Membrane Phospholipids and Polyphosphates as Cofactors and Binding Molecules of SERPINA12 (vaspin)
Abstract
1. Introduction
2. Results
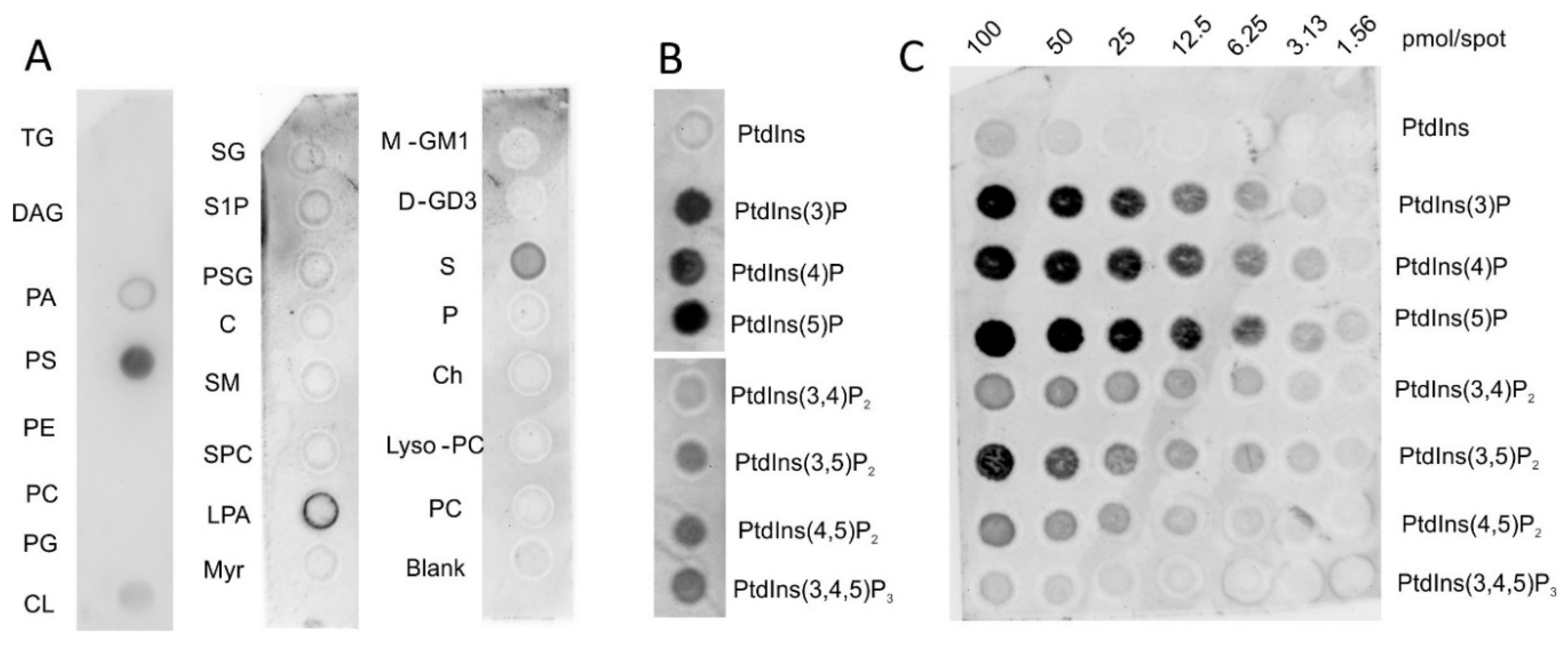
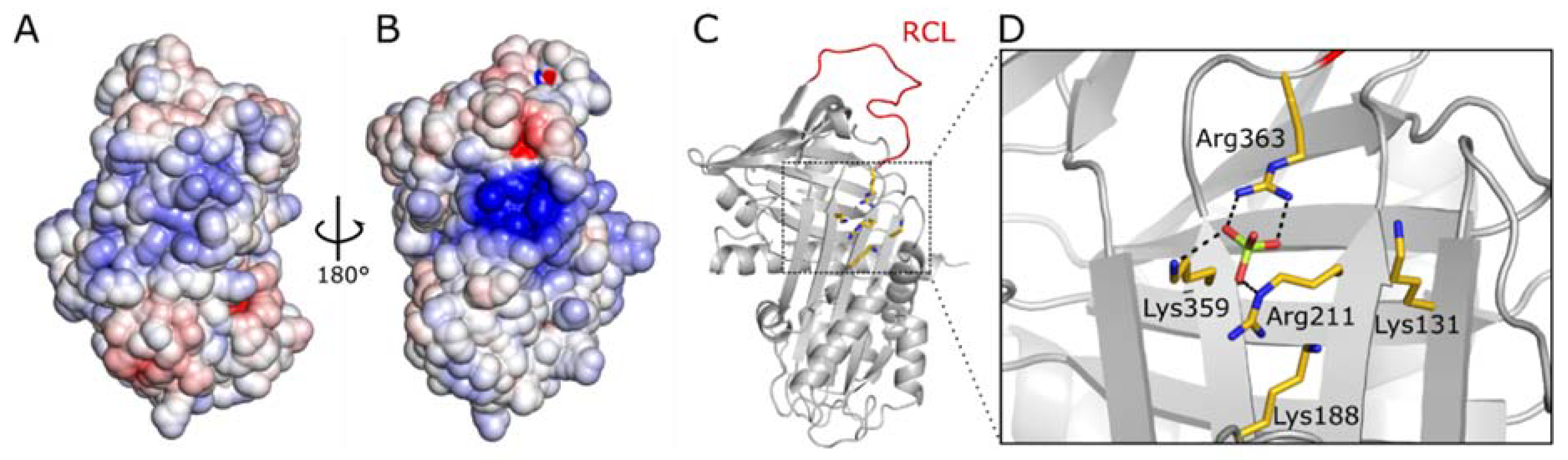
2.1. Vaspin Binds Membrane Phospholipids Phosphatidylinositol Phosphates and Phosphoserine
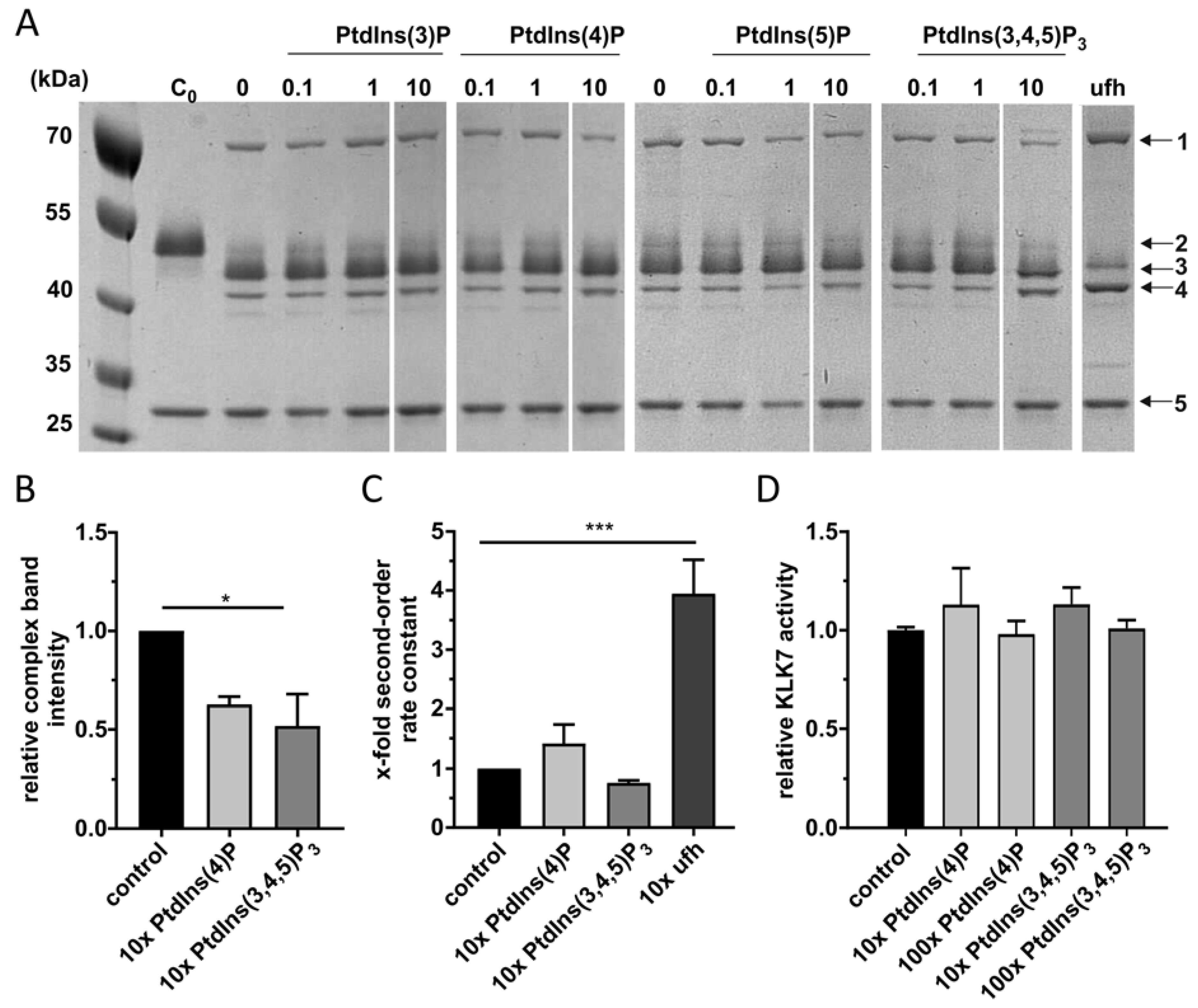
2.2. PtdInsPs Do Not Affect KLK7 Inhibition by Vaspin
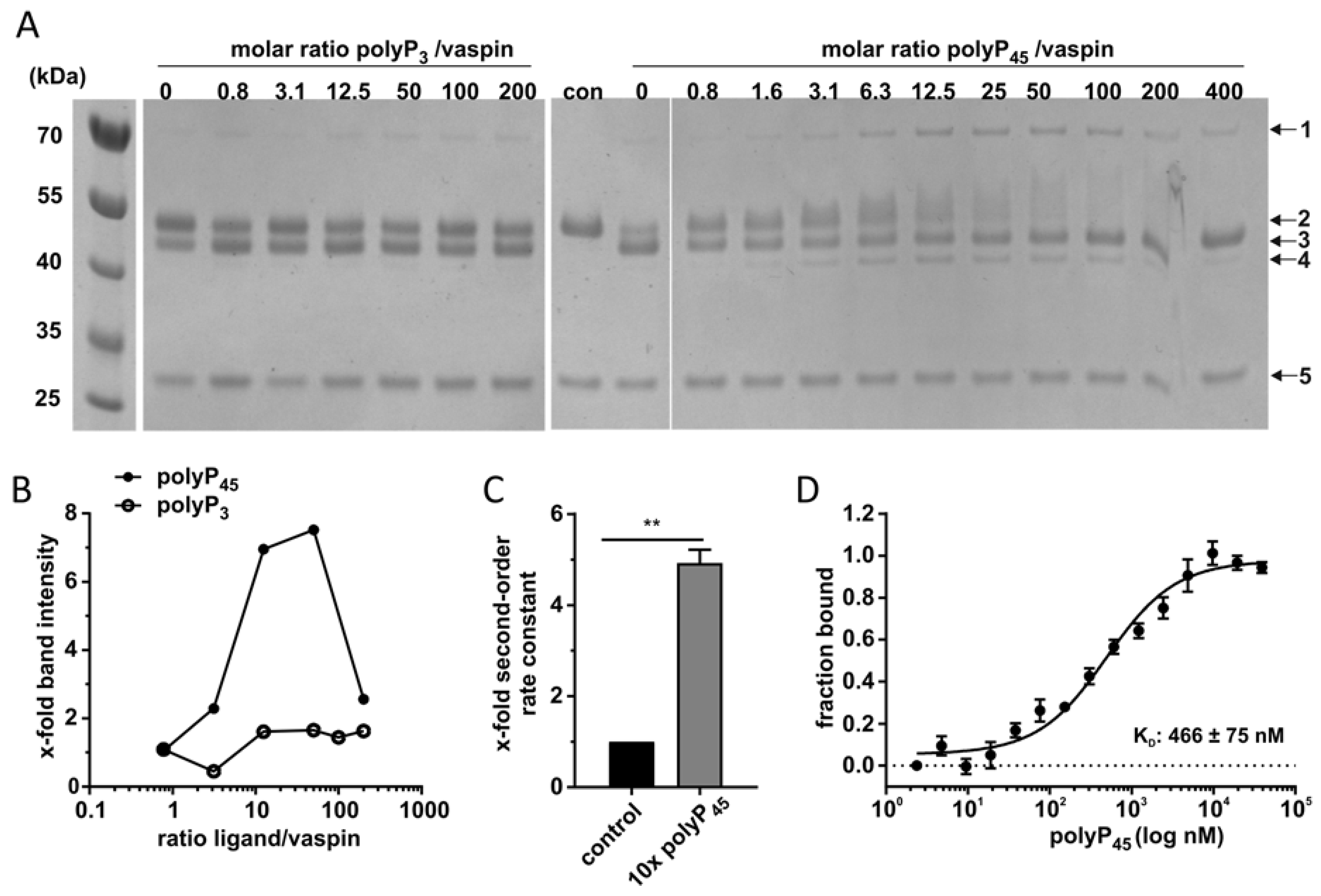
2.3. High-Affinity PolyP45 Binding Accelerates Vaspin-KLK7 Complex Formation
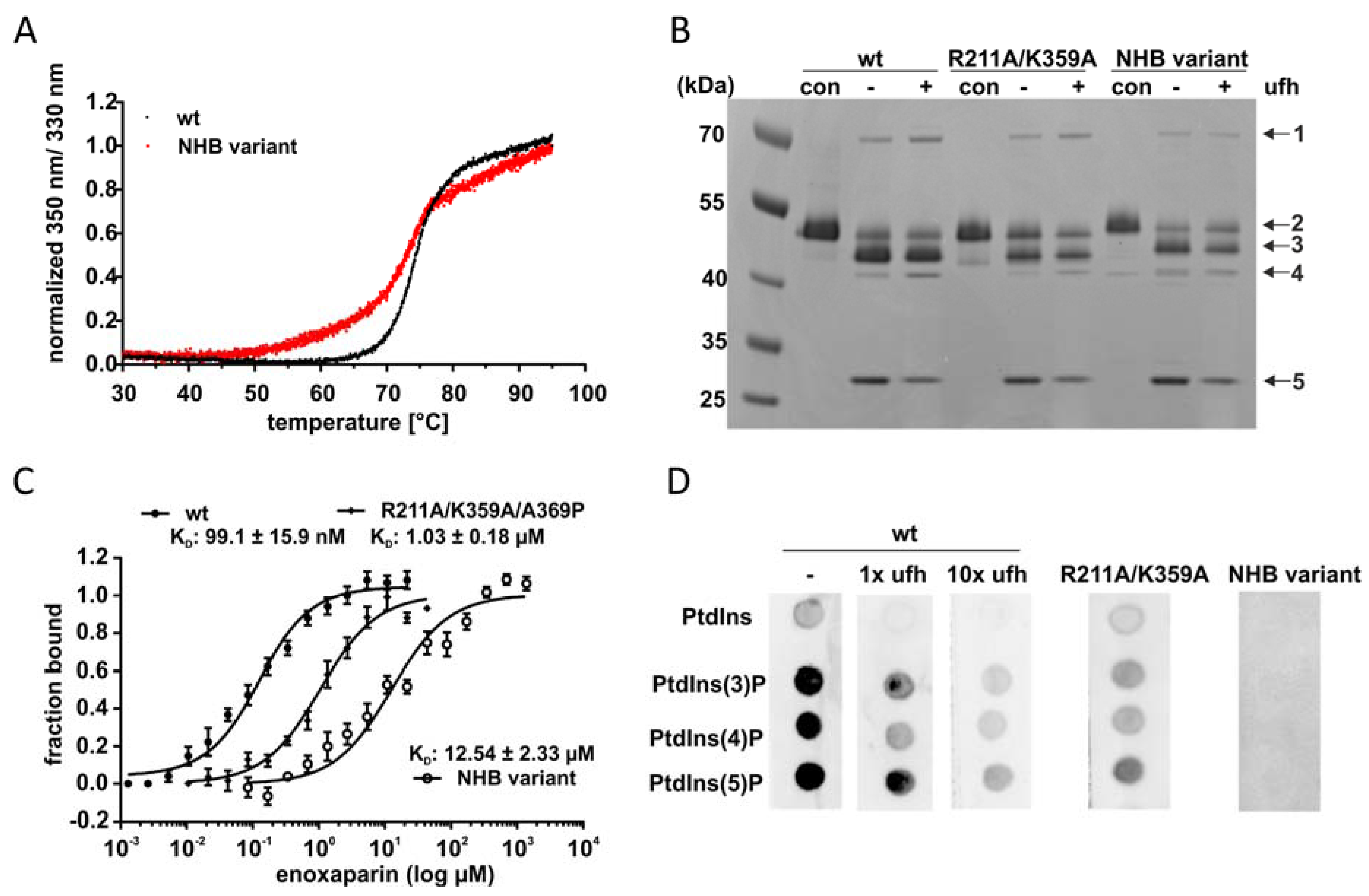
2.4. PtdInsPs and Heparin Share the Same Binding Site
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Lipid Overlay Assays
4.3. Complex Formation
4.4. Kinetics
4.5. Microscale Thermophoresis (MST)
4.6. Nano Differential Scanning Fluorimetry (NanoDSF)
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| KLK7 | kallikrein 7 |
| MST | microscale thermophoresis |
| PtdInsP | phosphatidylinositol phosphate |
| RCL | reactive center loop |
| PCI | protein C inhibitor |
| polyP | polyphosphate |
| ufh | unfractionated heparin |
References
- Law, R.H.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An overview of the serpin superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hida, K.; Wada, J.; Zhang, H.; Hiragushi, K.; Tsuchiyama, Y.; Shikata, K.; Makino, H. Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats. J. Lipid Res. 2000, 41, 1615–1622. [Google Scholar] [PubMed]
- Youn, B.S.; Kloting, N.; Kratzsch, J.; Lee, N.; Park, J.W.; Song, E.S.; Ruschke, K.; Oberbach, A.; Fasshauer, M.; Stumvoll, M.; et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008, 57, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Li, Y.; Wang, C.; Luo, C.; Liu, L.; Chuo, F.; Li, Q.; Sun, C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 88–94. [Google Scholar] [CrossRef]
- Weiner, J.; Zieger, K.; Pippel, J.; Heiker, J.T. Molecular Mechanisms of Vaspin Action-From Adipose Tissue to Skin and Bone, from Blood Vessels to the Brain. Adv. Exp. Med. Biol. 2019, 1111, 159–188. [Google Scholar] [PubMed]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [PubMed]
