Imperatorin as a Promising Chemotherapeutic Agent against Human Larynx Cancer and Rhabdomyosarcoma Cells
Abstract
:1. Introduction
2. Results
2.1. IMP Exhibits no Cytotoxic Effects to Normal Human Skin Fibroblasts (HSF) and Significantly Reduces the Viability of Human Rhabdomyosarcoma (TE671) and Larynx Cancer (RK33) Cells
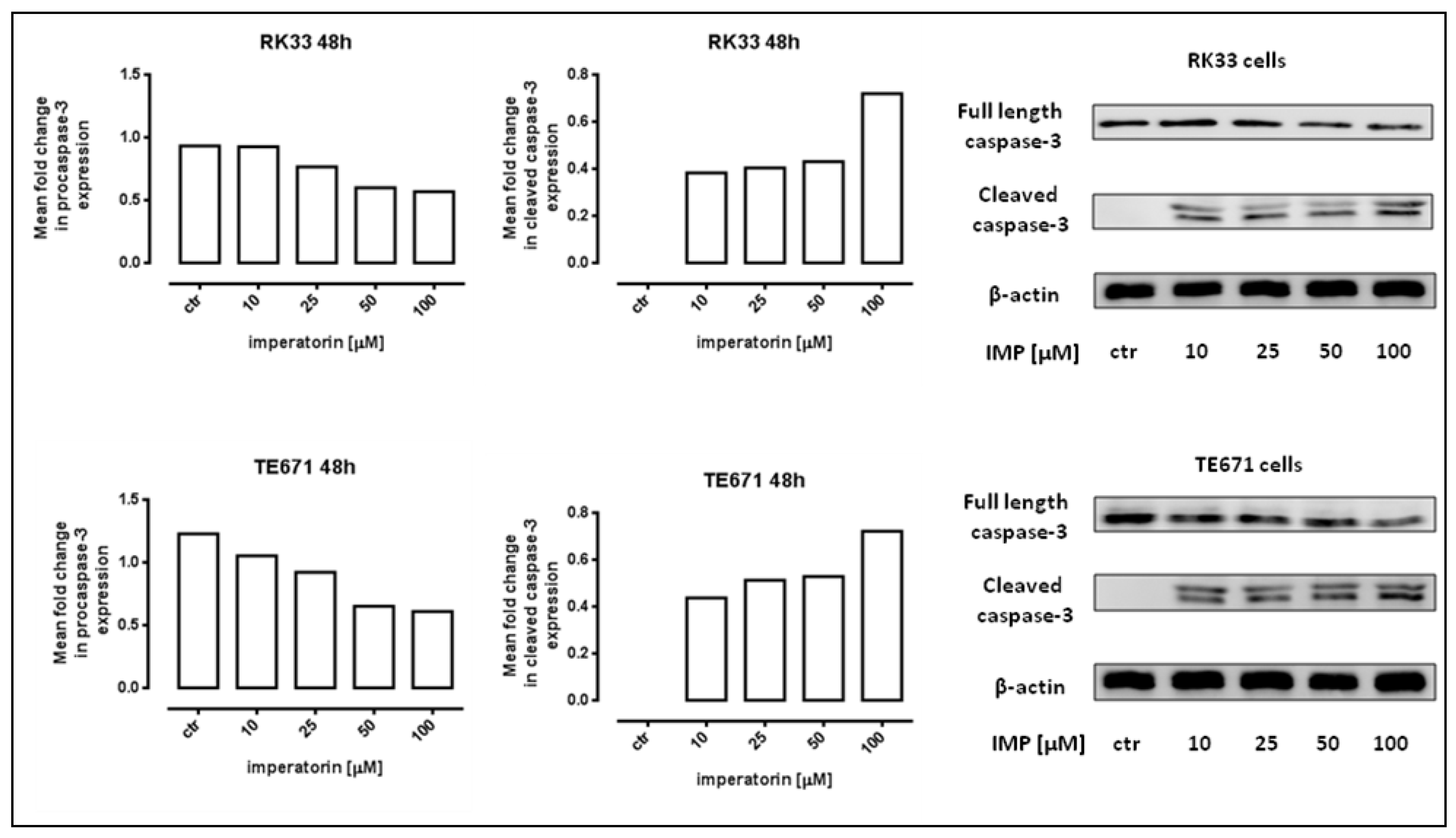
2.2. IMP Induced Apoptosis in Human Rhabdomyosarcoma and Larynx Cancer Cells
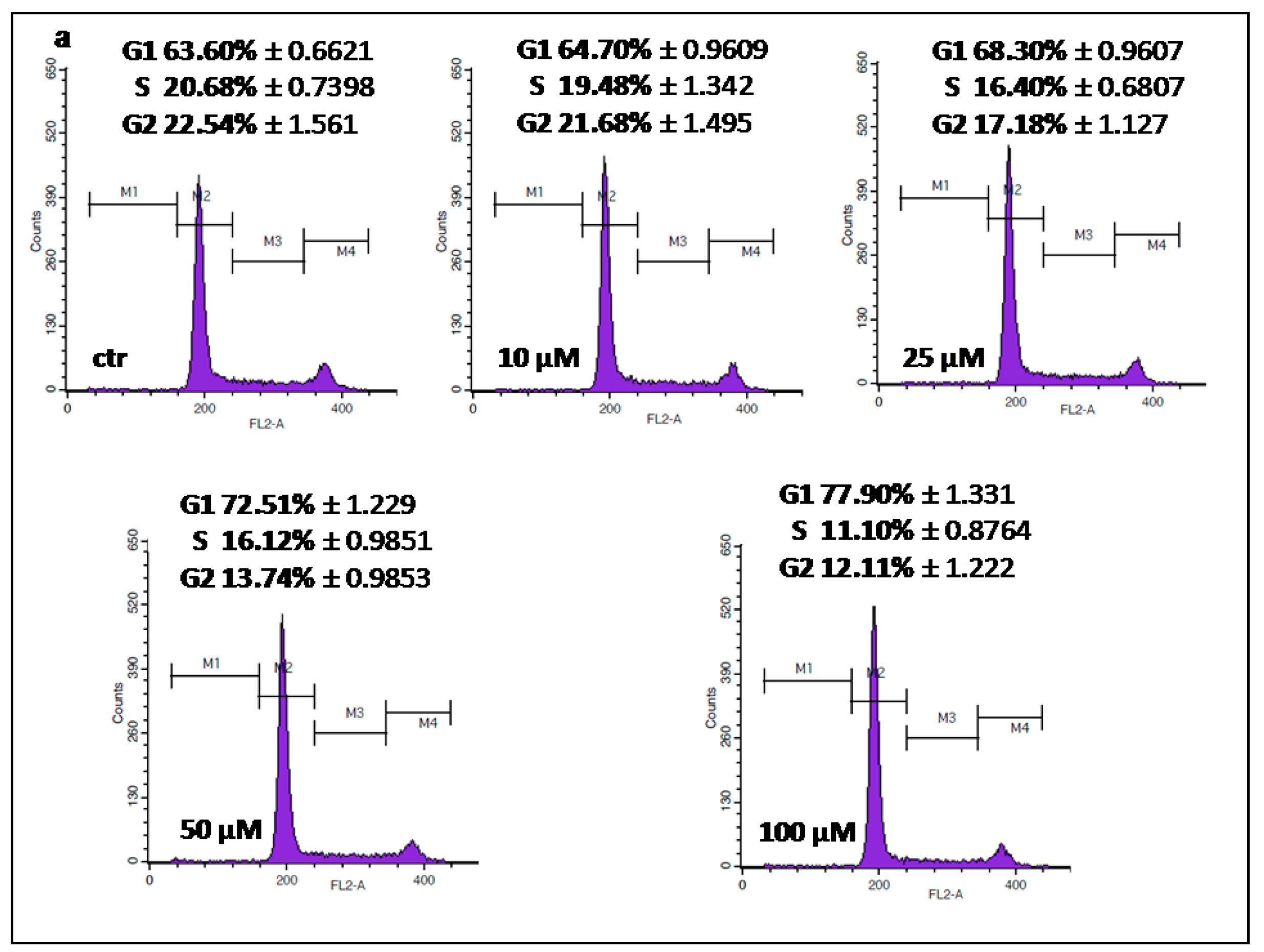
2.3. IMP Inhibited Cell Cycle Progression in the G1 Phase in Human Rhabdomyosarcoma and Larynx Cancer Cell Lines
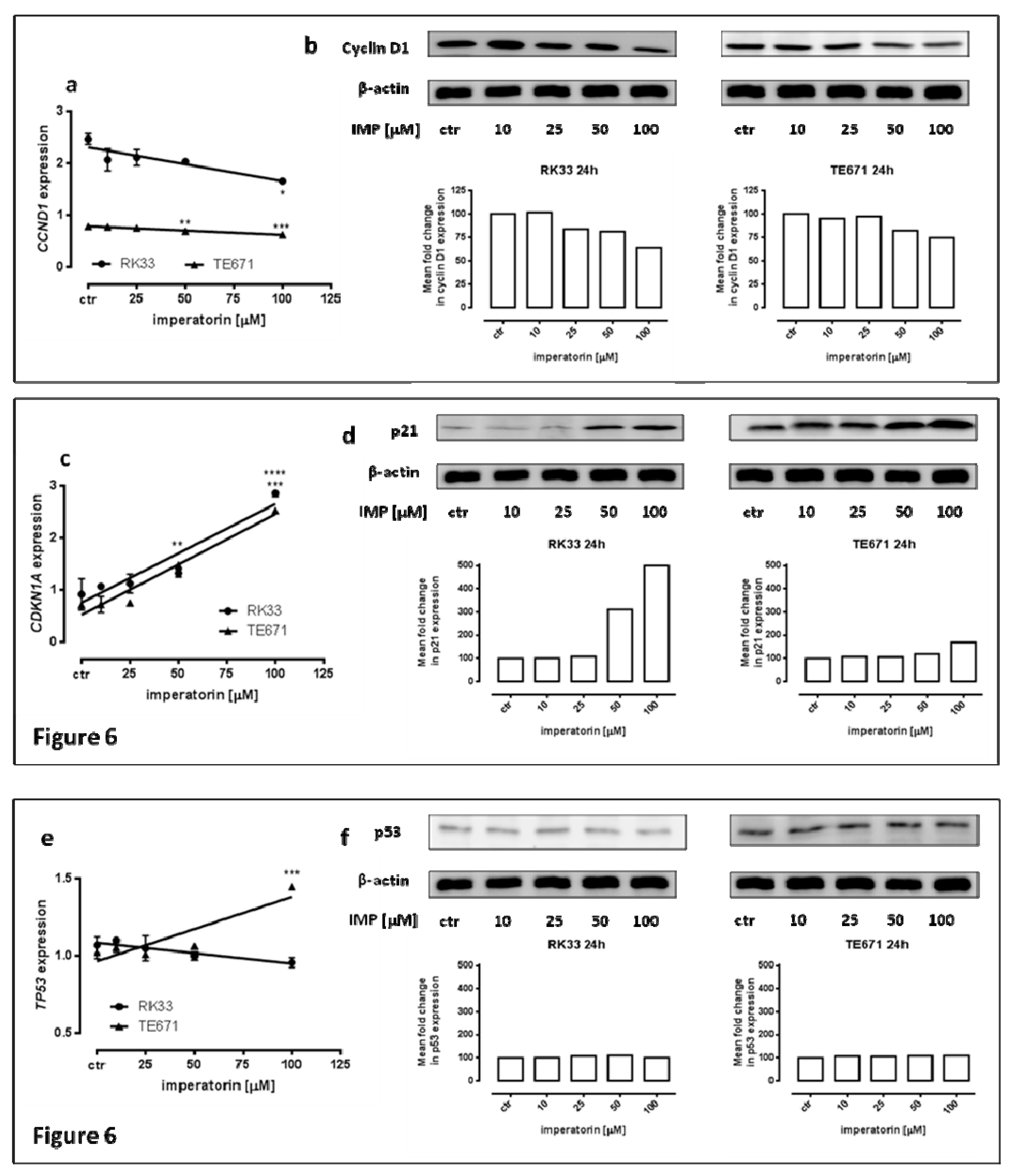
2.4. IMP Altered p21 and Cyclin D1 Expression on Protein and mRNA in Human Rhabdomyosarcoma and Larynx Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Cell Lines
4.4. Cell Viability Assessment
4.5. Cytotoxicity Assessment—LDH Assay
4.6. Assessment of Apoptosis
4.7. Cell Cycle Analysis
4.8. Protein Extraction and Western Blot Analysis
4.9. RNA Isolation and cDNA Synthesis
4.10. qPCR
4.11. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Fadlalla, K.; Watson, A.; Yehualaeshet, T.; Turner, T.; Samuel, T. Rutagraveolens extract induces DNA damage pathways and blocks Akt activation to inhibit cancer cell proliferation and survival. Anticancer Res. 2011, 31, 233–241. [Google Scholar]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.Q.; Yamazoe, Y. Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines. Acta Pharmacol. Sin. 2004, 25, 129–136. [Google Scholar] [PubMed]
