P. An ethnobotanical study of the medicinal plants used as anti-inflammatory remedies in Gampaha District-Western Province, Sri Lanka. Scientifica 2018, 2018, 9395052. Available online: https://www.hindawi.com/journals/scientifica/aip/9395052/ (accessed on 15 August 2019). [CrossRef]
- Jayaweera, D.M.A. Medicinal Plants (Indigenous and Exotic) Used in Ceylon, Part 1–5, 1st ed.; National Science Council: Colombo, Sri Lanka, 1980.
- Dharmasiri, M.G.; Ratnasooriya, W.D.; Thabrew, M.I. Anti-inflammatory activity of decoctions of leaves and stems of Anisomeles indica at preflowering and flowering stages. Pharm. Biol. 2002, 40, 433–439. [Google Scholar] [CrossRef]
- Dharmasiri, M.G.; Jayakody, J.R.; Galhena, G.; Liyanage, S.S.; Ratnasooriya, W.D. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J. Ethnopharmacol. 2003, 87, 199–206. [Google Scholar] [CrossRef]
- Hapuarachchi, S.D.; Suresh, T.S.; Senarath, W.T.P.S.K.; Hadunnetthi, S. Anti-inflammatory activity of the decoction of M. pinnata. In Proceedings of the National Ayurvedic Research Conference, Colombo, Sri Lanka, 20 January 2012; p. 34. [Google Scholar]
- Koeberle, A.; Werz, O. Natural products as inhibitors of prostaglandin E2 and pro-inflammatory 5-lipoxygenase-derived lipid mediator biosynthesis. Biotechnol. Adv. 2018, 36, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2014, 19, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Napagoda, M.; Gerstmeier, J.; Butschek, H.; Lorenz, S.; Kanatiwela, D.; De Soyza, S.D.; Qader, M.; Nagahawatte, A.; Wijayaratne, G.B.; Svatoš, A.; et al. Lipophilic extract of Leucas zeylanica, a multi-purpose medicinal plant in the tropics, inhibits key enzymes involved in inflammation and gout. J. Ethnopharmacol. 2018, 224, 474–481. [Google Scholar] [CrossRef]
- Napagoda, M.; Gerstmeier, J.; Wesely, S.; Popella, S.; Lorenz, S.; Scheubert, K.; Svatoš, A.; Werz, O. Inhibition of 5-lipoxygenase as anti-inflammatory mode of action of Plectranthus zeylanicus Benth and chemical characterization of ingredients by a mass spectrometric approach. J. Ethnopharmacol. 2014, 151, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Napagoda, M.; Gerstmeier, J.; Koeberle, A.; Wesely, S.; Popella, S.; Lorenz, S.; Scheubert, K.; Boecker, A.; Svatoš, A.; Werz, O. Munronia pinnata (Wall.) Theob. Unveiling phytochemistry and dual inhibition of 5-lipoxygenase and microsomal prostaglandin E2 synthase (mPGES)-1. J. Ethnopharmacol. 