HOCl Responsive Lanthanide Complexes Using Hydroquinone Caging Units
Abstract
:1. Introduction
2. Results and Discussion
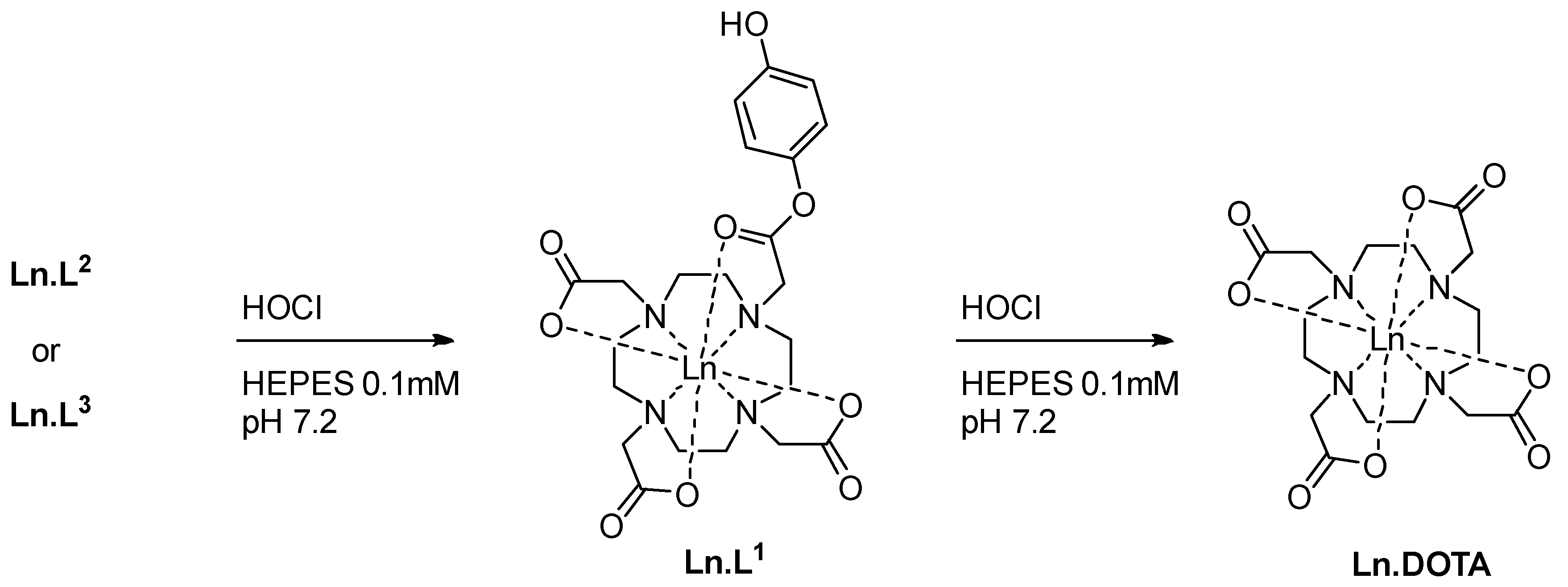
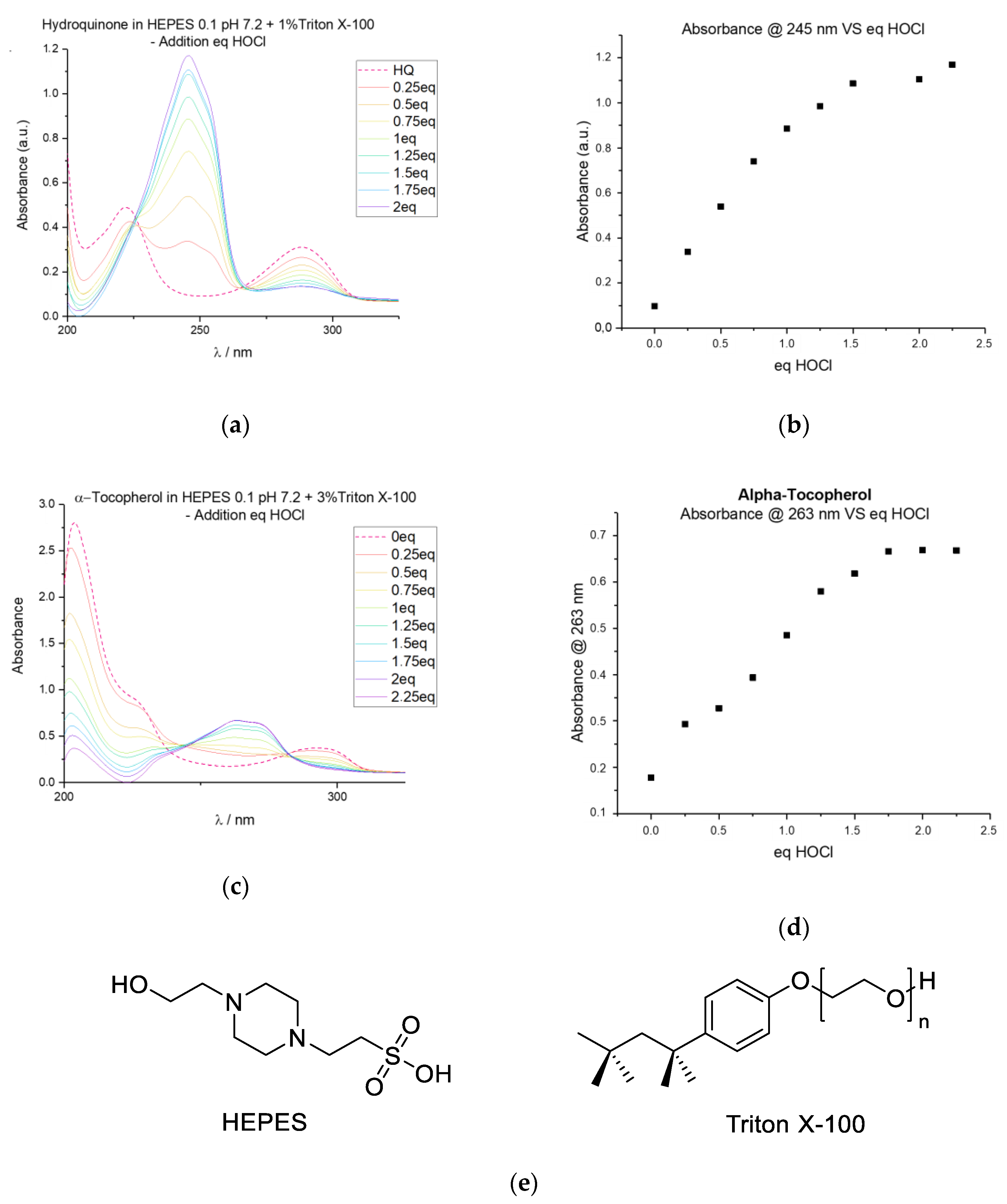
2.1. Oxidation Testbed
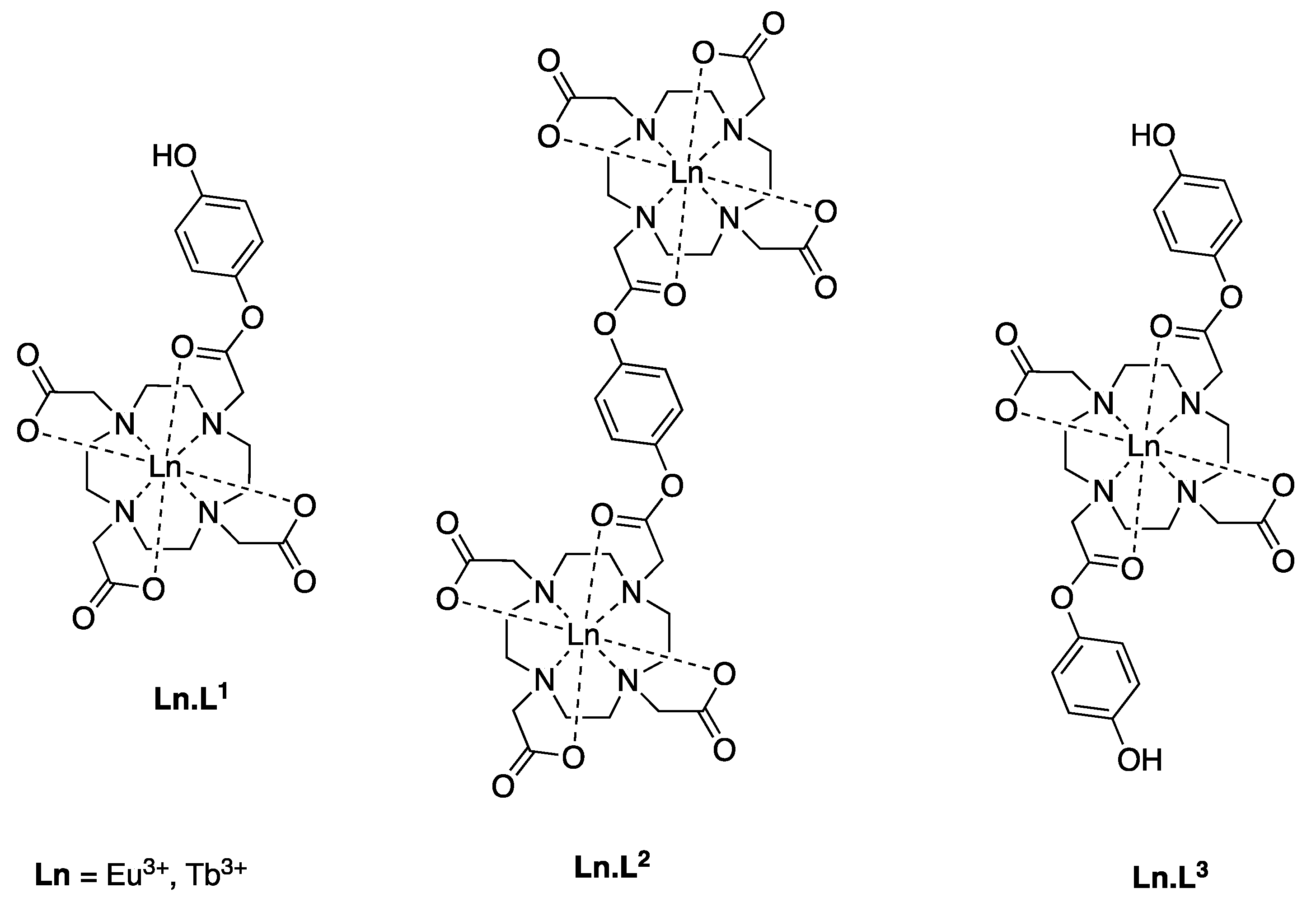
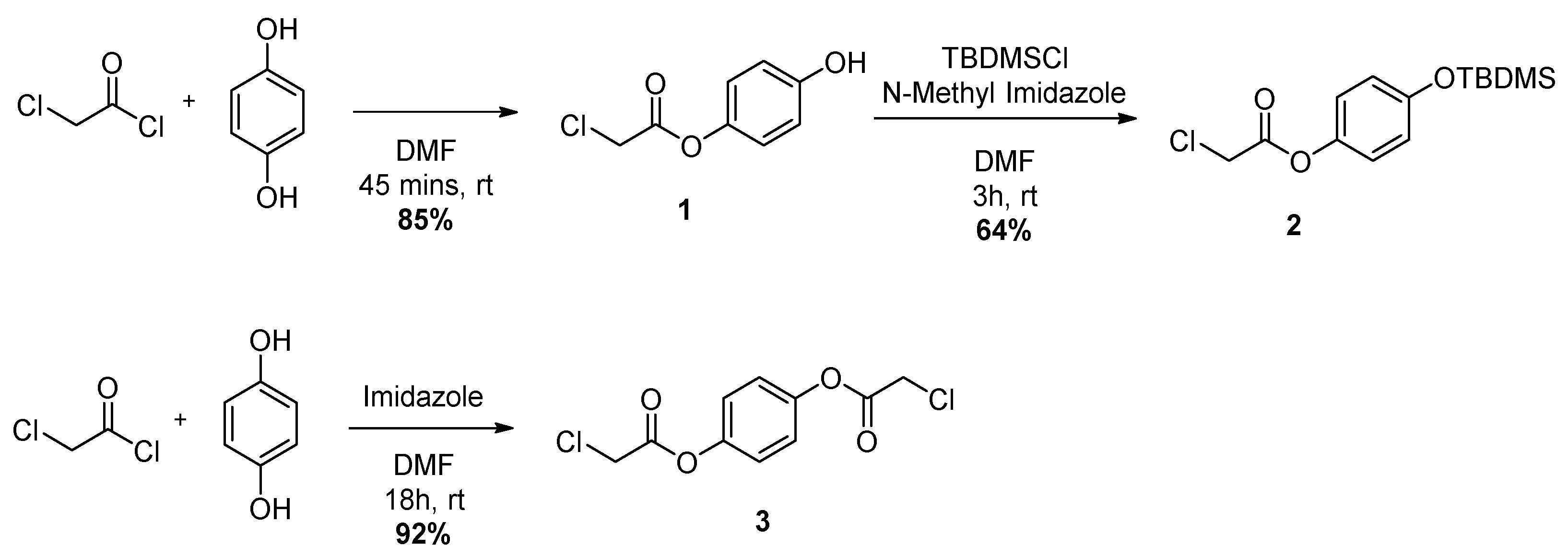
2.2. Synthesis
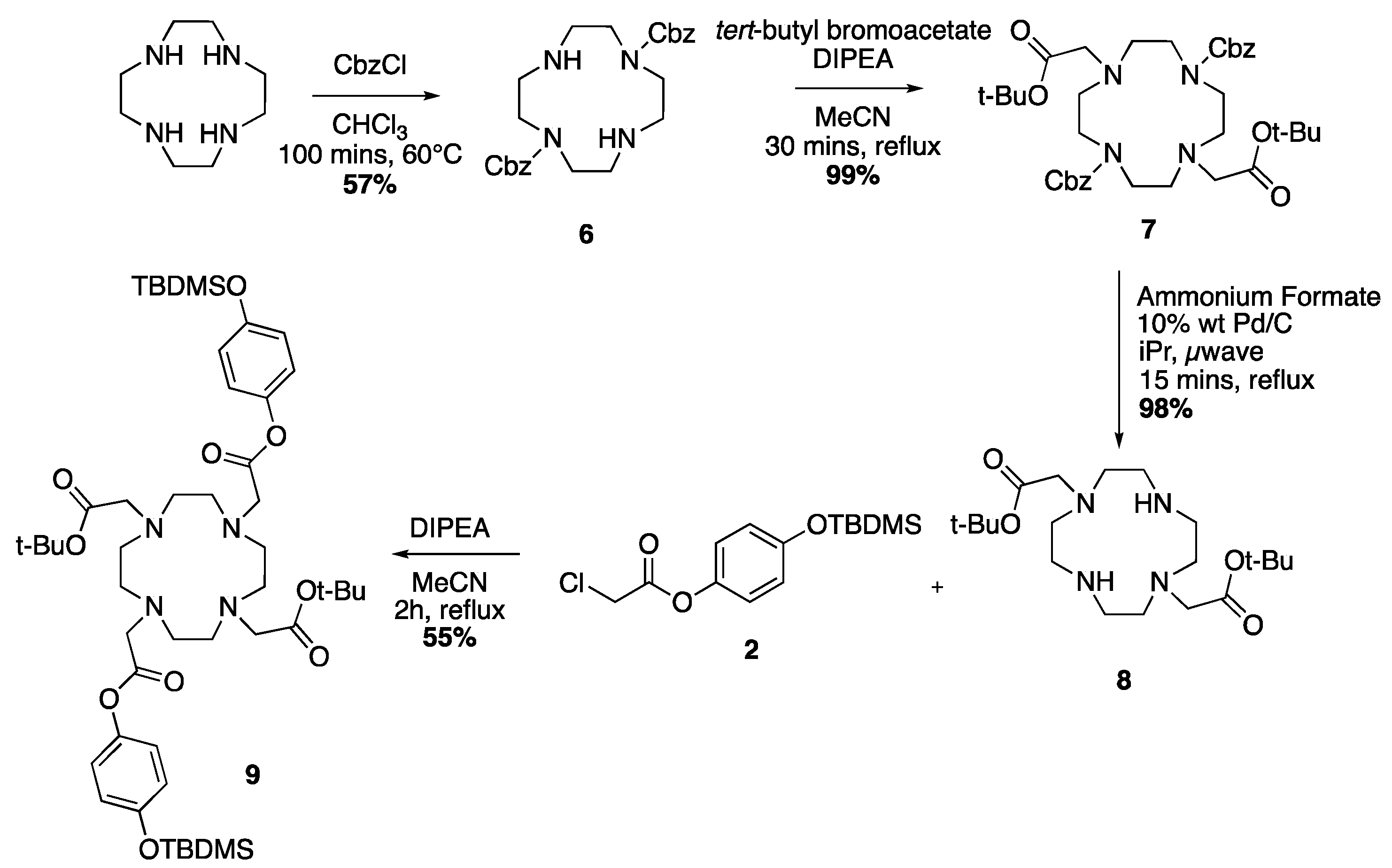
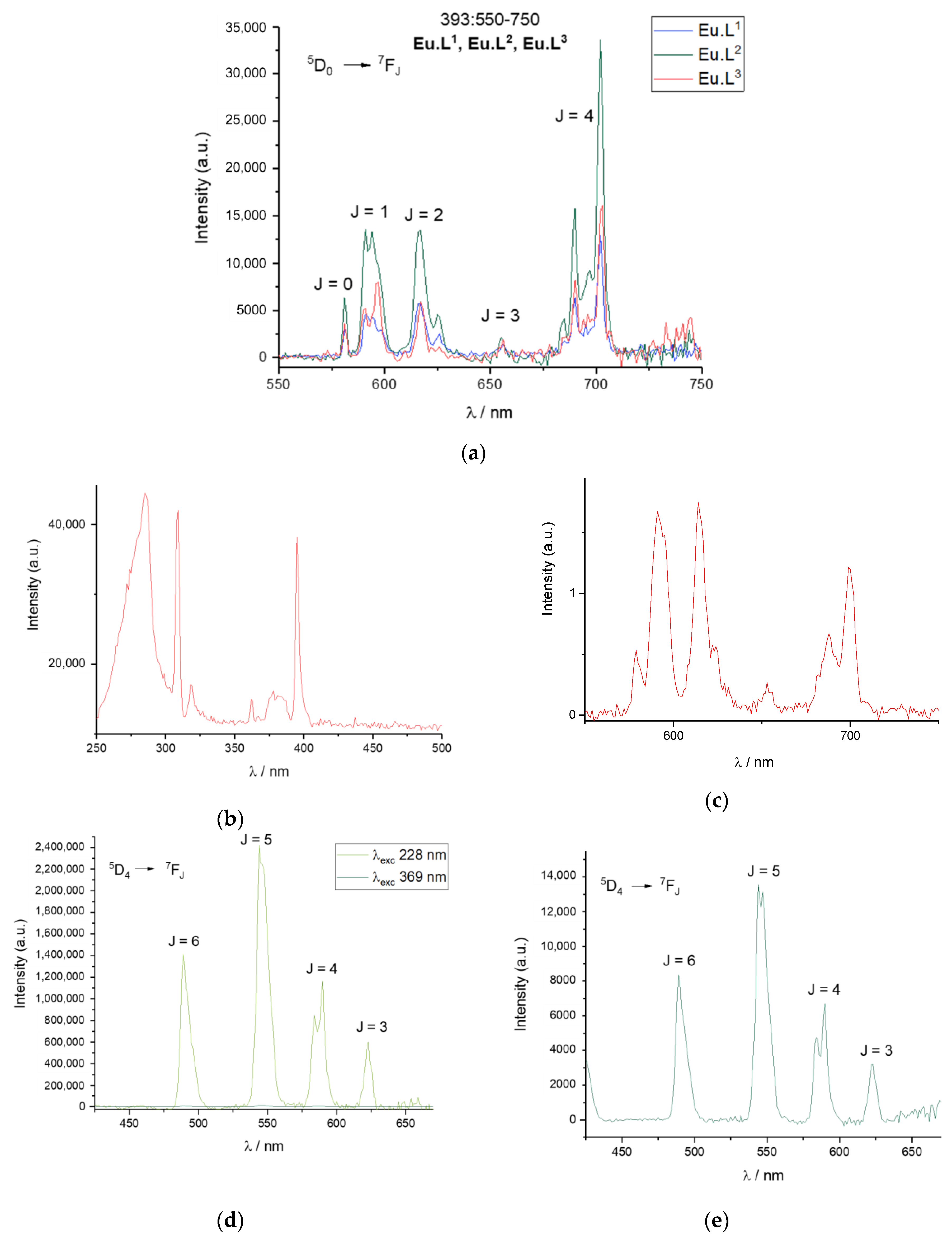
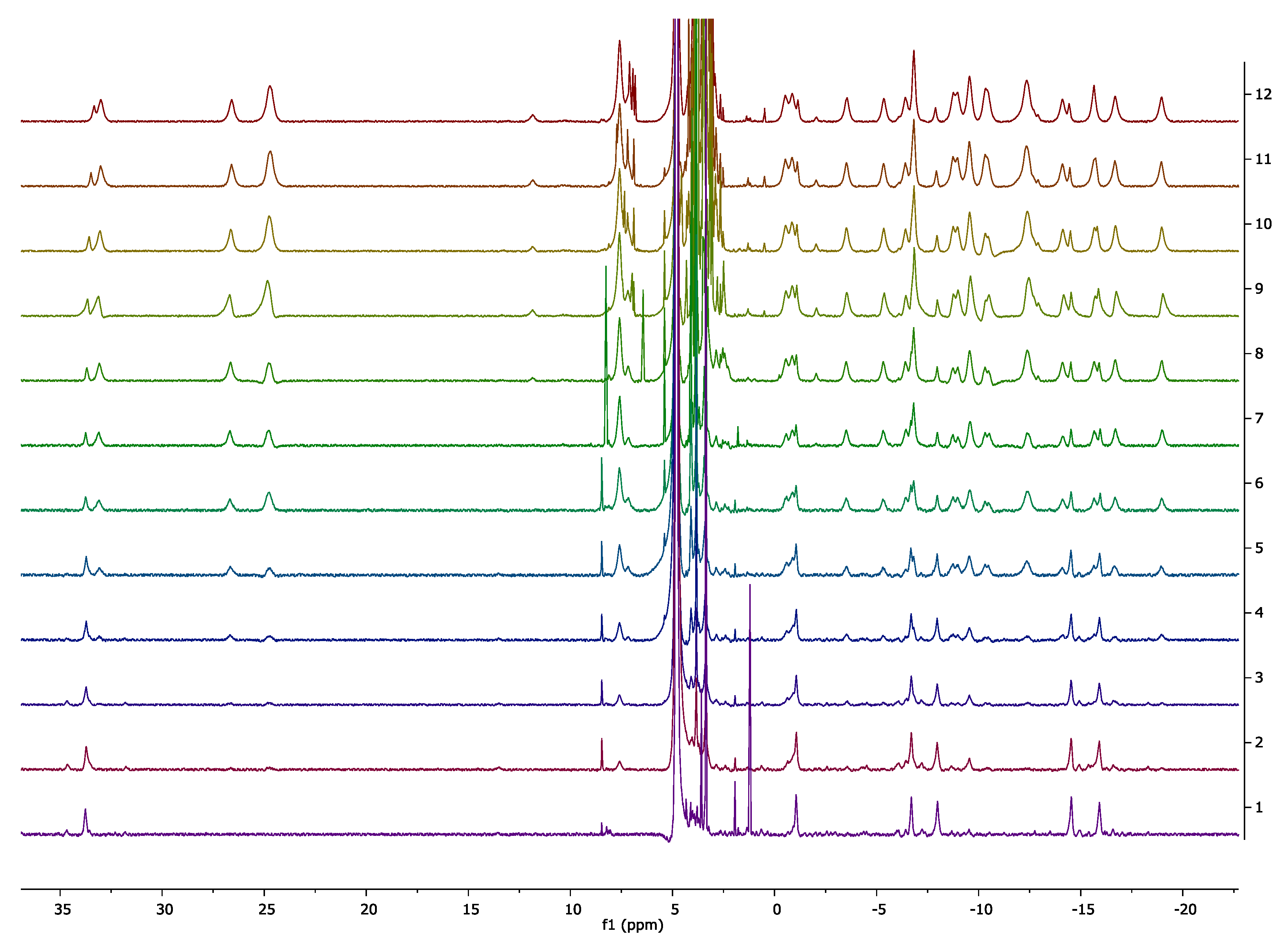
2.3. Characterisation
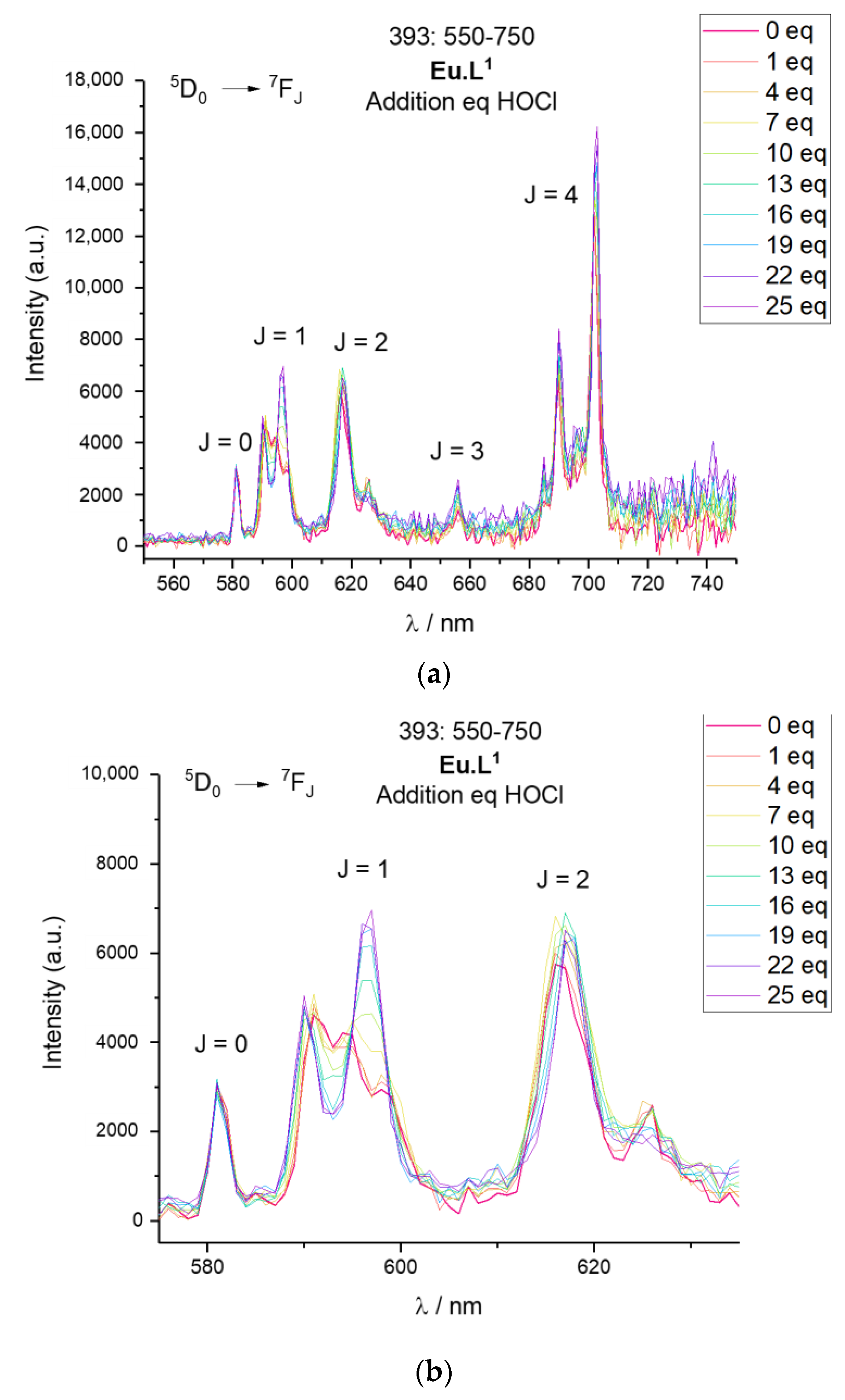
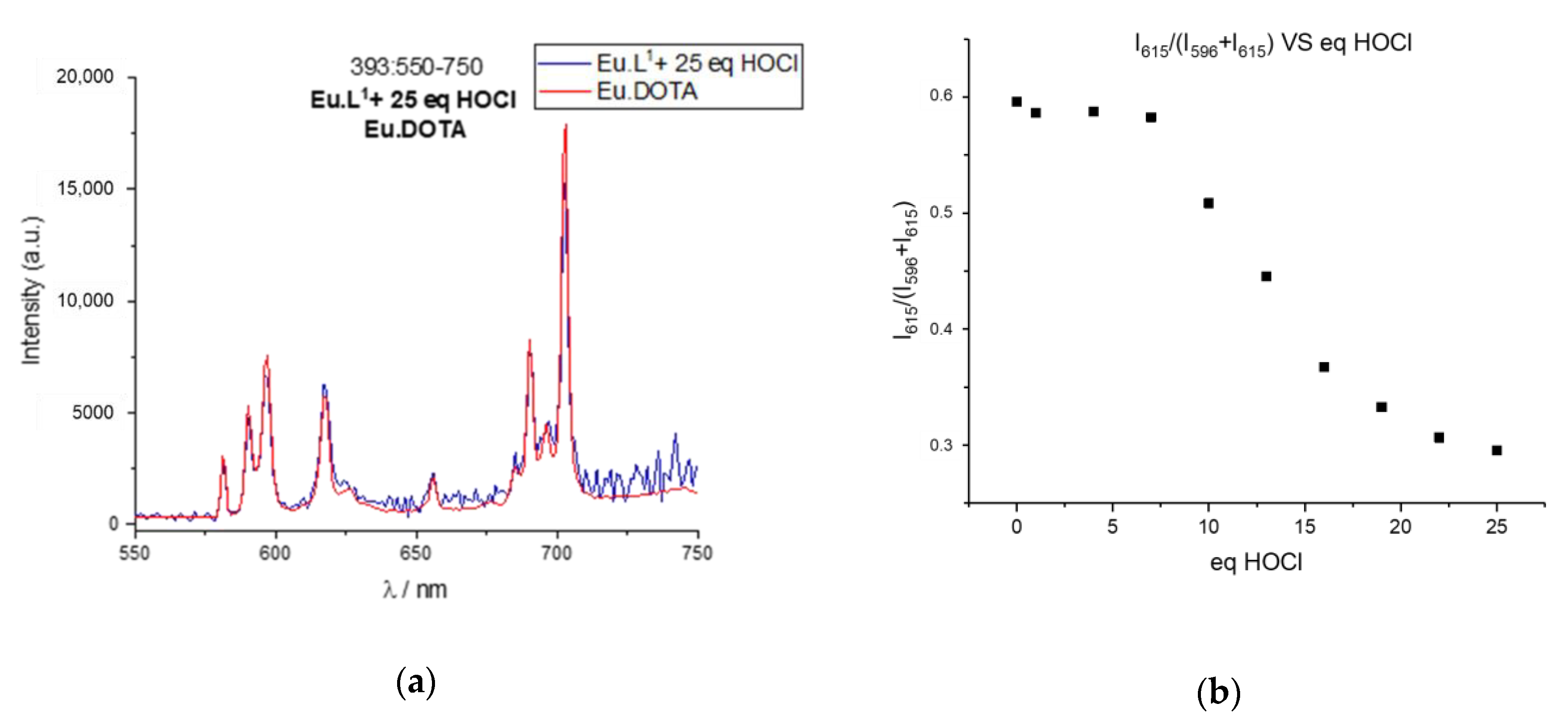
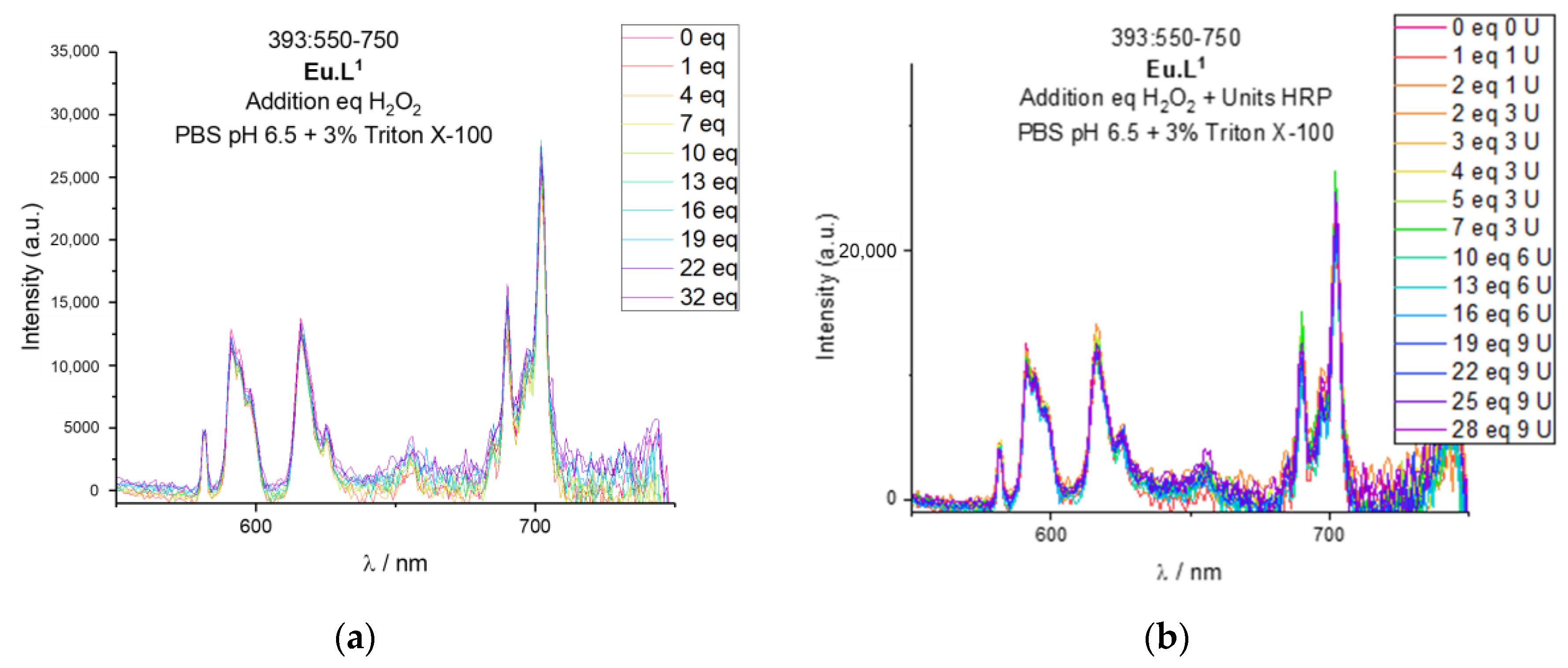
2.4. Response to Reactive Oxygen Species
3. Materials and Methods
3.1. General
3.2. Synthetic Procedures
3.2.1. Synthesis of 4-Hydroxyphenyl 2-chloroacetate (1)
3.2.2. Synthesis of 4-((Tert-butyldimethylsilyl)oxy)phenyl 2-chloroacetate (2)
3.2.3. Synthesis of 1,4-Phenylene bis(2-chloroacetate) (3)
3.2.4. Synthesis of Tri-tert-butyl 2,2′,2′′-(10-(2-(4-((tert-butyldimethylsilyl)oxy)phenoxy)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate (4)
3.2.5. Synthesis of Hexa-tert-butyl 2,2′,2′′-(10-(1,4-phenylene) bis-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)acetate (5)
3.2.6. Synthesis of Dibenzyl 1,4,7,10-tetraazacyclododecane-1,7-dicarboxylate (6)
3.2.7. Synthesis of Dibenzyl 4,10-bis(2-(tert-Butoxy)-2-Oxoethyl)-1,4,7,10-tetraazacyclododecane-1,7-dicarboxylate (7)
3.2.8. Synthesis of Di-tert-butyl 2,2′-(1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetate (8)
3.2.9. Synthesis of Di-tert-butyl 2,2′-(4,10-bis(2-(4-((tert-butyldimethylsilyl)oxy)phenoxy)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetate (9)