- Klenkar, J.; Molnar, M. Natural and synthetic coumarins as potential anticancer agents. J. Chem. Pharm. Res. 2015, 7, 1223–1238. [Google Scholar]
- Panno, M.L.; Giordano, F. Effects of psoralens as anti-tumoral agents in breast cancer cells. World J. Clin. Oncol. 2014, 5, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Traven, V.F. New synthetic routes to furocoumarins and their analogs: A review. Molecules 2004, 9, 50–66. [Google Scholar] [CrossRef] [Green Version]
- Shalaby, N.M.; Abd-Alla, H.I.; Aly, H.F.; Albalawy, M.A.; Shaker, K.H.; Bouajila, J. Preliminary in vitro and in vivo evaluation of antidiabetic activity of Ducrosia anethifolia Boiss. And its linear furanocoumarins. Biomed. Res. Int. 2014, 2014, 480545. [Google Scholar] [CrossRef] [Green Version]
- Luszczki, J.J.; Andres-Mach, M.; Glensk, M.; Skalicka-Wozniak, K. Anticonvulsant effects of four linear furanocoumarins, bergapten, imperatorin, oxypeucedanin, and xanthotoxin, in the mouse maximal electroshock-induced seizure model: A comparative study. Pharmacol. Rep. 2010, 62, 1231–1236. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Zagaja, M.; Głowniak, K.; Łuszczki, J.J. Purification and anticonvulsant activity of xanthotoxin (8-methoxypsolaren). Cent. Eur. J. Biol. 2014, 9, 431–436. [Google Scholar]
- Bartnik, M.; Slawinska-Brych, A.; Zurek, A.; Kandefer-Szerszen, M.; Zdzisinska, B. 8-methoxypsoralen reduces Akt phosphorylation, induces intrinsic and extrinsic apoptotic pathways, and suppresses cell growth of SK-N-AS neuroblastoma and SW620 metastatic colon cancer cells. J. Ethnopharmacol. 2017, 207, 19–29. [Google Scholar] [CrossRef]
