Porphyrin Derivative Nanoformulations for Therapy and Antiparasitic Agents
Abstract
:1. Introduction
2. General Aspects of the Porphyrin Properties
3. Structural Modifications Enabling Antiparasitic Activity
3.1. Porphyrins
3.2. Phthalocyanines
3.3. Expanded Porphyrins
4. Formulation Techniques
5. Applications of Porphyrins as Vector Control and Biosensors
6. Conclusions
Funding
Conflicts of Interest
References
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and Functionalization of Porphyrins through Organometallic Methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef] [PubMed]
- Urbani, M.; Gratzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Khan, M.A.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, R.K. Harper’s Biochemistry; Appleton & Lange: New Year, NY, USA, 2000. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry; W.H. Freeman and Company: New York, NY, USA, 2005. [Google Scholar]
- Huang, X.; Groves, J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [Google Scholar] [CrossRef] [Green Version]
- Stawski, W.; Kijewska, M.; Pawlicki, M. Multi-Cation Coordination in Porphyrinoids. Chem. Asian J. 2020, 15, 8–20. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Synthetic Chlorins, Possible Surrogates for Chlorophylls, Prepared by Derivatization of Porphyrins. Chem. Rev. 2017, 117, 344–535. [Google Scholar] [CrossRef]
- Graca, M.; Vicente, M.G.H. Porphyrins and derivatives: Synthetic strategies and reactivity profiles. Curr. Org. Chem. 2000, 4, 139–174. [Google Scholar]
- Kou, J.Y.; Dou, D.; Yang, L.M. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Liu, S.P.; Liu, Z.F.; Yang, J.D.; Zhu, J.H.; Qiao, M.; Hu, X.L. Fluorescence quenching and spectrophotometric methods for the determination of daunorubicin with meso-tera (4-sulphophenyl) porphyrin as probe. Spectrochim. Acta A 2014, 120, 7–13. [Google Scholar] [CrossRef]
- Martins, P.R.; Popolim, W.D.; Nagato, L.A.F.; Takemoto, E.; Araki, K.; Toma, H.E.; Angnes, L.; Penteado, M.D.C. Fast and reliable analyses of sulphite in fruit juices using a supramolecular amperometric detector encompassing in flow gas diffusion unit. Food Chem. 2011, 127, 249–255. [Google Scholar] [CrossRef]
- Esteves, C.H.A.; Iglesias, B.A.; Li, R.W.C.; Ogawa, T.; Araki, K.; Gruber, J. New composite porphyrin-conductive polymer gas sensors for application in electronic noses. Sens. Actuator B Chem. 2014, 193, 136–141. [Google Scholar] [CrossRef]
- Hamer, M.; Tomba, J.P.; Rezzano, I.N. Optical properties and sensor applications of bimetallic nanostructures of porphyrins. Sens. Actuator B Chem. 2014, 193, 121–127. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Arnaut, L.G.; Pereira, M.M.; Urbanska, K.; Stochel, G. Improved biodistribution, pharmacokinetics and photodynamic efficacy using a new photostable sulfonamide bacteriochlorin. Medchemcomm 2012, 3, 502–505. [Google Scholar] [CrossRef]
- Macdonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [Green Version]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef]
- Frochot, C.; Mordon, S. Update of the situation of clinical photodynamic therapy in Europe in the 2003–2018 period. J. Porphyr. Phthalocyanines 2019, 23, 347–357. [Google Scholar] [CrossRef]
- Baptista, M.S.; Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT) for the treatment of malaria, leishmaniasis and trypanosomiasis. Braz. J. Med. Biol. Res. 2011, 44, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Daskova, A.; Bogdanova, K.; Tomankova, K.; Binder, S.; Bajgar, R.; Kolar, M.; Mosinger, J.; Kolarova, H. Antimicrobial photodynamic therapy of porphyrins on bacterial cells. Eur. Biophys. J. 2011, 40, 43-43. [Google Scholar]
- Goslinski, T.; Piskorz, J. Fluorinated porphyrinoids and their biomedical applications. J. Photochem. Photobiol. C 2011, 12, 304–321. [Google Scholar] [CrossRef]
- Hanakova, A.; Bogdanova, K.; Tomankova, K.; Pizova, K.; Malohlava, J.; Binder, S.; Bajgar, R.; Langova, K.; Kolar, M.; Mosinger, J.; et al. The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiol. Res. 2014, 169, 163–170. [Google Scholar] [CrossRef]
- Baltazar, L.M.; Ray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J.; Nosanchuk, J.D. Antimicrobial photodynamic therapy: An effective alternative approach to control fungal infections. Front. Microbiol. 2015, 6, 202. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Marrero, V.; Gonzalez-Delgado, J.A.; Torres, T. Emerging Perspectives on Applications of Porphyrinoids for Photodynamic Therapy and Photoinactivation of Microorganisms. Macroheterocycles 2019, 12, 8–16. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef] [Green Version]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Elashnikov, R.; Radocha, M.; Panov, I.; Rirnpelova, S.; Ulbrich, P.; Michalcova, A.; Svorcik, V.; Lyutakov, O. Porphyrin-silver nanoparticles hybrids: Synthesis, characterization and antibacterial activity. Mater. Sci. Eng. C 2019, 102, 192–199. [Google Scholar] [CrossRef]
- Cruess, A.F.; Zlateva, G.; Pleil, A.M.; Wirostko, B. Photodynamic therapy with verteporfin in age-related macular degeneration: A systematic review of efficacy, safety, treatment modifications and pharmacoeconomic properties. Acta Ophthalmol. 2009, 87, 118–132. [Google Scholar] [CrossRef]
- Glowacka-Sobotta, A.; Wrotynski, M.; Kryjewski, M.; Sobotta, L.; Mielcarek, J. Porphyrinoids in photodynamic diagnosis and therapy of oral diseases. J. Porphyr. Phthalocyanines 2019, 23, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Invest. 2013, 17, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Trindade, A.C.; De Figueiredo, J.A.P.; Steier, L.; Weber, J.B.B. Photodynamic Therapy in Endodontics: A Literature Review. Photomed. Laser Surg. 2015, 33, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Al-Kheraif, A.A.; Qadri, T.; Hassan, M.I.A.; Ahmedef, A.; Warnakulasuriya, S.; Javed, F. Efficacy of photodynamic therapy in the management of oral premalignant lesions. A systematic review. Photodiagnosis Photodyn. Ther. 2015, 12, 150–159. [Google Scholar] [CrossRef] [PubMed]
- De Annunzio, S.R.; Costa, N.C.S.; Mezzina, R.D.; Graminha, M.A.S.; Fontana, C.R. Chlorin, Phthalocyanine, and Porphyrin Types Derivatives in Phototreatment of Cutaneous Manifestations: A Review. Int. J. Mol. Sci. 2019, 20, 3861. [Google Scholar] [CrossRef] [Green Version]
- Miguel-Gomez, L.; Vano-Galvan, S.; Perez-Garcia, B.; Carrillo-Gijon, R.; Jaen-Olasolo, P. Treatment of folliculitis decalvans with photodynamic therapy: Results in 10 patients. J. Am. Acad. Dermatol. 2015, 72, 1085–1087. [Google Scholar] [CrossRef]
- Jin, Y.G.; Zhang, X.H.; Zhang, B.L.; Kang, H.X.; Du, L.N.; Li, M. Nanostructures of an amphiphilic zinc phthalocyanine polymer conjugate for photodynamic therapy of psoriasis. Colloid Surf. B 2015, 128, 405–409. [Google Scholar] [CrossRef]