3.2.10. Synthesis of 2,2′,2′′-(10-(2-(4-Hydroxyphenoxy)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-Triyl)triacetic acid (L1)
3.2.11. Synthesis of 10-(1,4-Phenylene) bis-1,4,7,10-tetraazacyclododecane-1,4,7-Triyl)acetate (L2)
3.2.12. Synthesis of 2,2′-(4,10-Bis(2-(4-hydroxyphenoxy)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetic Acid (L3)
3.3. Synthesis of the Complexes
3.3.1. Eu.L1
3.3.2. Eu.L2
3.3.3. Eu.L3
3.3.4. Tb.L1
3.3.5. Tb.L2
3.3.6. Tb.L3
3.4. Oxidation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Andina, D.; Leroux, J.C.; Luciani, P. Ratiometric fluorescent probes for the detection of reactive oxygen species. Chemistry 2017, 23, 13549–13573. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.P.; Holmgren, A.; Larsson, N.G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Carroll, S.L.; Chen, J.; Wang, M.C.; Wang, J. Challenges and opportunities for small-molecule fluorescent probes in redox biology applications. Antioxid. Redox Signal. 2018, 29, 518–540. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Song, B.; Yuan, J. Bioanalytical methods for hypochlorous acid detection: Recent advances and challenges. TrAC Trends Anal. Chem. 2018, 99, 1–33. [Google Scholar] [CrossRef]
- Chen, X.; Tian, X.; Shin, I.; Yoon, J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2011, 40, 4783–4804. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ye, Z.; Wang, G.; Yuan, J. A ratiometric luminescence probe for highly reactive oxygen species based on lanthanide complexes. Inorg. Chem. 2012, 51, 2940–2946. [Google Scholar] [CrossRef] [PubMed]