- Heiker, J.T.; Kloting, N.; Kovacs, P.; Kuettner, E.B.; Strater, N.; Schultz, S.; Kern, M.; Stumvoll, M.; Bluher, M.; Beck-Sickinger, A.G. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell. Mol. Life Sci. 2013, 70, 2569–2583. [Google Scholar] [CrossRef]
- Kloting, N.; Kovacs, P.; Kern, M.; Heiker, J.T.; Fasshauer, M.; Schon, M.R.; Stumvoll, M.; Beck-Sickinger, A.G.; Bluher, M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011, 54, 1819–1823. [Google Scholar] [CrossRef]
- Brunetti, L.; Di Nisio, C.; Recinella, L.; Chiavaroli, A.; Leone, S.; Ferrante, C.; Orlando, G.; Vacca, M. Effects of vaspin, chemerin and omentin-1 on feeding behavior and hypothalamic peptide gene expression in the rat. Peptides 2011, 32, 1866–1871. [Google Scholar] [CrossRef]
- Luo, X.; Li, K.; Zhang, C.; Yang, G.; Yang, M.; Jia, Y.; Zhang, L.; Ma, Z.A.; Boden, G.; Li, L. Central administration of vaspin inhibits glucose production and augments hepatic insulin signaling in high-fat-diet-fed rat. Int. J. Obes. 2016, 40, 947–954. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, W.J.; Hwang, J.Y.; Seol, S.M.; Kim, Y.M.; Lee, Y.L.; Park, J.Y. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem. Biophys. Res. Commun. 2011, 413, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Phalitakul, S.; Okada, M.; Hara, Y.; Yamawaki, H. A novel adipocytokine, vaspin inhibits platelet-derived growth factor-BB-induced migration of vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2012, 423, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; Lee, Y.L.; Yoon, H.K.; Kang, S.W.; Lee, W.J.; Park, J.Y. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc. Diabetol. 2014, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dong, Y.; Wang, T.; Zhao, S.; Yang, K.; Chen, X.; Zheng, C. Vaspin inhibited proinflammatory cytokine induced activation of nuclear factor-kappa B and its downstream molecules in human endothelial EA.hy926 cells. Diabetes Res. Clin. Pract. 2014, 103, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Zieger, K.; Weiner, J.; Krause, K.; Schwarz, M.; Kohn, M.; Stumvoll, M.; Bluher, M.; Heiker, J.T. Vaspin suppresses cytokine-induced inflammation in 3T3-L1 adipocytes via inhibition of NFkappaB pathway. Mol. Cell. Endocrinol. 