- Choudhary, S.; Nouri, K.; Elsaie, M.L. Photodynamic therapy in dermatology: A review. Lasers Med. Sci. 2009, 24, 971–980. [Google Scholar] [CrossRef]
- Karrer, S.; Kohl, E.; Feise, K.; Hiepe-Wegener, D.; Lischner, S.; Philipp-Dormston, W.; Podda, M.; Prager, W.; Walker, T.; Szeimies, R.M. Photodynamic therapy for skin rejuvenation: Review and summary of the literature—results of a consensus conference of an expert group for aesthetic photodynamic therapy. J. Dtsch. Dermatol. Ges. 2013, 11, 137–148. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Andrade, C.G.; Figueiredo, R.C.B.Q.; Ribeiro, K.R.C.; Souza, L.I.O.; Sarmento-Neto, J.F.; Reboucas, J.S.; Santos, B.S.; Ribeiro, M.S.; Carvalho, L.B.; Fontes, A. Photodynamic effect of zinc porphyrin on the promastigote and amastigote forms of Leishmania braziliensis. Photochem. Photobiol. Sci. 2018, 17, 482–490. [Google Scholar] [CrossRef]
- Gomes, M.L.; DeFreitas-Silva, G.; dos Reis, P.G.; Melo, M.N.; Frezard, F.; Demicheli, C.; Idemori, Y.M. Synthesis and characterization of bismuth(III) and antimony(V) porphyrins: High antileishmanial activity against antimony-resistant parasite. J. Biol. Inorg. Chem. 2015, 20, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Egan, E.S.; Weekes, M.P.; Kanjee, U.; Manzo, J.; Srinivasan, A.; Lomas-Francis, C.; Westhoff, C.; Takahashi, J.; Tanaka, M.; Watanabe, S.; et al. Erythrocytes lacking the Langereis blood group protein ABCB6 are resistant to the malaria parasite Plasmodium falciparum. Commun. Biol. 2018, 1, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabris, C.; Ouedraogo, R.K.; Coppellotti, O.; Dabire, R.K.; Diabate, A.; Di Martino, P.; Guidolin, L.; Jori, G.; Lucantoni, L.; Lupidi, G.; et al. Efficacy of sunlight-activatable porphyrin formulates on larvae of Anopheles gambiae M and S molecular forms and An. arabiensis: A potential novel biolarvicide for integrated malaria vector control. Acta Trop. 2012, 123, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Feese, E.; Gracz, H.S.; Boyle, P.D.; Ghiladi, R.A. Towards microbe-targeted photosensitizers: Synthesis, characterization and in vitro photodynamic inactivation of the tuberculosis model pathogen M. smegmatis by porphyrin-peptide conjugates. J. Porphyr. Phthalocyanines 2019, 23, 1414–1439. [Google Scholar] [CrossRef]
- Dong, J.; Ghiladi, R.A.; Wang, Q.; Cai, Y.; Wei, Q. Protoporphyrin IX conjugated bacterial cellulose via diamide spacer arms with specific antibacterial photodynamic inactivation against Escherichia coli. Cellulose 2018, 25, 1673–1686. [Google Scholar] [CrossRef]
- Dong, J.; Ghiladi, R.A.; Wang, Q.; Cai, Y.; Wei, Q. Protoporphyrin-IX conjugated cellulose nanofibers that exhibit high antibacterial photodynamic inactivation efficacy. Nanotechnology 2018, 29, 265601. [Google Scholar] [CrossRef]
- Basilico, N.; Monti, D.; Olliaro, P.; Taramelli, D. Non-iron porphyrins inhibit beta-haematin (malaria pigment) polymerisation. FEBS Lett. 1997, 409, 297–299. [Google Scholar] [CrossRef] [Green Version]
- Monti, D.; Vodopivec, B.; Basilico, N.; Olliaro, P.; Taramelli, D. A novel endogenous antimalarial: Fe(II)-protoporphyrin IX alpha (heme) inhibits hematin polymerization to beta-hematin (malaria pigment) and kills malaria parasites. Biochemistry 1999, 38, 8858–8863. [Google Scholar] [CrossRef]
- Abada, Z.; Cojean, S.; Pomel, S.; Ferrie, L.; Akagah, B.; Lormier, A.T.; Loiseau, P.M.; Figadere, B. Synthesis and antiprotozoal activity of original porphyrin precursors and derivatives. Eur. J. Med. Chem. 2013, 67, 158–165. [Google Scholar] [CrossRef]
- Begum, K.; Kim, H.S.; Kumar, V.; Stojiljkovic, I.; Wataya, Y. In vitro antimalarial activity of metalloporphyrins against Plasmodium falciparum. Parasitol. Res. 2003, 90, 221–224. [Google Scholar] [CrossRef]
- Chemaly, S.M.; Chen, C.T.; van Zyl, R.L. Naturally occurring cobalamins have antimalarial activity. J. Inorg. Biochem. 2007, 101, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Stallivieri, A.; Le Guern, F.; Vanderesse, R.; Meledje, E.; Jori, G.; Frochot, C.; Acherar, S. Synthesis and photophysical properties of the photoactivatable cationic porphyrin 5-(4-N-dodecylpyridyl)-10,15,20-tri(4-N-methylpyridyl)-21H,23H-porphyrin tetraiodide for anti-malaria PDT. Photochem. Photobiol. Sci. 2015, 14, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Iglesias, B.A.; Deda, D.K.; Budu, A.; Matias, T.A.; Bueno, V.B.; Maluf, F.V.; Guido, R.V.; Oliva, G.; Catalani, L.H.; et al. Encapsulation of metalloporphyrins improves their capacity to block the viability of the human malaria parasite Plasmodium falciparum. Nanomedicine 2015, 11, 351–358. [Google Scholar] [CrossRef]
- Ziegler, J.; Pasierb, L.; Cole, K.A.; Wright, D.W. Metalloporphyrin probes for antimalarial drug action. J. Inorg. Biochem. 2003, 96, 478–486. [Google Scholar] [CrossRef]
- Tekwani, B.L.; Walker, L.A. Targeting the hemozoin synthesis pathway for new antimalarial drug discovery: Technologies for in vitro beta-hematin formation assay. Comb. Chem. High Throughput Screen. 2005, 8, 63–79. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Sharma, S.K.; Dai, T.H.; Chung, H.; Yaroslavsky, A.; Garcia-Diaz, M.; Chang, J.L.; Chiang, L.Y.; Hamblin, M.R. Can nanotechnology potentiate photodynamic therapy? Nanotechnol. Rev. 2012, 1, 111–146. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, F.M.; Rocha, S.V.; Toma, H.E.; Araki, K.; Baptista, M.S. Determination of n-octanol/water partition and membrane binding of cationic porphyrins. Int. J. Pharm. 2007, 329, 12–18. [Google Scholar] [CrossRef]
- Luciano, M.; Bruckner, C. Modifications of Porphyrins and Hydroporphyrins for Their Solubilization in Aqueous Media. Molecules 2017, 22, 980. [Google Scholar] [CrossRef] [Green Version]
- Uchoa, A.F.; de Oliveira, K.T.; Baptista, M.S.; Bortoluzzi, A.J.; Iamamoto, Y.; Serra, O.A. Chlorin Photosensitizers Sterically Designed to Prevent Self-Aggregation. J. Org. Chem. 2011, 76, 8824–8832. [Google Scholar] [CrossRef]
- Enakieva, Y.Y.; Volostnykh, M.V.; Nefedov, S.E.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y.; Bessmertnykh-Lemeune, A.G.; Stern, C.; Guilard, R. Gallium(III) and Indium(III) Complexes with meso-Monophosphorylated Porphyrins: Synthesis and Structure. A First Example of Dimers Formed by the Self-Assembly of meso-Porphyrinylphosphonic Acid Monoester. Inorg. Chem. 2017, 56, 3055–3070. [Google Scholar] [CrossRef]