2014, 151, 882–890. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Evans, C.H.; Stefanovic-Racic, M. Nitric oxide and inflammatory joint diseases. In Free Radicals and Inflammation; Winyard, P.G., Blake, D.R., Evans, C.H., Eds.; Birkhäuser: Basel, Switzerland, 2000; pp. 155–168. [Google Scholar]
- Palakawong, C.; Sophanodora, P.; Pisuchpen, S.; Phongpaichit, S. Antioxidant and antimicrobial activities of crude extracts from mangosteen (Garcinia mangostana L.) parts and some essential oils. Int. Food Res. J. 2010, 17, 583–589. [Google Scholar]
- Naves, V.M.L.; dos Santos, M.H.; Ribeiro, I.S.; da Silva, C.A.; Silva, N.C.; da Silva, M.A.; da Silva, G.A.; Dias, A.L.T.; Ionta, M.; Dias, D.F. Antimicrobial and antioxidant activity of Garcinia brasiliensis extracts. S. Afr. J. Bot. 2019, 124, 244–250. [Google Scholar] [CrossRef]
- Nagendra, K.; Kusum, R.; Ramachandran, H.D. Antimicrobial, antifungal and antioxidant activity of ethanolic extracts of Garcinia indica fruit. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 800–804. [Google Scholar]
- Israel, E.; Dermarkarian, R.; Rosenberg, M.; Sperling, R.; Taylor, G.; Rubin, P.; Drazen, J.M. The effects of a 5-lipoxygenase inhibitor on asthma induced by cold, dry air. N. Engl. J. Med. 1990, 323, 1740–1744. [Google Scholar] [CrossRef]
- Werz, O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef]
- Kröncke, K.-D.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Zingarelli, B.; Gilad, E.; Hake, P.; Salzman, A.L.; Szabó, C. Protective effect of melatonin in carrageenan-induced models of local inflammation. J. Pineal Res. 1997, 23, 106–116. [Google Scholar] [CrossRef]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J. Food Process. 2014, 2014, 918018. [Google Scholar] [CrossRef]
- Zoccolotti, J.O.; Tasso, C.O.; Arbeláez, M.I.A.; Malavolta, I.F.; Pereira, E.C.D.S.; Esteves, C.S.G.; Jorge, J.H. Properties of an acrylic resin after immersion in antiseptic soaps: Low-cost, easy-access procedure for the prevention of denture stomatitis. PLoS ONE 2018, 13, e0203187. [Google Scholar] [CrossRef]
- Koeberle, A.; Northoff, H.; Werz, O. Identification of 5- lipoxygenase and microsomal prostaglandin E2 synthase-1 as functional targets of the anti-inflammatory and anti-carcinogenic garcinol. Biochem. Pharmacol. 2009, 77, 1513–1521. [Google Scholar] [CrossRef]