2018, 460, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Saalbach, A.; Tremel, J.; Herbert, D.; Schwede, K.; Wandel, E.; Schirmer, C.; Anderegg, U.; Beck-Sickinger, A.G.; Heiker, J.T.; Schultz, S.; et al. Anti-Inflammatory Action of Keratinocyte-Derived Vaspin: Relevance for the Pathogenesis of Psoriasis. Am. J. Clin. Pathol. 2016, 186, 639–651. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 2012, 61, 2823–2832. [Google Scholar] [CrossRef]
- Ulbricht, D.; Tindall, C.A.; Oertwig, K.; Hanke, S.; Strater, N.; Heiker, J.T. Kallikrein-related peptidase 14 is the second KLK protease targeted by the serpin vaspin. Biol. Chem. 2018, 399, 1079–1084. [Google Scholar] [CrossRef]
- Huntington, J.A.; Read, R.J.; Carrell, R.W. Structure of a serpin-protease complex shows inhibition by deformation. Nature 2000, 407, 923–926. [Google Scholar] [CrossRef]
- Ulbricht, D.; Pippel, J.; Schultz, S.; Meier, R.; Strater, N.; Heiker, J.T. A unique serpin P1′ glutamate and a conserved β-sheet C arginine are key residues for activity, protease recognition and stability of serpinA12 (vaspin). Biochem. J. 2015, 470, 357–367. [Google Scholar] [CrossRef]
- Zieger, K.; Weiner, J.; Kunath, A.; Gericke, M.; Krause, K.; Kern, M.; Stumvoll, M.; Kloting, N.; Bluher, M.; Heiker, J.T. Ablation of kallikrein 7 (KLK7) in adipose tissue ameliorates metabolic consequences of high fat diet-induced obesity by counteracting adipose tissue inflammation in vivo. Cell. Mol. Life Sci. 2018, 75, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Kasparek, P.; Ileninova, Z.; Zbodakova, O.; Kanchev, I.; Benada, O.; Chalupsky, K.; Brattsand, M.; Beck, I.M.; Sedlacek, R. KLK5 and KLK7 Ablation Fully Rescues Lethality of Netherton Syndrome-Like Phenotype. PLoS Genet. 2017, 13, e1006566. [Google Scholar] [CrossRef] [PubMed]