- Haugland, R.P. Handbook of Fluorescent Probes and Research Chemicals, 11th ed.; Molecular Probes: Eugene, OR, USA, 2010. [Google Scholar]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., 2nd; Ischiropoulos, H.; et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef]
- Bunzli, J.C.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Zwier, J.M.; Bazin, H.; Lamarque, L.; Mathis, G. Luminescent lanthanide cryptates: From the bench to the bedside. Inorg. Chem. 2014, 53, 1854–1866. [Google Scholar] [CrossRef]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide probes for bioresponsive imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef] [Green Version]
- Stasiuk, G.J.; Long, N.J. The ubiquitous DOTA and its derivatives: The impact of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid on biomedical imaging. Chem. Commun. (Camb. Engl.) 2013, 49, 2732–2746. [Google Scholar] [CrossRef]
- Nielsen, L.G.; Junker, A.K.R.; Sørensen, T.J. Composed in the f-block: Solution structure and function of kinetically inert lanthanide(iii) complexes. Dalton Trans. 2018, 47, 10360–10376. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, T.J.; Faulkner, S. Multimetallic lanthanide complexes: Using kinetic control to define complex multimetallic arrays. Acc. Chem. Res. 2018, 51, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Brücher, E.; Baranyai, Z.; Tircsó, G. The future of biomedical imaging: Synthesis and chemical properties of the DTPA and DOTA derivative ligands and their complexes. In Biomedical Imaging: The Chemistry of Labels, Probes, and Contrast Agents; Braddock, M., Ed.; Royal Society of Chemistry: Cambridge, UK, 2012; Chapter 5.2; pp. 208–260. [Google Scholar] [CrossRef]