- Zheng, N.; Xie, D.; Wang, C.S.; Zhang, Z.Y.; Zheng, Y.B.; Lu, Q.; Bai, Y.G.; Li, Y.; Wang, A.G.; Song, W.Z. Water-Soluble, Zwitterionic Poly-photosensitizers as Carrier-Free, Photosensitizer-Self-Delivery System for in Vivo Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 44007–44017. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O. Nucleophilic substitution as a tool for the synthesis of unsymmetrical porphyrins. Acc. Chem. Res. 2005, 38, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, A.; Bhupathiraju, N.V.; Arianna, G.; Tiwari, K.; Drain, C.M. Glycosylated Porphyrins, Phthalocyanines, and Other Porphyrinoids for Diagnostics and Therapeutics. Chem. Rev. 2015, 115, 10261–10306. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Voon, S.H.; Kiew, L.V.; Lee, H.B.; Lim, S.H.; Noordin, M.I.; Kamkaew, A.; Burgess, K.; Chung, L.Y. In Vivo Studies of Nanostructure-Based Photosensitizers for Photodynamic Cancer Therapy. Small 2014, 10, 4993–5013. [Google Scholar] [CrossRef] [PubMed]
- Moret, F.; Reddi, E. Strategies for optimizing the delivery to tumors of macrocyclic photosensitizers used in photodynamic therapy (PDT). J. Porphyr. Phthalocyanines 2017, 21, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Srivatsan, A.; Missert, J.R.; Upadhyay, S.K.; Pandey, R.K. Porphyrin-based photosensitizers and the corresponding multifunctional nanoplatforms for cancer-imaging and phototherapy. J. Porphyr. Phthalocyanines 2015, 19, 109–134. [Google Scholar] [CrossRef]
- Tanaka, T.; Osuka, A. Conjugated porphyrin arrays: Synthesis, properties and applications for functional materials. Chem. Soc. Rev. 2015, 44, 943–969. [Google Scholar] [CrossRef]
- Schmitt, J.; Heitz, V.; Sour, A.; Bolze, F.; Ftouni, H.; Nicoud, J.F.; Flamigni, L.; Ventura, B. Diketopyrrolopyrrole-porphyrin conjugates with high two-photon absorption and singlet oxygen generation for two-photon photodynamic therapy. Angew Chem. Int. Ed. Engl. 2015, 54, 169–173. [Google Scholar] [CrossRef]
- Bill, N.L.; Ishida, M.; Bahring, S.; Lim, J.M.; Lee, S.; Davis, C.M.; Lynch, V.M.; Nielsen, K.A.; Jeppesen, J.O.; Ohkubo, K.; et al. Porphyrins fused with strongly electron-donating 1,3-dithiol-2-ylidene moieties: Redox control by metal cation complexation and anion binding. J. Am. Chem. Soc. 2013, 135, 10852–10862. [Google Scholar] [CrossRef]
- Purrello, R.; Gurrieri, S.; Lauceri, R. Porphyrin assemblies as chemical sensors. Coord. Chem. Rev. 1999, 190, 683–706. [Google Scholar] [CrossRef]
- He, H.S. Near-infrared emitting lanthanide complexes of porphyrin and BODIPY dyes. Coord. Chem. Rev. 2014, 273, 87–99. [Google Scholar] [CrossRef]
- Durot, S.; Taesch, J.; Heitz, V. Multiporphyrinic Cages: Architectures and Functions. Chem. Rev. 2014, 114, 8542–8578. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.K.; Broring, M.; Mathur, S.; Ravikanth, M. Boron dipyrrin-porphyrin conjugates. Coord. Chem. Re 2013, 257, 2348–2387. [Google Scholar] [CrossRef]
- Sessler, J.L.; Lawrence, C.M.; Jayawickramarajah, J. Molecular recognition via base-pairing. Chem. Soc. Rev. 2007, 36, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Ptaszynska, A.A.; Trytek, M.; Borsuk, G.; Buczek, K.; Rybicka-Jasinska, K.; Gryko, D. Porphyrins inactivate Nosema spp. microsporidia. Sci. Rep. 2018, 8, 5523. [Google Scholar] [CrossRef] [PubMed]
- Watterson, R.P. Porphyrin Therapy. Southwest Med. 1963, 44, 361–363. [Google Scholar]
- Varchi, G.; Foglietta, F.; Canaparo, R.; Ballestri, M.; Arena, F.; Sotgiu, G.; Guerrini, A.; Nanni, C.; Cicoria, G.; Cravotto, G.; et al. Engineered porphyrin loaded core-shell nanoparticles for selective sonodynamic anticancer treatment. Nanomedcine 2015, 10, 3483–3494. [Google Scholar] [CrossRef] [PubMed]
- Van Staden, J.F.; Stefan-van Staden, R.I. Application of porphyrins in flow-injection analysis: A review. Talanta 2010, 80, 1598–1605. [Google Scholar] [CrossRef]
- Biesaga, M.; Pyrzynska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef]
- Zucca, P.; Neves, C.M.; Simoes, M.M.; Neves Mda, G.; Cocco, G.; Sanjust, E. Immobilized Lignin Peroxidase-Like Metalloporphyrins as Reusable Catalysts in Oxidative Bleaching of Industrial Dyes. Molecules 2016, 21, 964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, F.C.; Liu, H.; Ding, M.L.; Lin, Q.P.; Jiang, H.L. Boosting Photocatalytic Hydrogen Production of Porphyrinic MOFs: The Metal Location in Metalloporphyrin Matters. ACS Catal. 2018, 8, 4583–4590. [Google Scholar] [CrossRef]
- De la Torre, G.; Bottari, G.; Sekita, M.; Hausmann, A.; Guldi, D.M.; Torres, T. A voyage into the synthesis and photophysics of homo- and heterobinuclear ensembles of phthalocyanines and porphyrins. Chem. Soc. Rev. 2013, 42, 8049–8105. [Google Scholar] [CrossRef]
- Saito, S.; Osuka, A. Expanded porphyrins: Intriguing structures, electronic properties, and reactivities. Angew Chem. Int. Ed. Engl. 2011, 50, 4342–4373. [Google Scholar] [CrossRef]
- Dini, D.; Calvete, M.J.; Hanack, M. Nonlinear Optical Materials for the Smart Filtering of Optical Radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef]
- Rajora, M.A.; Lou, J.W.H.; Zheng, G. Advancing porphyrin’s biomedical utility via supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 6433–6469. [Google Scholar] [CrossRef]
- Rengeng, L.; Qianyu, Z.; Yuehong, L.; Zhongzhong, P.; Libo, L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn Ther. 2017, 19, 159–166. [Google Scholar] [CrossRef]
- Hoogenboom, M.; Eikelenboom, D.; den Brok, M.H.; Heerschap, A.; Futterer, J.J.; Adema, G.J. Mechanical High-Intensity Focused Ultrasound Destruction of Soft Tissue: Working Mechanisms and Physiologic Effects. Ultrasound Med. Biol. 2015, 41, 1500–1517. [Google Scholar] [CrossRef]
- Milgrom, L.R. The Colours of Life. An Introduction to the Chemistry of Porphyrins and Related Compounds; Oxford university press: Oxford, UK, 1997. [Google Scholar]
- Bases, R.; Brodie, S.S.; Rubenfeld, S. Attempts at tumor localization using Cu 64-labeled copper porphyrins. Cancer 1958, 11, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Glidden, M.D.; Celli, J.P.; Massodi, I.; Rizvi, I.; Pogue, B.W.; Hasan, T. Image-Based Quantification of Benzoporphyrin Derivative Uptake, Localization, and Photobleaching in 3D Tumor Models, for Optimization of PDT Parameters. Theranostics 2012, 2, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [Green Version]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef]
- Su, S.; Ding, Y.; Li, Y.; Wu, Y.; Nie, G. Integration of photothermal therapy and synergistic chemotherapy by a porphyrin self-assembled micelle confers chemosensitivity in triple-negative breast cancer. Biomaterials 2016, 80, 169–178. [Google Scholar] [CrossRef]
- Senge, M.O.; Fazekas, M.; Notaras, E.G.A.; Blau, W.J.; Zawadzka, M.; Locos, O.B.; Mhuircheartaigh, E.M.N. Nonlinear optical properties of porphyrins. Adv. Mater. 2007, 19, 2737–2774. [Google Scholar] [CrossRef]