- Conforti, F.; Menichini, G.; Zanfini, L.; Tundis, R.; Statti, G.A.; Provenzano, E.; Menichini, F.; Somma, F.; Alfano, C. Evaluation of phototoxic potential of aerial components of the fig tree against human melanoma. Cell Prolif. 2012, 45, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiong, J.; Luo, W.; Yang, J.; Xi, T. 8-methoxypsoralen induces intrinsic apoptosis in HepG2 cells: Involvement of reactive oxygen species generation and ERK1/2 pathway inhibition. Cell Physiol. Biochem. 2015, 37, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Panno, M.L.; Giordano, F.; Palma, M.G.; Bartella, V.; Rago, V.; Maggiolini, M.; Sisci, D.; Lanzino, M.; De Amicis, F.; Ando, S. Evidence that bergapten, independently of its photoactivation, enhances p53 gene expression and induces apoptosis in human breast cancer cells. Curr. Cancer Drug Targets 2009, 9, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, C.; Cheng, B.; Jin, L.; Li, J.; Gong, Y.; Lin, W.; Pan, Z.; Pan, C. Imperatorin acts as a cisplatin sensitizer via downregulating Mcl-1 expression in HCC chemotherapy. Tumour. Biol. 2016, 37, 331–339. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.S.; Kim, S.H.; Shim, B.S.; Ahn, K.S. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, K.; Han, Y.; Zhang, G.; Dong, J.; Cui, Y.; Yang, Z. Effects of psoralen as an anti-tumor agent in human breast cancer MCF-7/ADR cells. Biol. Pharm. Bull. 2016, 39, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.J.; Chen, M.K.; Yu, Y.Y.; Sheu, G.T.; Chiou, H.L. Psoralen reverses docetaxel-induced multidrug resistance in A549/D16 human lung cancer cells lines. Phytomedicine 2014, 21, 970–977. [Google Scholar] [CrossRef]