- Parker, D. Luminescent lanthanide sensors for pH, pO2 and selected anions. Coord. Chem. Rev. 2000, 205, 109–130. [Google Scholar] [CrossRef] [Green Version]
- Junker, A.K.R.; Tropiano, M.; Faulkner, S.; Sørensen, T.J. Kinetically inert lanthanide complexes as reporter groups for binding of potassium by 18-crown-6. Inorg. Chem. 2016, 55, 12299–12308. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, G.; Chen, J.; Fu, X.; Zhang, W.; Yuan, J. Development of a novel terbium chelate-based luminescent chemosensor for time-resolved luminescence detection of intracellular Zn2+ ions. Biosens. Bioelectron. 2010, 26, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, K.; Liu, W.; Su, C.; Yan, X.; Tan, M.; Tang, Y. A novel luminescent chemosensor for detecting Hg(2+) based on the pendant benzo crown ether terbium complex. Dalton Trans. 2010, 39, 9763–9765. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.; Puckrin, R.; McMahon, B.K.; Pal, R.; Parker, D.; Pålsson, L.O. Induced circularly polarized luminescence arising from anion or protein binding to racemic emissive lanthanide complexes. Methods Appl. Fluoresc. 2014, 2. [Google Scholar] [CrossRef]
- Weitz, E.A.; Chang, J.Y.; Rosenfield, A.H.; Pierre, V.C. A selective luminescent probe for the direct time-gated detection of adenosine triphosphate. J. Am. Chem. Soc. 2012, 134, 16099–16102. [Google Scholar] [CrossRef]
- Hewitt, S.H.; Parris, J.; Mailhot, R.; Butler, S.J. A continuous luminescence assay for monitoring kinase activity: Signalling the ADP/ATP ratio using a discrete europium complex. Chem. Commun. (Camb. Engl.) 2017, 53, 12626–12629. [Google Scholar] [CrossRef] [Green Version]