- Ekholm, E.; Egelrud, T. Stratum corneum chymotryptic enzyme in psoriasis. Arch. Dermatol. Res. 1999, 291, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.; Saalbach, A.; Heiker, J.T.; Meier, R.; Zellmann, T.; Simon, J.C.; Beck-Sickinger, A.G. Proteolytic activation of prochemerin by kallikrein 7 breaks an ionic linkage and results in C-terminal rearrangement. Biochem. J. 2013, 452, 271–280. [Google Scholar] [CrossRef]
- Ulbricht, D.; Oertwig, K.; Arnsburg, K.; Saalbach, A.; Pippel, J.; Strater, N.; Heiker, J.T. Basic Residues of β-Sheet A Contribute to Heparin Binding and Activation of Vaspin (Serpin A12). J. Biol. Chem. 2017, 292, 994–1004. [Google Scholar] [CrossRef]
- Gettins, P.G.; Olson, S.T. Exosite determinants of serpin specificity. J. Biol. Chem. 2009, 284, 20441–20445. [Google Scholar] [CrossRef]
- Whinna, H.C.; Blinder, M.A.; Szewczyk, M.; Tollefsen, D.M.; Church, F.C. Role of lysine 173 in heparin binding to heparin cofactor II. J. Biol. Chem. 1991, 266, 8129–8135. [Google Scholar]
- Ehrlich, H.J.; Gebbink, R.K.; Keijer, J.; Pannekoek, H. Elucidation of structural requirements on plasminogen activator inhibitor 1 for binding to heparin. J. Biol. Chem. 1992, 267, 11606–11611. [Google Scholar]
- Belzar, K.J.; Zhou, A.; Carrell, R.W.; Gettins, P.G.; Huntington, J.A. Helix D elongation and allosteric activation of antithrombin. J. Biol. Chem. 2002, 277, 8551–8558. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, A.; Rao, N.N.; Ault-Riche, D. Inorganic polyphosphate: A molecule of many functions. Annu. Rev. Biochem. 1999, 68, 89–125. [Google Scholar] [CrossRef] [PubMed]
- Wijeyewickrema, L.C.; Lameignere, E.; Hor, L.; Duncan, R.C.; Shiba, T.; Travers, R.J.; Kapopara, P.R.; Lei, V.; Smith, S.A.; Kim, H.; et al. Polyphosphate is a novel cofactor for regulation of complement by a serpin, C1 inhibitor. Blood 2016, 128, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Mutch, N.J.; Schenk, W.A.; Smith, S.A.; Esterl, L.; Spronk, H.M.; Schmidbauer, S.; Gahl, W.A.; Morrissey, J.H.; Renne, T. Platelet Polyphosphates Are Proinflammatory and Procoagulant Mediators In Vivo. Cell 2009, 139, 1143–1156. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Wahlmuller, F.C.; Sokolikova, B.; Rieger, D.; Geiger, M. New lipid interaction partners stimulate the inhibition of activated protein C by cell-penetrating protein C inhibitor. Thromb. Haemost. 2014, 111, 41–52. [Google Scholar] [CrossRef]