- Kuimova, M.K.; Collins, H.A.; Balaz, M.; Dahlstedt, E.; Levitt, J.A.; Sergent, N.; Suhling, K.; Drobizhev, M.; Makarov, N.S.; Rebane, A.; et al. Photophysical properties and intracellular imaging of water-soluble porphyrin dimers for two-photon excited photodynamic therapy. Org. Biomol. Chem. 2009, 7, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Wang, X.M.; Li, B.; Jiang, W.L.; Yang, P.; Qian, S.X.; Tao, X.T.; Jiang, M.H. Two-photon absorption properties of substituted porphyrins. J. Porphyr. Phthalocyanines 2006, 10, 160–166. [Google Scholar] [CrossRef]
- Puangmalee, S.; Petsom, A.; Thamyongkit, P. A porphyrin derivative from cardanol as a diesel fluorescent marker. Dyes Pigm. 2009, 82, 26–30. [Google Scholar] [CrossRef]
- Giannangelo, C.; Anderson, D.; Wang, X.F.; Vennerstrom, J.L.; Charman, S.A.; Creek, D.J. Ozonide Antimalarials Alkylate Heme in the Malaria Parasite Plasmodium falciparum. ACS Infect. Dis. 2019, 5, 2076–2086. [Google Scholar] [CrossRef]
- Zhao, X.J.; Lustigman, S.; Kenney, M.E.; BenHur, E. Structure-activity and mechanism studies on silicon phthalocyanines with Plasmodium falciparum in the dark and under red light. Photochem. Photobiol. 1997, 66, 282–287. [Google Scholar] [CrossRef]
- Hooker, J.D.; Nguyen, V.H.; Taylor, V.M.; Cedeno, D.L.; Lash, T.D.; Jones, M.A.; Robledo, S.M.; Velez, I.D. New Application for Expanded Porphyrins: Sapphyrin and Heterosapphyrins as Inhibitors of Leishmania Parasites. Photochem. Photobiol. 2012, 88, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; Turchiello, R.D.F.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis Photodyn Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Kelly, J.X.; Ignatushchenko, M.V.; Bouwer, H.G.; Peyton, D.H.; Hinrichs, D.J.; Winter, R.W.; Riscoe, M. Antileishmanial drug development: Exploitation of parasite heme dependency. Mol. Biochem. Parasitol. 2003, 126, 43–49. [Google Scholar] [CrossRef]
- Gomes, A.T.P.C.; Cunha, A.C.; Domingues, M.D.M.; Neves, M.G.P.M.S.; Tome, A.C.; Silva, A.M.S.; Santos, F.D.; Souza, M.C.B.V.; Ferreira, V.F.; Cavaleiro, J.A.S. Synthesis and characterization of new porphyrin/4-quinolone conjugates. Tetrahedron 2011, 67, 7336–7342. [Google Scholar] [CrossRef]
- Bastos, M.M.; Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Santos, O.A.; Boechat, N.; Cavaleiro, J.A.S. Synthesis of beta-Substituted Porphyrin Derivatives Containing Heterocyclic Moieties as Potential Photosensitizers Against Cutaneous Leishmaniasis. Eur. J. Org. Chem. 2013, 2013, 1485–1493. [Google Scholar] [CrossRef]
- Frezard, F.; Martins, P.S.; Barbosa, M.C.M.; Pimenta, A.M.C.; Ferreira, W.A.; de Melo, J.E.; Mangrum, J.B.; Demicheli, C. New insights into the chemical structure and composition of the pentavalent antimonial drugs, meglumine antimonate and sodium stibogluconate. J. Inorg. Biochem. 2008, 102, 656–665. [Google Scholar] [CrossRef]
- Morgenthaler, J.B.; Peters, S.J.; Cedeno, D.L.; Constantino, M.H.; Edwards, K.A.; Kamowski, E.M.; Passini, J.C.; Butkus, B.E.; Young, A.M.; Lash, T.D.; et al. Carbaporphyrin ketals as potential agents for a new photodynamic therapy treatment of leishmaniasis. Bioorgan. Med. Chem. 2008, 16, 7033–7038. [Google Scholar] [CrossRef]
- Gardner, D.M.; Taylor, V.M.; Cedeno, D.L.; Padhee, S.; Robledo, S.M.; Jones, M.A.; Lash, T.D.; Velez, I.D. Association of Acenaphthoporphyrins with Liposomes for the Photodynamic Treatment of Leishmaniasis. Photochem. Photobiol. 2010, 86, 645–652. [Google Scholar] [CrossRef]
- Sartorello, R.; Budu, A.; Bagnaresi, P.; Fernandes, C.A.H.; Sato, P.M.; Bueno, V.B.; Fontes, M.R.M.; Oliveira, P.L.; Paiva-Silva, G.O.; Alves, S.V.; et al. In vivo uptake of a haem analogue Zn protoporphyrin IX by the human malaria parasite P. falciparum-infected red blood cells. Cell Biol. Int. 2010, 34, 859–865. [Google Scholar] [CrossRef]
- Cabello-Donayre, M.; Orrego, L.M.; Herraez, E.; Vargas, P.; Martinez-Garcia, M.; Campos-Salinas, J.; Perez-Victoria, I.; Vicente, B.; Marin, J.J.G.; Perez-Victoria, J.M. Leishmania heme uptake involves LmFLVCRb, a novel porphyrin transporter essential for the parasite. Cell Mol. Life Sci. 2019. [Google Scholar] [CrossRef]
- Bohle, D.S.; Dodd, E.L.; Stephens, P. Structure of Malaria Pigment and Related Propanoate-Linked Metalloporphyrin Dimers. Chem. Biodivers. 2012, 9, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Dodd, E.L.; Bohle, D.S. Extended structure of indium(III) protoporphyrin IX acetate mimics dimer structure of hematin anhydride. Polyhedron 2016, 108, 36–42. [Google Scholar] [CrossRef]
- Bristowa, C.A.; Hudson, R.; Paget, T.A.; Boyle, R.W. Potential of cationic porphyrins for photodynamic treatment of cutaneous Leishmaniasis. Photodiagnosis Photodyn. Ther. 2006, 3, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Brown, S.B. Photosensitized inactivation of microorganisms. Photochem. Photobiol. Sci. 2004, 3, 403–405. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [Green Version]
- Lustigman, S.; BenHur, E. Photosensitized inactivation of Plasmodium falciparum in human red cells by phthalocyanines. Transfusion 1996, 36, 543–546. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, D.; Kolli, B.K.; Chang, K.P. Photodynamic sensitization of Leishmania amazonensis in both extracellular and intracellular stages with aluminum phthalocyanine chloride for photolysis in vitro. Antimicrob. Agents Chemother. 2005, 49, 4474–4484. [Google Scholar] [CrossRef] [Green Version]
- Escobar, P.; Hernandez, I.P.; Rueda, C.M.; Martinez, F.; Paez, E. Photodynamic activity of aluminium (III) and zinc (II) phthalocyanines in Leishmania promastigotes. Biomedica 2006, 26 (Suppl. 1), 49–56. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.G.; Soares, C.P.; Mittmann, J. Assessment of Leishmania major and Leishmania braziliensis promastigote viability after photodynamic treatment with aluminum phthalocyanine tetrasulfonate (AlPcS4). J. Venomous Anim. Toxins Incl. Trop. Dis. 2011, 17, 300–307. [Google Scholar]
- Bhat, A.R.; Athar, F.; Van Zyl, R.L.; Chen, C.T.; Azam, A. Synthesis and biological evaluation of novel 4-substituted 1-{[4-(10,15,20-triphenylporphyrin-5-yl)phenyl]methylidene}thiosemicarbazides as new class of potential antiprotozoal agents. Chem. Biodivers. 2008, 5, 764–776. [Google Scholar] [CrossRef]
- Cole, K.A.; Ziegler, J.; Evans, C.A.; Wright, D.W. Metalloporphyrins inhibit beta-hematin (hemozoin) formation. J. Inorg. Biochem. 2000, 78, 109–115. [Google Scholar] [CrossRef]
- Marginedas-Freixa, I.; Hattab, C.; Bouyer, G.; Halle, F.; Chene, A.; Lefevre, S.D.; Cambot, M.; Cueff, A.; Schmitt, M.; Gamain, B.; et al. TSPO ligands stimulate ZnPPIX transport and ROS accumulation leading to the inhibition of P. falciparum growth in human blood. Sci. Rep. 2016, 6, 33516. [Google Scholar] [CrossRef] [Green Version]