- Di Nunzio, M.R.; Cohen, B.; Douhal, A. Structural photodynamics of camptothecin, an anticancer drug in aqueous solutions. J. Phys. Chem. A 2011, 115, 5094–5104. [Google Scholar] [CrossRef] [PubMed]
- Haeggström, J.Z.; Rinaldo-Matthis, A.; Wheelock, C.E.; Wetterholm, A. Advances in eicosanoid research, novel therapeutic implications. Biochem. Biophys. Res. Commun. 2010, 396, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B.; Morgenstern, R.; Jakobsson, P.J. Membrane prostaglandin E synthase-1: A novel therapeutic target. Pharmacol. Rev. 2007, 59, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B.; Dahlén, S.E.; Lindgren, J.A.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science 1987, 237, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, G.A. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2003, 2, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Werz, O. Perspective of microsomal prostaglandin E2 synthase-1 as drug target in inflammation-related disorders. Biochem. Pharmacol. 2015, 98, 1–15. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Inhibitors of the microsomal prostaglandin E(2) synthase-1 as alternative to nonsteroidal anti-inflammatory drugs (NSAIDs)—A critical review. Curr. Med. Chem. 2009, 16, 4274–4296. [Google Scholar] [CrossRef]
- Svouraki, A.; Garscha, U.; Kouloura, E.; Pace, S.; Pergola, C.; Krauth, V.; Rossi, A.; Sautebin, L.; Halabalaki, M.; Werz, O.; et al. Evaluation of dual 5-lipoxygenase/microsomal prostaglandin E2 synthase-1 inhibitory effect of natural and synthetic acronychia-type isoprenylated acetophenones. J. Nat. Prod. 2017, 80, 699–706. [Google Scholar] [CrossRef]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 1: Medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenasecyclooxygenase. Phytother. Res. 2005, 19, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Yang, D.Z.; Lv, C. Anti-inflammatory effects of gambogic acid in murine collagen-induced arthritis through PI3K/Akt signaling pathway. Mol. Med. Rep. 2018, 17, 4791–4796. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Lerner, E. Nitric oxide inhibition strategies. Future Sci. OA 2015, 1, FSO35. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Baliga, M.S. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: A preliminary study. Phytother. Res. 2004, 18, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Mehandzhiyski, A.; Batovska, D.; Dimitrov, D.; Evstatieva, L.; Danova, K. Nitric oxide-scavenging activity of in vitro cultured Balkan medicinal and aromatic plants. Bulg. J. Agric. Sci. 2013, 19, 31–34. [Google Scholar]