- Skalicka-Wozniak, K.; Garrard, I. Counter-current chromatography for the separation of terpenoids: A comprehensive review with respect to the solvent systems employed. Phytochem. Rev. 2014, 13, 547–572. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.D.; Nahar, L. Natural medicine: The genus Angelica. Curr. Med. Chem. 2004, 11, 1479–1500. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Zou, Y.; Sarem, M.; Xiang, S.; Hu, H.; Xu, W.; Shastri, V.P. Autophagy inhibition enhances matrine derivative MASM induced apoptosis in cancer cells via a mechanism involving reactive oxygen species-mediated PI3K/Akt/mTOR and Erk/p38 signaling. BMC Cancer 2019, 19, 949. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, J.; Nakano, D.; Fujioka, T.; Okabe, H. Screening of promising chemotherapeutic candidates from plants extracts. J. Nat. Med. 2016, 70, 335–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkata Sairam, K.; Gurupadayya, B.M.; Chandan, R.S.; Nagesha, D.K.; Vishwanathan, B. A review on chemical profile of coumarins and their therapeutic role in the treatment of cancer. Curr. Drug Deliv. 2016, 13, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Kielbus, M.; Skalicka-Wozniak, K.; Grabarska, A.; Jeleniewicz, W.; Dmoszynska-Graniczka, M.; Marston, A.; Polberg, K.; Gawda, P.; Klatka, J.; Stepulak, A. 7-substituted coumarins inhibit proliferation and migration of laryngeal cancer cells in vitro. Anticancer Res. 2013, 33, 4347–4356. [Google Scholar]