- Deda, D.K.; Pavani, C.; Carita, E.; Baptista, M.S.; Toma, H.E.; Araki, K. Control of Cytolocalization and Mechanism of Cell Death by Encapsulation of a Photosensitizer. J. Biomed. Nanotechnol. 2013, 9, 1307–1317. [Google Scholar] [CrossRef]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Delivery Rev. 2004, 56, 675–711. [Google Scholar] [CrossRef]
- Ding, J.X.; Chen, J.J.; Gao, L.Q.; Jiang, Z.Y.; Zhang, Y.; Li, M.Q.; Xiao, Q.C.; Lee, S.S.; Chen, X.S. Engineered nanomedicines with enhanced tumor penetration. Nano Today 2019, 29, 100800. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Althubiti, M.A. Cancer Nanomedicine: A New Era of Successful Targeted Therapy. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Majidinia, M.; Mirza-Aghazadeh-Attari, M.; Rahimi, M.; Mihanfar, A.; Karimian, A.; Safa, A.; Yousefi, B. Overcoming multidrug resistance in cancer: Recent progress in nanotechnology and new horizons. IUBMB Life. 2020, 72, 855–871. [Google Scholar] [CrossRef]
- Tian, B.R.; Hua, S.Y.; Liu, J.Y. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Jia, X.; Jia, L. Nanoparticles Improve Biological Functions of Phthalocyanine Photosensitizers Used for Photodynamic Therapy. Curr. Drug Metab. 2012, 13, 1119–1122. [Google Scholar] [CrossRef]
- Garapati, C.; Clarke, B.; Zadora, S.; Burney, C.; Cameron, B.D.; Fournier, R.; Baugh, R.F.; Boddu, S.H.S. Development and characterization of erythrosine nanoparticles with potential for treating sinusitis using photodynamic therapy. Photodiagnosis Photodyn. Ther. 2015, 12, 9–18. [Google Scholar] [CrossRef]
- Aditya, N.P.; Vathsala, P.G.; Vieira, V.; Murthy, R.S.R.; Souto, E.B. Advances in nanomedicines for malaria treatment. Adv. Colloid Interface Sci. 2013, 201, 1–17. [Google Scholar] [CrossRef]
- Nance, E.A.; Woodworth, G.F.; Sailor, K.A.; Shih, T.Y.; Xu, Q.G.; Swaminathan, G.; Xiang, D.; Eberhart, C.; Hanes, J. A Dense Poly(Ethylene Glycol) Coating Improves Penetration of Large Polymeric Nanoparticles Within Brain Tissue. Sci. Transl. Med. 2012, 4, 149ra119. [Google Scholar] [CrossRef] [Green Version]
- Coma-Cros, E.M.; Biosca, A.; Lantero, E.; Manca, M.L.; Caddeo, C.; Gutierrez, L.; Ramirez, M.; Borgheti-Cardoso, L.N.; Manconi, M.; Fernandez-Busquets, X. Antimalarial Activity of Orally Administered Curcumin Incorporated in Eudragit((R))-Containing Liposomes. Int. J. Mol. Sci. 2018, 19, 1361. [Google Scholar] [CrossRef] [Green Version]
- Barabadi, H.; Alizadeh, Z.; Rahimi, M.T.; Barac, A.; Maraolo, A.E.; Robertson, L.J.; Masjedi, A.; Shahrivar, F.; Ahmadpour, E. Nanobiotechnology as an emerging approach to combat malaria: A systematic review. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 221–233. [Google Scholar] [CrossRef]
- Deda, D.K.; Budu, A.; Cruz, L.N.; Araki, K.; Garcia, C.R.S. Strategies for Development of Antimalarials Based on Encapsulated Porphyrin Derivatives. Mini-Rev. Med. Chem. 2014, 14, 1055–1071. [Google Scholar] [CrossRef]
- Mhlwatika, Z.; Aderibigbe, B.A. Polymeric Nanocarriers for the Delivery of Antimalarials. Molecules 2018, 23, 2527. [Google Scholar] [CrossRef] [Green Version]
- Mvango, S.; Matshe, W.M.R.; Balogun, A.O.; Pilcher, L.A.; Balogun, M.O. Nanomedicines for Malaria Chemotherapy: Encapsulation vs. Polymer Therapeutics. Pharm. Res. 2018, 35, 237. [Google Scholar] [CrossRef] [Green Version]
- Puttappa, N.; Kumar, R.S.; Kuppusamy, G.; Radhakrishnan, A. Nano-facilitated drug delivery strategies in the treatment of plasmodium infection. Acta Trop. 2019, 195, 103–114. [Google Scholar] [CrossRef]
- Rahman, K.; Khan, S.U.; Fahad, S.; Chang, M.X.; Abbas, A.; Khan, W.U.; Rahman, L.; Ul Haq, Z.; Nabi, G.; Khan, D. Nano-biotechnology: A new approach to treat and prevent malaria. Int. J. Nanomed. 2019, 14, 1401–1410. [Google Scholar] [CrossRef] [Green Version]
- Peeters, P.A.M.; Deleest, K.; Eling, W.M.C.; Crommelin, D.J.A. Chloroquine Blood-Levels after Administration of the Liposome-Encapsulated Drug in Relation to Therapy of Murine Malaria. Pharm. Res. 1989, 6, 787–793. [Google Scholar] [CrossRef]
- Peeters, P.A.M.; Huiskamp, C.W.E.M.; Eling, W.M.C.; Crommelin, D.J.A. Chloroquine Containing Liposomes in the Chemotherapy of Murine Malaria. Parasitology 1989, 98, 381–386. [Google Scholar] [CrossRef]
- Fotoran, W.L.; Muntefering, T.; Kleiber, N.; Miranda, B.N.M.; Liebau, E.; Irvine, D.J.; Wunderlich, G. A multilamellar nanoliposome stabilized by interlayer hydrogen bonds increases antimalarial drug efficacy. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102099. [Google Scholar] [CrossRef]
- Wang, F.Y.; Zhang, G.P.; Zang, C.; Pan, H.; Ma, L.N.; Li, C.; Hou, H.P.; Su, P.; Gao, Y.H.; Sun, J.; et al. Preparation and In Vitro/ Vivo Evaluation of Nano-Liposomal Form of Febrifugine Hydrochloride. J. Nanosci. Nanotechnol. 2020, 20, 2558–2566. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef]
- Devalapally, H.; Chakilam, A.; Amiji, M.M. Role of nanotechnology in pharmaceutical product development. J. Pharm. Sci. 2007, 96, 2547–2565. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Sun, X.Y.; Punj, V.; Sriramoju, B.; Mohan, R.R.; Zhou, S.F.; Chauhan, A.; Kanwar, R.K. Nanoparticles in the treatment and diagnosis of neurological disorders: Untamed dragon with fire power to heal. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 399–414. [Google Scholar] [CrossRef]
- Coma-Cros, E.M.; Lancelot, A.; San Anselmo, M.; Borgheti-Cardoso, L.N.; Valle-Delgado, J.J.; Serrano, J.L.; Fernandez-Busquets, X.; Sierra, T. Micelle carriers based on dendritic macromolecules containing bis-MPA and glycine for antimalarial drug delivery. Biomater. Sci. 2019, 7, 1661–1674. [Google Scholar] [CrossRef] [Green Version]
- Kannan, D.; Yadav, N.; Ahmad, S.; Namdev, P.; Bhattacharjee, S.; Lochab, B.; Singh, S. Pre-clinical study of iron oxide nanoparticles fortified artesunate for efficient targeting of malarial parasite. EBioMedicine 2019, 45, 261–277. [Google Scholar] [CrossRef] [Green Version]
- Kingsley, J.D.; Dou, H.Y.; Morehead, J.; Rabinow, B.; Gendelman, H.E.; Destache, C.J. Nanotechnology: A Focus on Nanoparticles as a Drug Delivery System. J. Neuroimmune Pharmacol. 2006, 1, 340–350. [Google Scholar] [CrossRef]
- Bamrungsap, S.; Zhao, Z.L.; Chen, T.; Wang, L.; Li, C.M.; Fu, T.; Tan, W.H. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine 2012, 7, 1253–1271. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Ourique, A.F.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Tretinoin-loaded nanocapsules: Preparation, physicochemical characterization, and photostability study. Int. J. Pharm. 2008, 352, 1–4. [Google Scholar] [CrossRef]
- Akhtar, F.; Rizvi, M.M.A.; Kar, S.K. Oral delivery of curcumin bound to chitosan nanoparticles cured Plasmodium yoelii infected mice. Biotechnol. Adv. 2012, 30, 310–320. [Google Scholar] [CrossRef]