- Mailhot, R.; Traviss-Pollard, T.; Pal, R.; Butler, S.J. Cationic europium complexes for visualizing fluctuations in mitochondrial atp levels in living cells. Chem. A Eur. J. 2018, 24, 10745–10755. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, S.H.; Ali, R.; Mailhot, R.; Antonen, C.R.; Dodson, C.A.; Butler, S.J. A simple, robust, universal assay for real-time enzyme monitoring by signalling changes in nucleoside phosphate anion concentration using a europium(iii)-based anion receptor. Chem. Sci. 2019, 10, 5373–5381. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, S.H.; Macey, G.; Mailhot, R.; Elsegood, M.R.J.; Duarte, F.; Kenwright, A.M.; Butler, S.J. Tuning the anion binding properties of lanthanide receptors to discriminate nucleoside phosphates in a sensing array. Chem. Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Page, S.E.; Wilke, K.T.; Pierre, V.C. Sensitive and selective time-gated luminescence detection of hydroxyl radical in water. Chem. Commun. (Camb. Engl.) 2010, 46, 2423–2425. [Google Scholar] [CrossRef]
- Lippert, A.R.; Gschneidtner, T.; Chang, C.J. Lanthanide-based luminescent probes for selective time-gated detection of hydrogen peroxide in water and in living cells. Chem. Commun. (Camb. Engl.) 2010, 46, 7510–7512. [Google Scholar] [CrossRef]
- Smith, D.G.; McMahon, B.K.; Pal, R.; Parker, D. Live cell imaging of lysosomal pH changes with pH responsive ratiometric lanthanide probes. Chem. Commun. (Camb. Engl.) 2012, 48, 8520–8522. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Z.; Xin, C.; Yuan, J. Development of a ratiometric time-resolved luminescence sensor for pH based on lanthanide complexes. Anal. Chim. Acta 2013, 761, 149–156. [Google Scholar] [CrossRef]