- Pemberton, J.G.; Balla, T. Polyphosphoinositide-Binding Domains: Insights from Peripheral Membrane and Lipid-Transfer Proteins. Adv. Exp. Med. Biol. 2019, 1111, 77–137. [Google Scholar]
- Malleier, J.M.; Oskolkova, O.; Bochkov, V.; Jerabek, I.; Sokolikova, B.; Perkmann, T.; Breuss, J.; Binder, B.R.; Geiger, M. Regulation of protein C inhibitor (PCI) activity by specific oxidized and negatively charged phospholipids. Blood 2007, 109, 4769–4776. [Google Scholar] [CrossRef]
- Wang, J.; Gambhir, A.; Hangyas-Mihalyne, G.; Murray, D.; Golebiewska, U.; McLaughlin, S. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J. Biol. Chem. 2002, 277, 34401–34412. [Google Scholar] [CrossRef]
- Laux, T.; Fukami, K.; Thelen, M.; Golub, T.; Frey, D.; Caroni, P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol. 2000, 149, 1455–1472. [Google Scholar] [CrossRef]
- Yeung, T.; Terebiznik, M.; Yu, L.; Silvius, J.; Abidi, W.M.; Philips, M.; Levine, T.; Kapus, A.; Grinstein, S. Receptor activation alters inner surface potential during phagocytosis. Science 2006, 313, 347–351. [Google Scholar] [CrossRef]
- McLaughlin, S.; Murray, D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 2005, 438, 605–611. [Google Scholar] [CrossRef]
- McCrea, H.J.; De Camilli, P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology 2009, 24, 8–16. [Google Scholar] [CrossRef]
- Balla, T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hortsman, H.; Seet, L.; Wong, S.H.; Hong, W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat. Cell. Biol. 2001, 3, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.W.; Di Paolo, G.; Diaz, E.; Cestra, G.; Diaz, M.E.; Lindau, M.; De Camilli, P.; Toomre, D. Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proc. Natl. Acad. Sci. USA 2005, 102, 5204–5209. [Google Scholar] [CrossRef]
- Milosevic, I.; Sorensen, J.B.; Lang, T.; Krauss, M.; Nagy, G.; Haucke, V.; Jahn, R.; Neher, E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 2005, 25, 2557–2565. [Google Scholar] [CrossRef]