- Dandekar, P.P.; Jain, R.; Patil, S.; Dhumal, R.; Tiwari, D.; Sharma, S.; Vanage, G.; Patravale, V. Curcumin-Loaded Hydrogel Nanoparticles: Application in Anti-Malarial Therapy and Toxicological Evaluation. J. Pharm. Sci. 2010, 99, 4992–5010. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Loiseau, P.M.; Bories, C.; Legrand, P.; Devissaguet, J.P.; Barratt, G. Efficacy and pharmacokinetics of intravenous nanocapsule formulations of halofantrine in Plasmodium berghei-infected mice. Antimicrob. Agents Chemother. 2004, 48, 1222–1228. [Google Scholar] [CrossRef] [Green Version]
- Mosqueira, V.C.F.; Legrand, P.; Barratt, G. Surface-modified and conventional nanocapsules as novel, formulations for parenteral delivery of halofantrine. J. Nanosci. Nanotechnol. 2006, 6, 3193–3202. [Google Scholar] [CrossRef]
- Hoennscheidt, C.; Kreyenschulte, D.; Margaritis, A.; Krull, R. Production of stable quinine nanodispersions using esterified gamma-polyglutamic acid biopolymer. Biochem. Eng. J. 2013, 79, 259–266. [Google Scholar] [CrossRef]
- Wang, H.X.; Li, Q.G.; Reyes, S.; Zhang, J.; Zeng, Q.; Zhang, P.; Xie, L.; Lee, P.J.; Roncal, N.; Melendez, V.; et al. Nanoparticle formulations of decoquinate increase antimalarial efficacy against liver stage Plasmodium infections in mice. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 57–65. [Google Scholar] [CrossRef]
- Chen, G.; Shen, J.; Ohulchanskyy, T.Y.; Patel, N.J.; Kutikov, A.; Li, Z.; Song, J.; Pandey, R.K.; Agren, H.; Prasad, P.N.; et al. (α-NaYbF4:Tm3+)/CaF2 Core/Shell Nanoparticles with Efficient Near-Infrared to Near-Infrared Upconversion for High-Contrast Deep Tissue Bioimaging. ACS Nano. 2012, 6, 8280–8287. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.D.; Lindstrom, A.; Li, Y.P. Porphyrin-Based Nanomedicines for Cancer Treatment. Bioconjugate Chem. 2019, 30, 1585–1603. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Liang, X.L.; Dai, Z.F. Porphyrin-loaded nanoparticles for cancer theranostics. Nanoscale 2016, 8, 12394–12405. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef]
- Lassalle, H.P.; Dumas, D.; Grafe, S.; D’Hallewin, M.A.; Guillemin, F.; Bezdetnaya, L. Correlation between in vivo pharmacokinetics, intratumoral distribution and photodynamic efficiency of liposomal mTHPC. J. Control. Release 2009, 134, 118–124. [Google Scholar] [CrossRef]
- Reshetov, V.; Lassalle, H.P.; Francois, A.; Dumas, D.; Hupont, S.; Grafe, S.; Filipe, V.; Jiskoot, W.; Guillemin, F.; Zorin, V.; et al. Photodynamic therapy with conventional and PEGylated liposomal formulations of mTHPC (temoporfin): Comparison of treatment efficacy and distribution characteristics in vivo. Int. J. Nanomed. 2013, 8, 3817–3831. [Google Scholar] [CrossRef] [Green Version]
- Lamch, L.; Bazylinska, U.; Kulbacka, J.; Pietkiewicz, J.; Biezunska-Kusiak, K.; Wilk, K.A. Polymeric micelles for enhanced Photofrin II (R) delivery, cytotoxicity and pro-apoptotic activity in human breast and ovarian cancer cells. Photodiagnosis Photodyn. Ther. 2014, 11, 570–585. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.X.; Qiao, Z.Y.; An, H.W.; Qiao, S.L.; Wang, L.; Rajapaksha, R.P.Y.J.; Wang, H. Self-Assembled Autophagy-Inducing Polymeric Nanoparticles for Breast Cancer Interference In-Vivo. Adv. Mater. 2015, 27, 2627–2634. [Google Scholar] [CrossRef]
- Temizel, E.; Sagir, T.; Ayan, E.; Isik, S.; Ozturk, R. Delivery of lipophilic porphyrin by liposome vehicles: Preparation and photodynamic therapy activity against cancer cell lines. Photodiagnosis Photodyn. Ther. 2014, 11, 537–545. [Google Scholar] [CrossRef]
- Molinari, A.; Colone, M.; Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Arancia, G.; Mannino, S.; Mangiola, A.; Maira, G.; Bombelli, C.; et al. Cationic liposomes, loaded with m-THPC, in photodynamic therapy for malignant glioma. Toxicol. In Vitro 2007, 21, 230–234. [Google Scholar] [CrossRef]
- Bovis, M.J.; Woodhams, J.H.; Loizidou, M.; Scheglmann, D.; Bown, S.G.; MacRobert, A.J. Improved in vivo delivery of m-THPC via pegylated liposomes for use in photodynamic therapy. J. Control. Release 2012, 157, 196–205. [Google Scholar] [CrossRef]
- Broekgaarden, M.; de Kroon, A.I.P.M.; van Gulik, T.M.; Heger, M. Development and In Vitro Proof-of-Concept of Interstitially Targeted Zinc-Phthalocyanine Liposomes for Photodynamic Therapy. Curr. Med. Chem. 2014, 21, 377–391. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.A.; Kohn, L.K.; Antonio, M.A.; Carvalho, J.E.; Moreira, M.R.; Machado, A.E.H.; Pessine, F.B.T. Photoinactivation of different human tumor cell lines and sheep red blood cells in vitro by liposome-bound Zn(II) Phthalocyanine: Effects of cholesterol. J. Photochem. Photobiol. B 2010, 100, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Souto, C.A.Z.; Madeira, K.P.; Rettori, D.; Baratti, M.O.; Rangel, L.B.A.; Razzo, D.; da Silva, A.R. Improved photodynamic action of nanoparticles loaded with indium (III) phthalocyanine on MCF-7 breast cancer cells. J. Nanopart. Res. 2013, 15, 1879. [Google Scholar] [CrossRef]
- Deda, D.K.; Uchoa, A.F.; Carita, E.; Baptista, M.S.; Toma, H.E.; Araki, K. A new micro/nanoencapsulated porphyrin formulation for PDT treatment. Int. J. Pharm. 2009, 376, 76–83. [Google Scholar] [CrossRef]
- Deda, D.K.; Pavani, C.; Carita, E.; Baptista, M.S.; Toma, H.E.; Araki, K. Correlation of photodynamic activity and singlet oxygen quantum yields in two series of hydrophobic monocationic porphyrins. J. Porphyr. Phthalocyanines 2012, 16, 55–63. [Google Scholar] [CrossRef]
- Sutoris, K.; Rakusan, J.; Karaskova, M.; Mattova, J.; Benes, J.; Nekvasil, M.; Jezek, P.; Zadinova, M.; Pouckova, P.; Vetvicka, D. Novel Topical Photodynamic Therapy of Prostate Carcinoma Using Hydroxy-aluminum Phthalocyanine Entrapped in Liposomes. Anticancer Res. 2013, 33, 1563–1568. [Google Scholar]
- Sutoris, K.; Vetvicka, D.; Horak, L.; Benes, J.; Nekvasil, M.; Jezek, P.; Zadinova, M.; Pouckova, P. Evaluation of Topical Photodynamic Therapy of Mammary Carcinoma with an Experimental Gel Containing Liposomal Hydroxyl-aluminium Phthalocyanine. Anticancer Res. 2012, 32, 3769–3774. [Google Scholar]
- Derycke, A.S.L.; de Witte, P.A.M. Liposomes for photodynamic therapy. Adv. Drug Delivery Rev. 2004, 56, 17–30. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yu, Y.; Ma, J.; Zhang, H.R.; Zhang, H.; Wang, X.Q.; Wang, J.C.; Zhang, X.; Zhang, Q. LyP-1 Modification To Enhance Delivery of Artemisinin or Fluorescent Probe Loaded Polymeric Micelles to Highly Metastatic Tumor and Its Lymphatics. Mol. Pharm. 2012, 9, 2646–2657. [Google Scholar] [CrossRef]
- Xu, J.P.; Ji, J.; Chen, W.D.; Shen, J.C. Novel biomimetic polymersomes as polymer therapeutics for drug delivery. J. Control. Release 2005, 107, 502–512. [Google Scholar] [CrossRef]