- Breen, C.; Pal, R.; Elsegood, M.R.J.; Teat, S.J.; Iza, F.; Wende, K.; Buckley, B.R.; Butler, S.J. Time-resolved luminescence detection of peroxynitrite using a reactivity-based lanthanide probe. Chem. Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Wolfbeis, O.S.; Dürkop, A.; Wu, M.; Lin, Z. A europium-ion-based luminescent sensing probe for hydrogen peroxide. Angew. Chem. Int. Ed. 2002, 41, 4495–4498. [Google Scholar] [CrossRef]
- Hong, J.; Zhuang, Y.; Ji, X.; Guo, X. A long-lived luminescence and EPR bimodal lanthanide-based probe for free radicals. Analyst 2011, 136, 2464–2470. [Google Scholar] [CrossRef] [Green Version]
- Pershagen, E.; Nordholm, J.; Borbas, K.E. Luminescent lanthanide complexes with analyte-triggered antenna formation. J. Am. Chem. Soc. 2012, 134, 9832–9835. [Google Scholar] [CrossRef]
- Kaur, A.; Kolanowski, J.L.; New, E.J. Reversible fluorescent probes for biological redox states. Angew. Chem. 2016, 55, 1602–1613. [Google Scholar] [CrossRef]
- Shuvaev, S.; Starck, M.; Parker, D. Responsive, water-soluble Europium(III) luminescent probes. Chem. A Eur. J. 2017, 23, 9974–9989. [Google Scholar] [CrossRef]
- Szijjarto, C.; Pershagen, E.; Ilchenko, N.O.; Borbas, K.E. A versatile long-wavelength-absorbing scaffold for Eu-based responsive probes. Chemistry 2013, 19, 3099–3109. [Google Scholar] [CrossRef]
- Cui, G.; Ye, Z.; Chen, J.; Wang, G.; Yuan, J. Development of a novel terbium(III) chelate-based luminescent probe for highly sensitive time-resolved luminescence detection of hydroxyl radical. Talanta 2011, 84, 971–976. [Google Scholar] [CrossRef]