- Balla, T.; Szentpetery, Z.; Kim, Y.J. Phosphoinositide signaling: New tools and insights. Physiology 2009, 24, 231–244. [Google Scholar] [CrossRef]
- Wenk, M.R.; De Camilli, P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: Insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. USA 2004, 101, 8262–8269. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Kim, Y.J.; Varnai, P.; Szentpetery, Z.; Knight, Z.; Shokat, K.M.; Balla, T. Maintenance of hormone-sensitive phosphoinositide pools in the plasma membrane requires phosphatidylinositol 4-kinase IIIalpha. Mol. Biol. Cell 2008, 19, 711–721. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Leevers, S.J.; Ahmadi, K.; Timms, J.; Katso, R.; Driscoll, P.C.; Woscholski, R.; Parker, P.J.; Waterfield, M.D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001, 70, 535–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, F.; Pei, H.X.; Zhu, X.; Lin, X.; Song, C.Y.; Liang, Q.H.; Liao, E.Y.; Yuan, L.Q. Vaspin regulates the osteogenic differentiation of MC3T3-E1 through the PI3K-Akt/miR-34c loop. Sci. Rep. 2016, 6, 25578. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.P.; Casati, M.Z.; Nociti, F.H., Jr.; Sallum, A.W.; Sallum, E.A.; Aukhil, I.; Wallet, S.M.; Shaddox, L.M. Connective tissue graft plus resin-modified glass ionomer restoration for the treatment of gingival recession associated with non-carious cervical lesions: Microbiological and immunological results. Clin. Oral Investig. 2013, 17, 67–77. [Google Scholar] [CrossRef]
- Rezaie, A.R. Calcium enhances heparin catalysis of the antithrombin-factor Xa reaction by a template mechanism. Evidence that calcium alleviates Gla domain antagonism of heparin binding to factor Xa. J. Biol. Chem. 1998, 273, 16824–16827. [Google Scholar] [CrossRef] [PubMed]
- Bray, B.; Lane, D.A.; Freyssinet, J.M.; Pejler, G.; Lindahl, U. Anti-thrombin activities of heparin. Effect of saccharide chain length on thrombin inhibition by heparin cofactor II and by antithrombin. Biochem. J. 1989, 262, 225–232. [Google Scholar] [CrossRef]