- Jarzab, A.; Grabarska, A.; Kielbus, M.; Jeleniewicz, W.; Dmoszynska-Graniczka, M.; Skalicka-Wozniak, K.; Sieniawska, E.; Polberg, K.; Stepulak, A. Osthole induces apoptosis, suppresses cell-cycle progression and proliferation of cancer cells. Anticancer Res. 2014, 34, 6473–6480. [Google Scholar]
- Nakano, D.; Ishitsuka, K.; Matsuda, N.; Kouguchi, A.; Tsuchihashi, R.; Okawa, M.; Okabe, H.; Tamura, K.; Kinjo, J. Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (v): Coumarins and alkaloids from Boenninghausenia japonica and Ruta graveolens. J. Nat. Med. 2017, 71, 170–180. [Google Scholar] [CrossRef]
- Sigurdsson, S.; Ogmundsdottir, H.M.; Gudbjarnason, S. Antiproliferative effect of Angelica archangelica fruits. Z Naturforsch C 2004, 59, 523–527. [Google Scholar] [CrossRef]
- Koziol, E.; Skalicka-Wozniak, K. Imperatorin-pharmacological meaning and analytical clues: Profound investigation. Phytochem. Rev. 2016, 15, 627–649. [Google Scholar] [CrossRef] [Green Version]
- Badziul, D.; Jakubowicz-Gil, J.; Paduch, R.; Glowniak, K.; Gawron, A. Combined treatment with quercetin and imperatorin as a potent strategy for killing HeLa and Hep-2 cells. Mol. Cell Biochem. 2014, 392, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Rzeski, W.; Paduch, R.; Klatka, J.; Kandefer-Szerszen, M.; Stepulak, A.; Pozarowski, P.; Zdzisinska, B. Establishment and preliminary characterization of two cell lines derived from larynx carcinoma. Folia Histochem. Cytobiol. 2002, 40, 195–196. [Google Scholar] [PubMed]