- Liu, X.; Guo, L.; Song, B.; Tang, Z.; Yuan, J. Development of a novel europium complex-based luminescent probe for time-gated luminescence imaging of hypochlorous acid in living samples. Methods Appl. Fluoresc. 2017, 5. [Google Scholar] [CrossRef]
- Peterson, K.L.; Margherio, M.J.; Doan, P.; Wilke, K.T.; Pierre, V.C. Basis for sensitive and selective time-delayed luminescence detection of hydroxyl radical by lanthanide complexes. Inorg. Chem. 2013, 52, 9390–9398. [Google Scholar] [CrossRef] [Green Version]
- Tropiano, M.; Kilah, N.L.; Morten, M.; Rahman, H.; Davis, J.J.; Beer, P.D.; Faulkner, S. Reversible luminescence switching of a redox-active ferrocene-europium dyad. J. Am. Chem. Soc. 2011, 133, 11847–11849. [Google Scholar] [CrossRef]
- Lou, Z.; Li, P.; Pan, Q.; Han, K. A reversible fluorescent probe for detecting hypochloric acid in living cells and animals: Utilizing a novel strategy for effectively modulating the fluorescence of selenide and selenoxide. Chem. Commun. (Camb. Engl.) 2013, 49, 2445–2447. [Google Scholar] [CrossRef]
- Koide, Y.; Kawaguchi, M.; Urano, Y.; Hanaoka, K.; Komatsu, T.; Abo, M.; Terai, T.; Nagano, T. A reversible near-infrared fluorescence probe for reactive oxygen species based on Te-rhodamine. Chem. Commun. (Camb. Engl.) 2012, 48, 3091–3093. [Google Scholar] [CrossRef]
- Hopper, L.E.; Allen, M.J. Rapid synthesis of 1,7-bis(t-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (DO2A-t-Bu ester). Tetrahedron Lett. 2014, 55, 5560–5561. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, L.G.; Sørensen, T.J. Including and declaring structural fluctuations in the study of Lanthanide(III) coordination chemistry in solution. Inorg. Chem. 2019. [Google Scholar] [CrossRef]