- Huang, X.; Rezaie, A.R.; Broze, G.J., Jr.; Olson, S.T. Heparin is a major activator of the anticoagulant serpin, protein Z-dependent protease inhibitor. J. Biol. Chem. 2011, 286, 8740–8751. [Google Scholar] [CrossRef]
- Muhl, L.; Galuska, S.P.; Oorni, K.; Hernandez-Ruiz, L.; Andrei-Selmer, L.C.; Geyer, R.; Preissner, K.T.; Ruiz, F.A.; Kovanen, P.T.; Kanse, S.M. High negative charge-to-size ratio in polyphosphates and heparin regulates factor VII-activating protease. FEBS J. 2009, 276, 4828–4839. [Google Scholar] [CrossRef]
- Ruiz, F.A.; Lea, C.R.; Oldfield, E.; Docampo, R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J. Biol. Chem. 2004, 279, 44250–44257. [Google Scholar] [CrossRef]
- Moreno-Sanchez, D.; Hernandez-Ruiz, L.; Ruiz, F.A.; Docampo, R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J. Biol. Chem. 2012, 287, 28435–28444. [Google Scholar] [CrossRef]
- Nickel, K.F.; Ronquist, G.; Langer, F.; Labberton, L.; Fuchs, T.A.; Bokemeyer, C.; Sauter, G.; Graefen, M.; Mackman, N.; Stavrou, E.X.; et al. The polyphosphate-factor XII pathway drives coagulation in prostate cancer-associated thrombosis. Blood 2015, 126, 1379–1389. [Google Scholar] [CrossRef]
- Verhoef, J.J.; Barendrecht, A.D.; Nickel, K.F.; Dijkxhoorn, K.; Kenne, E.; Labberton, L.; McCarty, O.J.; Schiffelers, R.; Heijnen, H.F.; Hendrickx, A.P.; et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood 2017, 129, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jakob, U. Inorganic polyphosphate, a multifunctional polyanionic protein scaffold. J. Biol. Chem. 2019, 294, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Lee, W.; Rezaie, A.R. Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models. J. Thromb. Haemost. 2012, 10, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Hassanian, S.M.; Qureshi, S.H.; Manithody, C.; Eissenberg, J.C.; Yang, L.; Rezaie, A.R. Polyphosphate amplifies proinflammatory responses of nuclear proteins through interaction with receptor for advanced glycation end products and P2Y1 purinergic receptor. Blood 2014, 123, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, B.; Leuck, J.; Kohl, D.; Muller, W.E.; Schroder, H.C. Anti-HIV-1 activity of inorganic polyphosphates. J. Acquir. Immune Defic. Syndr. 