- Ahmed, F.; Pakunlu, R.I.; Brannan, A.; Bates, F.; Minko, T.; Discher, D.E. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J. Control. Release 2006, 116, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG-PLA and PEG-PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Christian, D.A.; Cai, S.; Bowen, D.M.; Kim, Y.; Pajerowski, J.D.; Discher, D.E. Polymersome carriers: From self-assembly to siRNA and protein therapeutics. Eur. J. Pharm. Biopharm. 2009, 71, 463–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biosca, A.; Dirscherl, L.; Moles, E.; Imperial, S.; Fernandez-Busquets, X. An ImmunoPEGliposome for Targeted Antimalarial Combination Therapy at the Nanoscale. Pharmaceutics 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, R.Z.; Ma, L.; Zhang, L.L.; Li, C.Y.; Liu, W.D.; Wei, M.; Yan, D.; Evans, D.G.; Duan, X. A monomeric photosensitizer for targeted cancer therapy. Chem. Commun. 2014, 50, 14983–14986. [Google Scholar] [CrossRef]
- Abdelghany, S.M.; Schmid, D.; Deacon, J.; Jaworski, J.; Fay, F.; McLaughlin, K.M.; Gormley, J.A.; Burrows, J.F.; Longley, D.B.; Donnelly, R.F.; et al. Enhanced Antitumor Activity of the Photosensitizer meso-Tetra(N-methyl-4-pyridyl) Porphine Tetra Tosylate through Encapsulation in Antibody-Targeted Chitosan/Alginate Nanoparticles. Biomacromolecules 2013, 14, 302–310. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, J.; Song, H.; Zhuo, L.G.; Wang, G.Q.; Liao, W.; Feng, Y.; Wei, H.Y.; Chen, Y.; Yang, Y.C.; et al. Uptake and light-induced cytotoxicity of hyaluronic acid-grafted liposomes containing porphyrin in tumor cells. J. Drug Delivery Sci. Technol. 2018, 47, 137–143. [Google Scholar] [CrossRef]
- Jung, S.; Jung, S.; Kim, D.M.; Lim, S.H.; Shim, Y.H.; Kwon, H.; Kim, D.H.; Lee, C.M.; Kim, B.H.; Jeong, Y.I. Hyaluronic Acid-Conjugated with Hyperbranched Chlorin e6 Using Disulfide Linkage and Its Nanophotosensitizer for Enhanced Photodynamic Therapy of Cancer Cells. Materials 2019, 12, 3080. [Google Scholar] [CrossRef] [Green Version]
- Alven, S.; Aderibigbe, B. Combination Therapy Strategies for the Treatment of Malaria. Molecules 2019, 24, 3601. [Google Scholar] [CrossRef] [Green Version]
- Oyeyemi, O.; Morenkeji, O.; Afolayan, F.; Dauda, K.; Busari, Z.; Meena, J.; Panda, A. Curcumin-Artesunate Based Polymeric Nanoparticle; Antiplasmodial and Toxicological Evaluation in Murine Model. Front. Pharm. 2018, 9, 562. [Google Scholar] [CrossRef]
- Aditya, N.P.; Chimote, G.; Gunalan, K.; Banerjee, R.; Patankar, S.; Madhusudhan, B. Curcuminoids-loaded liposomes in combination with arteether protects against Plasmodium berghei infection in mice. Exp. Parasitol. 2012, 131, 292–299. [Google Scholar] [CrossRef]
- Isacchi, B.; Bergonzi, M.C.; Grazioso, M.; Righeschi, C.; Pietretti, A.; Severini, C.; Bilia, A.R. Artemisinin and artemisinin plus curcumin liposomal formulations: Enhanced antimalarial efficacy against Plasmodium berghei-infected mice. Eur. J. Pharm. Biopharm. 2012, 80, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, W.; Na, K. Doxorubicin loaded singlet-oxygen producible polymeric micelle based on chlorine e6 conjugated pluronic F127 for overcoming drug resistance in cancer. Biomaterials 2014, 35, 7963–7969. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.B.; Xie, A.J.; Xiao, Y.Z.; Li, S.K.; Huang, F.Z.; Shen, Y.H. Novel core-shell dextran-hemin crosslinked micelles: Synthesis, photo-controlled drug release and excellent (synergetic) antitumor effect. J. Mater. Chem. B 2015, 3, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.C.; Brown, S.B. Haem degradation in human haemoglobin in vitro. Separation of the contribution of the alpha- and beta-subunits. Biochem. J. 1984, 222, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, E.; Maluf, F.V.; Bueno, V.B.; Guido, R.V.; Oliva, G.; Singh, M.; Scarpelli, P.; Costa, F.; Sartorello, R.; Catalani, L.H.; et al. Biliverdin targets enolase and eukaryotic initiation factor 2 (eIF2alpha) to reduce the growth of intraerythrocytic development of the malaria parasite Plasmodium falciparum. Sci. Rep. 2016, 6, 22093. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.H.; Dhingra, S.K.; Lewis, I.A.; Singh, M.K.; Siriwardana, A.; Dalal, S.; Rubiano, K.; Klein, M.S.; Baska, K.S.; Krishna, S.; et al. Evidence for Regulation of Hemoglobin Metabolism and Intracellular Ionic Flux by the Plasmodium falciparum Chloroquine Resistance Transporter. Sci Rep. 2018, 8, 13578. [Google Scholar] [CrossRef] [Green Version]
- Awad, H.H.; El-Tayeb, T.A.; El-Aziz, N.M.A.; Abdelkader, M.H. A semi-field study on the effect of novel hematoporphyrin formula on the control of Culex pipiens larvae. J. Agric. Sci 2008, 4, 85–88. [Google Scholar]
- Lucantoni, L.; Magaraggia, M.; Lupidi, G.; Ouedraogo, R.K.; Coppellotti, O.; Esposito, F.; Fabris, C.; Jori, G.; Habluetzel, A. Novel, meso-substituted cationic porphyrin molecule for photo-mediated larval control of the dengue vector Aedes aegypti. PLoS Negl.Trop. Dis. 2011, 5, e1434. [Google Scholar] [CrossRef]
- Ben Amor, T.; Jori, G. Sunlight-activated insecticides: Historical background and mechanisms of phototoxic activity. Insect Biochem. Mol. Biol. 2000, 30, 915–925. [Google Scholar] [CrossRef]
- Richter, P.R.; Strauch, S.M.; Azizullah, A.; Häder, D.-P. Chlorophyllin as a possible measure against vectors of human parasites and fish parasites. Front. Environ. Sci. 2014, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Erzinger, G.S.; Wohllebe, S.; Vollrath, F.; Souza, S.C.; Richter, P.; Lebert, M.; Hader, D.P. Optimizing conditions for the use of chlorophyll derivatives for photodynamic control of parasites in aquatic ecosystems. Parasitol. Res. 2011, 109, 781–786. [Google Scholar] [CrossRef]
- Wohllebe, S.; Richter, R.; Richter, P.; Hader, D.P. Photodynamic control of human pathogenic parasites in aquatic ecosystems using chlorophyllin and pheophorbid as photodynamic substances. Parasitol. Res. 2009, 104, 593–600. [Google Scholar] [CrossRef]
- Abdel-Kader, M.H.; El-Tayeb, T.A. Field implementation using chlorophyll derivatives with sunlight for malaria, filaria and dengue fever vectors control in infested Africa swamps. Malar. J. 2012, 11, P42. [Google Scholar] [CrossRef] [Green Version]
- Buonasera, K.; Lambreva, M.; Rea, G.; Touloupakis, E.; Giardi, M.T. Technological applications of chlorophyll a fluorescence for the assessment of environmental pollutants. Anal. Bioanal. Chem. 2011, 401, 1139–1151. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [Green Version]
- Prakash, J.S.; Srivastava, A.; Strasser, R.J.; Mohanty, P. Senescence-induced alterations in the photosystem II functions of Cucumis sativus cotyledons: Probing of senescence driven alterations of photosystem II by chlorophyll a fluorescence induction O-J-I-P transients. Indian J. Biochem. Biophys. 2003, 40, 160–168. [Google Scholar]