- Kyaw, B.M.; Arora, S.; Lim, C.S. Bactericidal antibiotic- phytochemical combinations against methicillin resistant Staphylococcus aureus. Braz. J. Microbiol. 2012, 43, 938–945. [Google Scholar] [CrossRef]
- Taleb-Contini, S.H.; Salvador, M.J.; Watanabe, E.; Ito, I.Y.; Oliveira, D.C.R. Antimicrobial activity of flavonoids and steroids isolated from two Chromolaena species. Braz. J. Pharm. Sci. 2003, 39, 403–408. [Google Scholar] [CrossRef]
- Makade, C.S.; Shenoi, P.R.; Morey, E.; Paralikar, A.V. Evaluation of antimicrobial activity and efficacy of herbal oils and extracts in disinfection of gutta percha cones before obturation. Restor. Dent. Endod. 2017, 42, 264–272. [Google Scholar] [CrossRef]
- Elersek, T.; Ženko, M.; Filipič, M. Ecotoxicity of disinfectant benzalkonium chloride and its mixture with antineoplastic drug 5-fluorouracil towards alga Pseudokirchneriella subcapitata. PeerJ 2018, 6, e4986. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Amended safety assessment of dodecylbenzenesulfonate, decylbenzenesulfonate, and tridecylbenzenesulfonate salts as used in cosmetics. Int. J. Toxicol. 2010, 29 (Suppl. 6), 288S–305S. [Google Scholar] [CrossRef]
- Gertsch, E.J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Goetzl, J.E. Vitamin E modulates the lipoxygenation of arachidonic acid in leukocytes. Nature 1980, 288, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, M.; Tosa, H.; Tanaka, T.; Kanamaru, S.; Asai, F.; Kobayashi, Y.; Miyauchi, K.; Shimano, R. Antibacterial activity of some Garcinia benzophenone derivatives against Methicillin-Resistant Staphylococcus aureus. Biol. Pharm. Bull. 1996, 19, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, M.D.; Fosberg, F.R. A Revised Handbook to the Flora of Ceylon- Complete Set; Taylor & Francis: Oxfordshire, UK, 1980; Volume 5. [Google Scholar]
- Fischer, L.; Szellas, D.; Radmark, O.; Steinhilber, D.; Werz, O. Phosphorylation- and stimulus-dependent inhibition of cellular 5-lipoxygenase activity by nonredox-type inhibitors. FASEB J. 2003, 17, 949–951. [Google Scholar] [CrossRef]