- Wang, K.S.; Lv, Y.; Wang, Z.; Ma, J.; Mi, C.; Li, X.; Xu, G.H.; Piao, L.X.; Zheng, S.Z.; Jin, X. Imperatorin efficiently blocks TNF-alpha-mediated activation of ROS/PI3K/Akt/NF-kappaB pathway. Oncol. Rep. 2017, 37, 3397–3404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.M.; Lu, A.X.; Shen, J.Z.; Kwok, A.H.; Ho, W.S. Imperatorin exhibits anticancer activities in human colon cancer cells via the caspase cascade. Oncol. Rep. 2016, 35, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Hinson, A.R.; Jones, R.; Crose, L.E.; Belyea, B.C.; Barr, F.G.; Linardic, C.M. Human rhabdomyosarcoma cell lines for rhabdomyosarcoma research: Utility and pitfalls. Front. Oncol. 2013, 3, 183. [Google Scholar] [CrossRef] [Green Version]
- Felix, C.A.; Kappel, C.C.; Mitsudomi, T.; Nau, M.M.; Tsokos, M.; Crouch, G.D.; Nisen, P.D.; Winick, N.J.; Helman, L.J. Frequency and diversity of p53 mutations in childhood rhabdomyosarcoma. Cancer Res. 1992, 52, 2243–2247. [Google Scholar]
- Zhou, X.; Hao, Q.; Lu, H. Mutant p53 in cancer therapy-the barrier or the path. J. Mol. Cell. Biol. 2019, 11, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, M.; Fu, S.; Li, T.; Wang, S.; Zhao, M.; Ding, W.; Wang, C.; Wang, Q. Simultaneous determination of imperatorin and its metabolite xanthotoxol in rat plasma by using HPLC-ESI-MS coupled with hollow fiber liquid phase microextraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 945–946, 185–192. [Google Scholar] [CrossRef]
- Zhao, G.; Peng, C.; Du, W.; Wang, S. Simultaneous determination of imperatorin and its metabolites in vitro and in vivo by a GC-MS method: Application to a bioavailability and protein binding ability study in rat plasma. Biomed. Chromatogr. 2014, 28, 947–956. [Google Scholar] [CrossRef]