1997, 14, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Santi, M.J.; Montilla, M.; Carroza, M.A.; Ruiz, F.A. Novel assay for prothrombotic polyphosphates in plasma reveals their correlation with obesity. Thromb. Res. 2016, 144, 53–55. [Google Scholar] [CrossRef]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 2010, 1, 100. [Google Scholar] [CrossRef]
- Seidel, S.A.; Dijkman, P.M.; Lea, W.A.; van den Bogaart, G.; Jerabek-Willemsen, M.; Lazic, A.; Joseph, J.S.; Srinivasan, P.; Baaske, P.; Simeonov, A.; et al. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods 2013, 59, 301–315. [Google Scholar] [CrossRef]
- Oertwig, K.; Ulbricht, D.; Hanke, S.; Pippel, J.; Bellmann-Sickert, K.; Strater, N.; Heiker, J.T. Glycosylation of human vaspin (SERPINA12) and its impact on serpin activity, heparin binding and thermal stability. Biochim. Biophys. Acta. Proteins. Proteom. 2017, 1865, 1188–1194. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tindall, C.A.; Dommel, S.; Riedl, V.; Ulbricht, D.; Hanke, S.; Sträter, N.; Heiker, J.T. Membrane Phospholipids and Polyphosphates as Cofactors and Binding Molecules of SERPINA12 (vaspin). Molecules 2020, 25, 1992. https://doi.org/10.3390/molecules25081992
Tindall CA, Dommel S, Riedl V, Ulbricht D, Hanke S, Sträter N, Heiker JT. Membrane Phospholipids and Polyphosphates as Cofactors and Binding Molecules of SERPINA12 (vaspin). Molecules. 2020; 25(8):1992. https://doi.org/10.3390/molecules25081992
Chicago/Turabian StyleTindall, Catherine A., Sebastian Dommel, Veronika Riedl, David Ulbricht, Stefanie Hanke, Norbert Sträter, and John T. Heiker. 2020. "Membrane Phospholipids and Polyphosphates as Cofactors and Binding Molecules of SERPINA12 (vaspin)" Molecules 25, no. 8: 1992. https://doi.org/10.3390/molecules25081992
APA StyleTindall, C. A., Dommel, S., Riedl, V., Ulbricht, D., Hanke, S., Sträter, N., & Heiker, J. T. (2020). Membrane Phospholipids and Polyphosphates as Cofactors and Binding Molecules of SERPINA12 (vaspin). Molecules, 25(8), 1992. https://doi.org/10.3390/molecules25081992