- Koeberle, A.; Zettl, H.; Greiner, C.; Wurglics, M.; Schubert-Zsilavecz, M.; Werz, O. Pirinixic acid derivatives as novel dual inhibitors of microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. J. Med. Chem. 2008, 51, 8068–8076. [Google Scholar] [CrossRef]
- Asanuma, M.; Nishibayashi-Asanuma, S.; Miyazaki, I.; Kohno, M.; Ogawa, N. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J. Neurochem. 2001, 76, 1895–1904. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, R.; Gupta, P.K.; Rana, J.K.; Sharma, M.; Taneja, N. Comparative efficacy evaluation of disinfectants routinely used in hospital practice: India. Indian J. Crit. Care Med. 2012, 16, 123–129. [Google Scholar]
Sample Availability: All the plant extracts used in this study are available from the authors. |
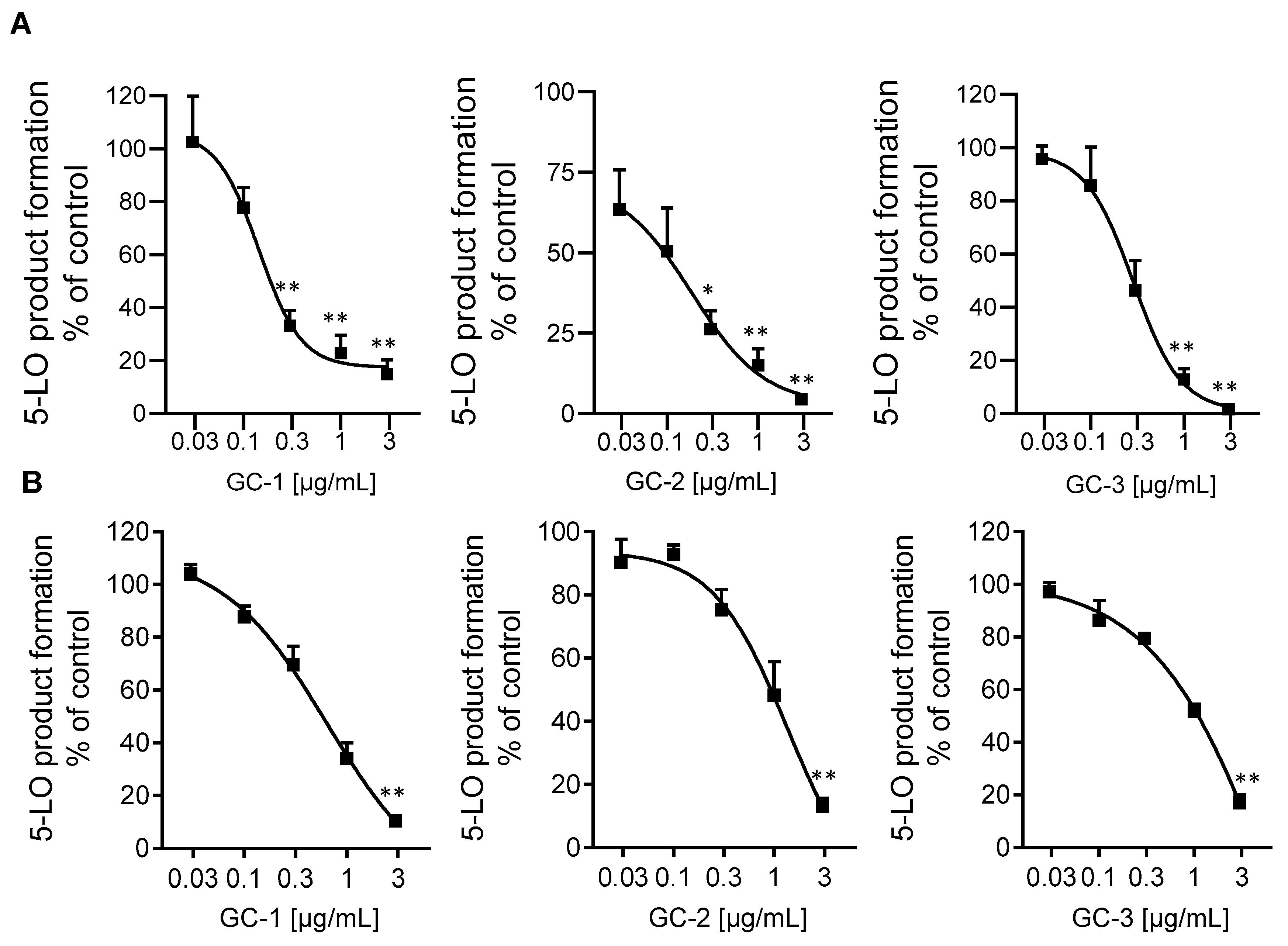
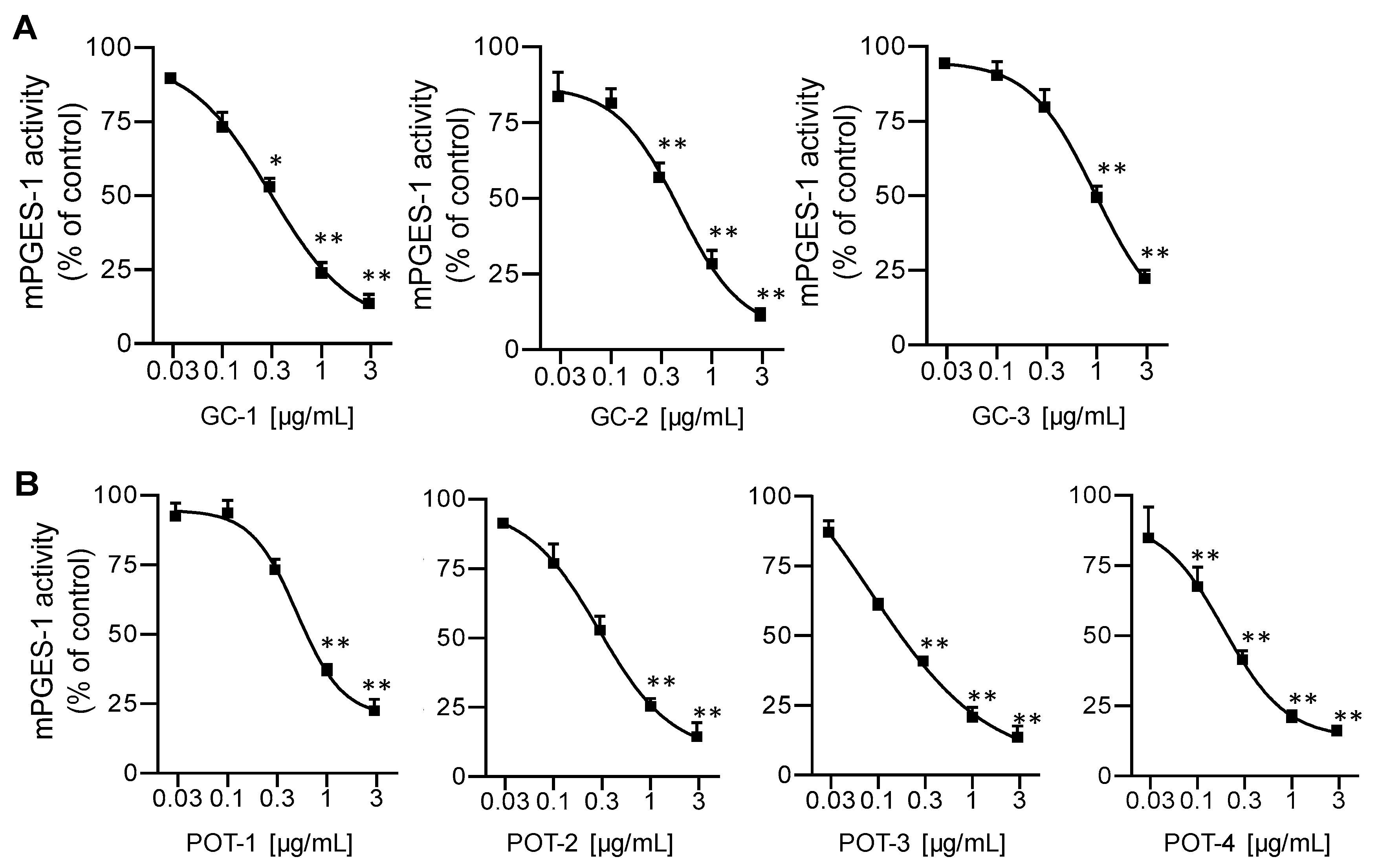

| Plant and Plant Extracts | IC50 Values (μg/mL) | ||
|---|---|---|---|
| 5-LO (Cell-Free) | 5-LO (Cell-Based) | mPGES-1 (Cell-Free) | |
| A. populifolia | |||
| n-Hexane | - | - | - |
| DCM | - | - | - |
| Ethyl acetate | - | - | - |
| Methanol | - | - | - |
| G. cambogia | |||
| n-Hexane | 0.15 | 0.92 | 0.29 |
| DCM | 0.16 | 1.39 | 0.49 |
| Ethyl acetate | 0.24 | 1.55 | 0.85 |
| Methanol | - | - | - |
| H. furcatus | |||
| n-Hexane | - | - | - |
| DCM | - | - | - |
| Ethyl acetate | 1.0 | 10.0 | - |
| Methanol | - | - | - |
| M. cerviana | |||
| n-Hexane | 5.5 | 6.8 | - |
| DCM | - | - | - |
| Ethyl acetate | - | - | - |
| Methanol | - | - | - |
| N. arbor-tristis | |||
| n-Hexane | - | - | - |
| DCM | - | - | - |
| Ethyl acetate | - | - | - |
| Methanol | - | - | - |
| O. mungos | - | ||
| n-Hexane | - | - | - |
| DCM | - | - | - |
| Ethyl acetate | - | - | - |
| Methanol | 1.6 | 8.7 | - |
| P. scandens | |||
| n-Hexane | - | - | 0.50 |
| DCM | - | - | 0.27 |
| Ethyl acetate | - | - | 0.10 |
| Methanol | - | - | 0.20 |
| MK886 | - | - | 1.2 |
| Zileuton | 0.11 | 0.13 | - |
| Plant Extract | IC50 (μg/mL) |
|---|---|
| H. furcatus-DCM | 142.9 |
| H. furcatus-methanol | 229.4 |
| M. cerviana-DCM | 347.7 |
| M. cerviana-ethyl acetate | 760 |
| M. cerviana-methanol | 231.7 |
| N. arbor-tristis-methanol | 171.2 |
| O. mungos–methanol | 85.8 |
| Aspirin (reference drug) | 3.5 |
| Extract/Drug | MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus | S. saprophyticus | E. feacalis | MRSA 1 | MRSA 2 | MRSA 3 | MRSA 4 | |