- Chen, L.; Jian, Y.; Wei, N.; Yuan, M.; Zhuang, X.; Li, H. Separation and simultaneous quantification of nine furanocoumarins from Radix Angelicae dahuricae using liquid chromatography with tandem mass spectrometry for bioavailability determination in rats. J. Sep. Sci. 2015, 38, 4216–4224. [Google Scholar] [CrossRef]
- Zhao, A.H.; Zhang, Y.B.; Yang, X.W. Simultaneous determination and pharmacokinetics of sixteen Angelicae dahurica coumarins in vivo by LC-ESI-MS/MS following oral delivery in rats. Phytomedicine 2016, 23, 1029–1036. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, G.; Zheng, C.; Song, M.; Liu, F.; Huang, X.; Bai, S.; Lin, C.; Zhu, C.; Hu, Y.; et al. Activating the pregnane X receptor by imperatorin attenuates dextran sulphate sodium-induced colitis in mice. Br. J. Pharmacol. 2018, 175, 3563–3580. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Shin, M.S.; Ham, I.; Choi, H.Y. Investigation of the mechanisms of Angelica dahurica root extract-induced vasorelaxation in isolated rat aortic rings. BMC Complement. Altern Med. 2015, 15, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marur, S.; Forastiere, A.A. Head and neck squamous cell carcinoma: Update on epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, B.; Boguszewska-Czubara, A.; Kruk-Slomka, M.; Skalicka-Wozniak, K.; Michalak, A.; Musik, I.; Biala, G.; Glowniak, K. Effects of imperatorin on nicotine-induced anxiety- and memory-related responses and oxidative stress in mice. Physiol. Behav. 2013, 122, 46–55. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