- Beeby, A.; Clarkson, I.M.; Dickins, R.S.; Faulkner, S.; Parker, D.; Royle, L.; de Sousa, A.S.; Williams, J.A.G.; Woods, M. Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: An improved luminescence method for establishing solution hydration states. J. Chem. Soc. Perkin Trans. 1999, 2, 493–504. [Google Scholar] [CrossRef]
- Tropiano, M.; Blackburn, O.A.; Tilney, J.A.; Hill, L.R.; Just Sørensen, T.; Faulkner, S. Exploring the effect of remote substituents and solution structure on the luminescence of three lanthanide complexes. J. Lumin. 2015, 167, 296–304. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Sudnick, D.R. Lanthanide ion probes of structure in biology. Laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water molecules. J. Am. Chem. Soc. 1979, 101, 334–340. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| Compound | 0.1 M HEPES pH 7.2 | D2O 1 |
|---|---|---|
| Eu.L1 | 0.524 | 1.391 |
| Eu.L2 | 0.086 (17%)/0.546 (83%) | 0.792 |
| Eu.L3 | 0.259 | 0.917 |
| Tb.L1 | 1.273 | 1.931 |
| Tb.L2 | 0.420 | 0.776 |
| Tb.L3 | 0.366 | 0.929 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giorgio, E.; Sørensen, T.J. HOCl Responsive Lanthanide Complexes Using Hydroquinone Caging Units. Molecules 2020, 25, 1959. https://doi.org/10.3390/molecules25081959
Del Giorgio E, Sørensen TJ. HOCl Responsive Lanthanide Complexes Using Hydroquinone Caging Units. Molecules. 2020; 25(8):1959. https://doi.org/10.3390/molecules25081959
Chicago/Turabian StyleDel Giorgio, Elena, and Thomas Just Sørensen. 2020. "HOCl Responsive Lanthanide Complexes Using Hydroquinone Caging Units" Molecules 25, no. 8: 1959. https://doi.org/10.3390/molecules25081959