| n-Hexane | 31.25 | 31.25 | 250 | 31.25 | 62.5 | 125 | 125 |
| DCM | 62.5 | 62.5 | 250 | 62.5 | 125 | 125 | 125 |
| Ethyl acetate | 500 | 500 | - | - | - | - | - |
| Methanol | 500 | 500 | - | - | - | - | - |
| Cefotaxime | 0.93 | 3.72 | 3.72 | 31.25 | 62.5 | 62.5 | 31.25 |
| Gentamicin | 7.5 | 7.5 | 31.25 | 31.25 | 31.25 | 31.25 | 15.6 |
| Tested Sample | Disinfectant Efficiency (%) against Different Microorganisms on Various Surfaces | |||||
|---|---|---|---|---|---|---|
| S. aureus | MRSA Strain 1 | MRSA Strain 2 | ||||
| Smooth Surface | Rough Surface | Smooth Surface | Rough Surface | Smooth Surface | Rough Surface | |
| n-Hexane extract | 100 | 100 | 87.5 | 100 | 80 | 78.5 |
| DCM extract | 58.3 | 60 | 90.6 | 100 | 69.2 | 100 |
| Lifebuoy® soap | 86.3 | 40 | 92 | 55.5 | 100 | 20 |
| Lysol® (benzalkonium Chloride 0.10%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Teepol™ (sodium dodecylbenzene sulfonate 10%) | 100 | 100 | 100 | 100 | 100 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napagoda, M.; Gerstmeier, J.; Butschek, H.; De Soyza, S.; Pace, S.; Lorenz, S.; Qader, M.; Witharana, S.; Nagahawatte, A.; Wijayaratne, G.; et al. The Anti-Inflammatory and Antimicrobial Potential of Selected Ethnomedicinal Plants from Sri Lanka. Molecules 2020, 25, 1894. https://doi.org/10.3390/molecules25081894
Napagoda M, Gerstmeier J, Butschek H, De Soyza S, Pace S, Lorenz S, Qader M, Witharana S, Nagahawatte A, Wijayaratne G, et al. The Anti-Inflammatory and Antimicrobial Potential of Selected Ethnomedicinal Plants from Sri Lanka. Molecules. 2020; 25(8):1894. https://doi.org/10.3390/molecules25081894
Chicago/Turabian StyleNapagoda, Mayuri, Jana Gerstmeier, Hannah Butschek, Sudhara De Soyza, Simona Pace, Sybille Lorenz, Mallique Qader, Sanjeeva Witharana, Ajith Nagahawatte, Gaya Wijayaratne, and et al. 2020. "The Anti-Inflammatory and Antimicrobial Potential of Selected Ethnomedicinal Plants from Sri Lanka" Molecules 25, no. 8: 1894. https://doi.org/10.3390/molecules25081894
APA StyleNapagoda, M., Gerstmeier, J., Butschek, H., De Soyza, S., Pace, S., Lorenz, S., Qader, M., Witharana, S., Nagahawatte, A., Wijayaratne, G., Svatoš, A., Jayasinghe, L., Koeberle, A., & Werz, O. (2020). The Anti-Inflammatory and Antimicrobial Potential of Selected Ethnomedicinal Plants from Sri Lanka. Molecules, 25(8), 1894. https://doi.org/10.3390/molecules25081894