Sample Availability: Samples of the compounds imperatorin and xanthotoxin are available from the authors. |


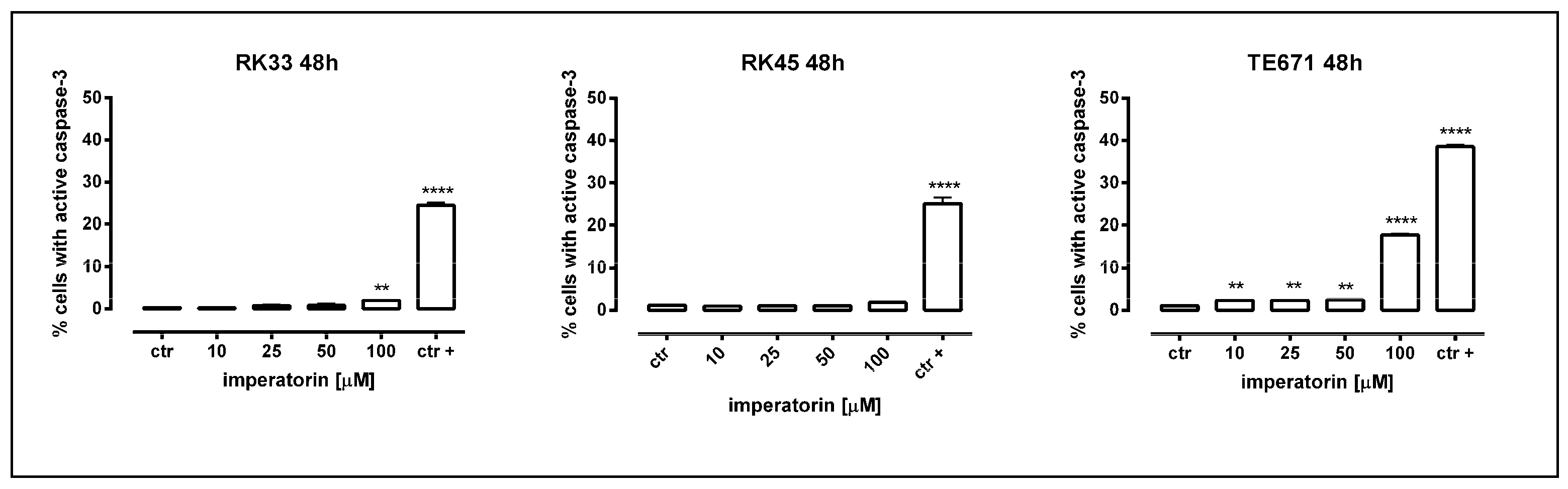

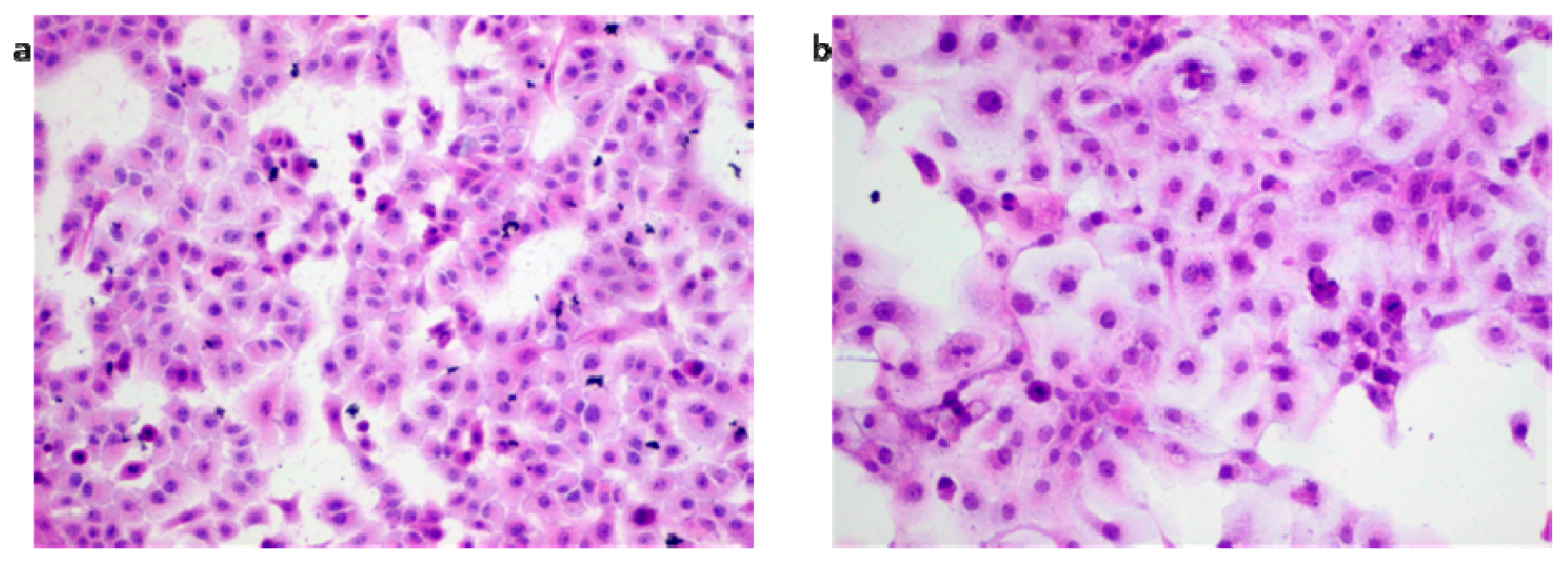
| ctr | ctr+ | 10 µM | 25 µM | 50 µM | 100 µM | |
|---|---|---|---|---|---|---|
| RK33 | 0.3100 | 24.51 | 0.2600 | 0.7533 | 0.8400 | 2.033 |
| RK45 | 1.190 | 25.12 | 1.000 | 1.090 | 1.157 | 1.870 |
| TE671 | 1.067 | 38.61 | 2.345 | 2.348 | 2.497 | 17.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabarska, A.; Skalicka-Woźniak, K.; Kiełbus, M.; Dmoszyńska-Graniczka, M.; Miziak, P.; Szumiło, J.; Nowosadzka, E.; Kowalczuk, K.; Khalifa, S.; Smok-Kalwat, J.; et al. Imperatorin as a Promising Chemotherapeutic Agent against Human Larynx Cancer and Rhabdomyosarcoma Cells. Molecules 2020, 25, 2046. https://doi.org/10.3390/molecules25092046
Grabarska A, Skalicka-Woźniak K, Kiełbus M, Dmoszyńska-Graniczka M, Miziak P, Szumiło J, Nowosadzka E, Kowalczuk K, Khalifa S, Smok-Kalwat J, et al. Imperatorin as a Promising Chemotherapeutic Agent against Human Larynx Cancer and Rhabdomyosarcoma Cells. Molecules. 2020; 25(9):2046. https://doi.org/10.3390/molecules25092046
Chicago/Turabian StyleGrabarska, Aneta, Krystyna Skalicka-Woźniak, Michał Kiełbus, Magdalena Dmoszyńska-Graniczka, Paulina Miziak, Justyna Szumiło, Ewa Nowosadzka, Krystyna Kowalczuk, Sherief Khalifa, Jolanta Smok-Kalwat, and et al. 2020. "Imperatorin as a Promising Chemotherapeutic Agent against Human Larynx Cancer and Rhabdomyosarcoma Cells" Molecules 25, no. 9: 2046. https://doi.org/10.3390/molecules25092046