- Merz, D.; Geyer, M.; Moss, D.A.; Ache, H.J. Chlorophyll fluorescence biosensor for the detection of herbicides. Anal. Bioanal. Chem. 1996, 354, 299–305. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The Role of Chlorophyll Fluorescence in the Detection of Stress Conditions in Plants. CRC Crit. Rev. Anal. Chem. 1988, 19, S29–S85. [Google Scholar] [CrossRef]
- Prasad, P.V.D.; Potluri, S.D.P. Influence of proline and hydroxyproline on salt-stressed axillary bud cultures of two varieties of potato (Solanum tuberosum). In Vitro Cell Dev Biol.—Plant 1996, 32, 47–50. [Google Scholar] [CrossRef]
- Roshchina, V.V.; Melnikova, E.V.; Kovaleva, L.V. Autofluorescence in pollen-pistil system in Hippeastrum hybridum. Dokl Akad Nauk+ 1996, 349, 118–120. [Google Scholar]
- Rodgers, K.R. Heme-based sensors in biological systems. Curr. Opin. Chem. Biol. 1999, 3, 158–167. [Google Scholar] [CrossRef]
- Fetzner, S.; Lingens, F. Bacterial dehalogenases: Biochemistry, genetics, and biotechnological applications. Microbiol. Rev. 1994, 58, 641–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, C.E.; Wade, R.S.; Belser, N.O. Biodehalogenation: Reactions of cytochrome P-450 with polyhalomethanes. Biochemistry 1985, 24, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.S.; Castro, C.E. Oxidation of Iron(Ii) Porphyrins by Alkyl-Halides. J. Am. Chem. Soc. 1973, 95, 226–230. [Google Scholar] [CrossRef]
- Wade, R.S.; Castro, C.E. Oxidation of Heme Proteins by Alkyl-Halides. J. Am. Chem. Soc. 1973, 95, 231–234. [Google Scholar] [CrossRef]
- Ahr, H.J.; King, L.J.; Nastainczyk, W.; Ullrich, V. The mechanism of chloroform and carbon monoxide formation from carbon tetrachloride by microsomal cytochrome P-450. Biochem. Pharm. 1980, 29, 2855–2861. [Google Scholar] [CrossRef]
- Klecka, G.M.; Gonsior, S.J. Reductive Dechlorination of Chlorinated Methanes and Ethanes by Reduced Iron (Ii) Porphyrins. Chemosphere 1984, 13, 391–402. [Google Scholar] [CrossRef]
- Xie, H.; Li, Y.T.; Lei, Y.M.; Liu, Y.L.; Xiao, M.M.; Gao, C.; Pang, D.W.; Huang, W.H.; Zhang, Z.Y.; Zhang, G.J. Real-Time Monitoring of Nitric Oxide at Single-Cell Level with Porphyrin-Functionalized Graphene Field-Effect Transistor Biosensor. Anal. Chem. 2016, 88, 11115–11122. [Google Scholar] [CrossRef]
- Dobson, D.J.; Saini, S. Porphyrin-modified electrodes as biomimetic sensors for the determination of organohalide pollutants in aqueous samples. Anal. Chem. 1997, 69, 3532–3538. [Google Scholar] [CrossRef]
- Mesaros, S.; Grunfeld, S.; Mesarosova, A.; Bustin, D.; Malinski, T. Determination of nitric oxide saturated (stock) solution by chronoamperometry on a porphyrine microelectrode. Anal. Chim. Acta 1997, 339, 265–270. [Google Scholar] [CrossRef]
- Malinski, T.; Taha, Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature 1992, 358, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Malinski, T.; Taha, Z.; Grunfeld, S.; Burewicz, A.; Tomboulian, P.; Kiechle, F. Measurements of Nitric-Oxide in Biological-Materials Using a Porphyrinic Microsensor. Anal. Chim. Acta 1993, 279, 135–140. [Google Scholar] [CrossRef]
- Sugawara, K.; Yamamoto, F.; Tanaka, S.; Nakamura, H. Electrochemical-Behavior of Sugar Investigated Using a Carbon-Paste Electrode Modified with Copper(Ii)-Porphyrin. J. Electroanal. Chem. 1995, 394, 263–265. [Google Scholar] [CrossRef]
- Guerra, S.V.; Xavier, C.R.; Nakagaki, S.; Kubota, L.T. Electrochemical behavior of copper porphyrin synthesized into zeolite cavity: A sensor for hydrazine. Electroanalysis 1998, 10, 462–466. [Google Scholar] [CrossRef]
- Dall’Orto, V.C.; Danilowicz, C.; Hurst, J.; Lo Balbo, A.; Rezzano, I. Studies of the interaction between metalloporphyrin films and phenols in a preconcentration type sensor. Electroanalysis 1998, 10, 127–131. [Google Scholar] [CrossRef]
- Qu, F.; Li, N.Q.; Jiang, Y.Y. Electrochemical studies of CuTMAP interaction with DNA and determination of DNA. Microchem. J. 1998, 58, 39–51. [Google Scholar] [CrossRef]
- Duong, B.; Arechabaleta, R.; Tao, N.J. In situ AFM/STM characterization of porphyrin electrode films for electrochemical detection of neurotransmitters. J. Electroanal. Chem. 1998, 447, 63–69. [Google Scholar] [CrossRef]
- Frey, H.H.; McNeil, C.J.; Keay, R.W.; Bannister, J.V. Characterization of a copper detecting amperometric electrode. Electroanalysis 1998, 10, 480–485. [Google Scholar] [CrossRef]
- Ciszewski, A.; Milczarek, G. Electrocatalytic oxidation of alcohols on glassy carbon electrodes electrochemically modified by conductive polymeric nickel(II) tetrakis(3-methoxy-4-hydroxyphenyl) porphyrin film. J. Electroanal. Chem. 1996, 413, 137–142. [Google Scholar] [CrossRef]
- Watanabe, H.; Ohmori, H. Dual-wavelength spectrophotometric determination of copper in sea-water with alpha,beta,gamma, degrees -tetrakis(1-methylpyridinium-4-yl) porphine. Talanta 1981, 28, 774–776. [Google Scholar] [CrossRef]
- Giovannetti, R.; Bartocci, V.; Pucciarelli, F.; Ricciutelli, M. Reactions of anionic porphyrin with group 11 elements: A spectrophotometric and electrospray ionization mass spectrometry study. Talanta 2004, 63, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Igarashi, S.; Yotsuyanagi, T. Acceleration Effect of L-Tryptophan on Metal-Ion Exchange-Reaction of Cadmium(Ii) with Water-Soluble-Porphyrin-Lead(I) Complex and Its Application to Stopped-Flow Spectrophotometric Determination on Nm Level of Cadmium(Ii). Anal. Sci. 1988, 4, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.Q.; Wang, Y.Y.; Zhen, C.M.; Zhang, J.; Yang, Y.H.; Guo, Y.P. Preparation of Si1-x-yGexCy semiconductor films on Si by ion implantation and solid phase epitaxy. Acta Phys. Sin.-Ch. Ed. 2002, 51, 2340–2343. [Google Scholar]
- Yoon, I.J.; Shin, J.H.; Paeng, I.S.R.; Nam, H.H.; Cha, G.S.; Paeng, K.J. Potentiometric behavior of metalloporphyrin-based ion-selective electrodes: Use of silicone rubber matrix for serum chloride analysis. Anal. Chim. Acta 1998, 367, 175–181. [Google Scholar] [CrossRef]






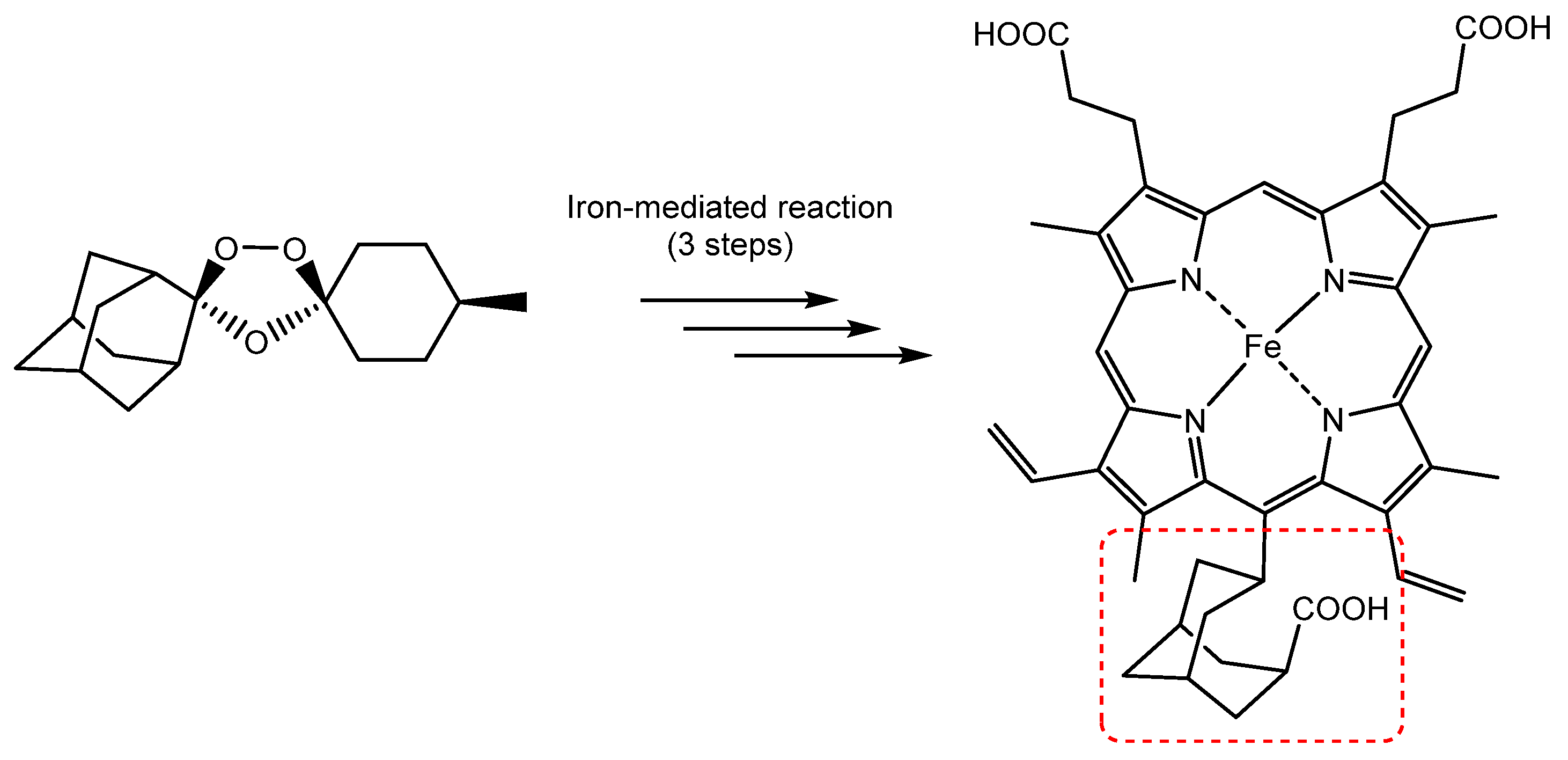










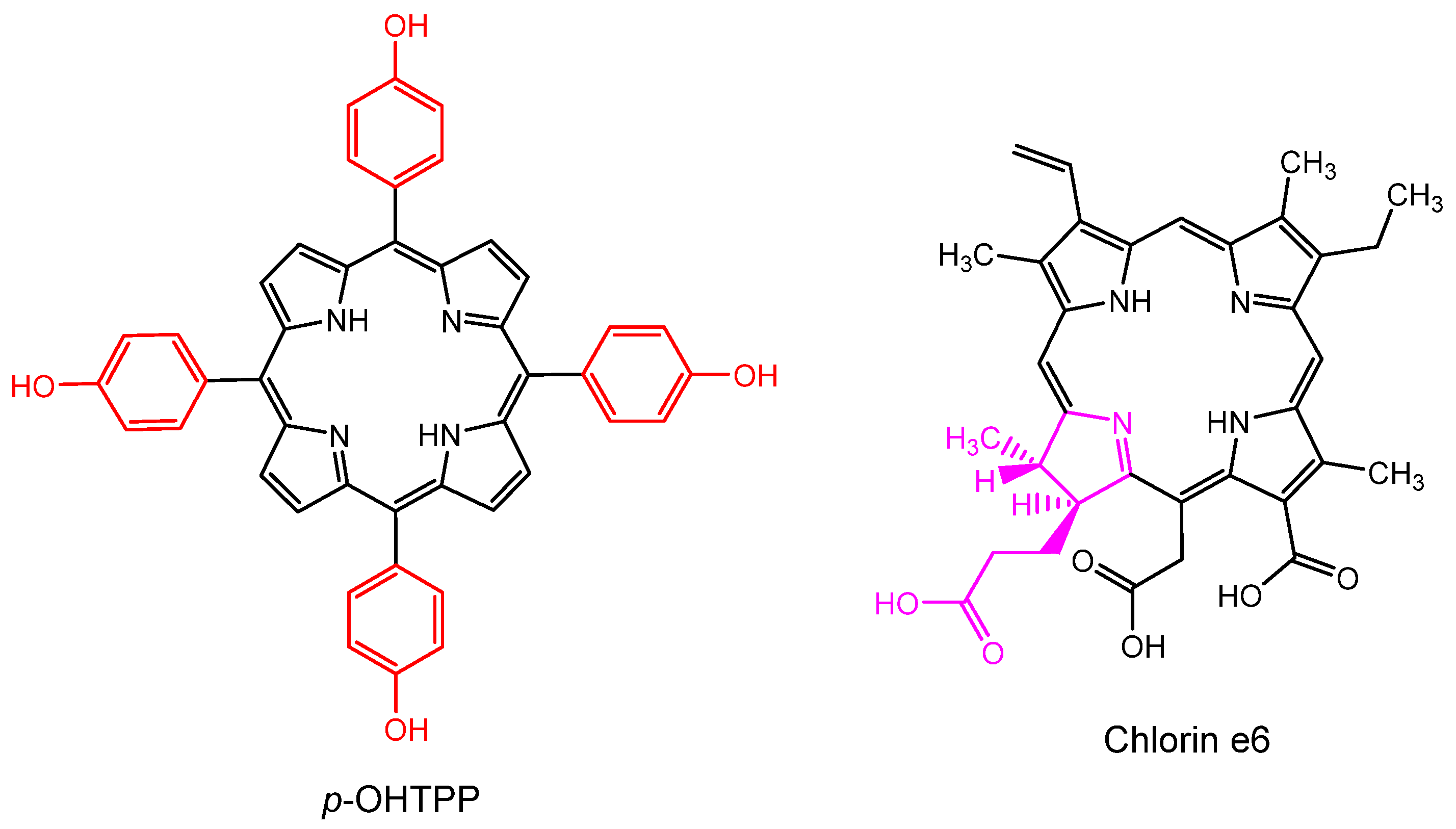

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deda, D.K.; Iglesias, B.A.; Alves, E.; Araki, K.; Garcia, C.R.S. Porphyrin Derivative Nanoformulations for Therapy and Antiparasitic Agents. Molecules 2020, 25, 2080. https://doi.org/10.3390/molecules25092080
Deda DK, Iglesias BA, Alves E, Araki K, Garcia CRS. Porphyrin Derivative Nanoformulations for Therapy and Antiparasitic Agents. Molecules. 2020; 25(9):2080. https://doi.org/10.3390/molecules25092080
Chicago/Turabian StyleDeda, Daiana K., Bernardo A. Iglesias, Eduardo Alves, Koiti Araki, and Celia R. S. Garcia. 2020. "Porphyrin Derivative Nanoformulations for Therapy and Antiparasitic Agents" Molecules 25, no. 9: 2080. https://doi.org/10.3390/molecules25092080
APA StyleDeda, D. K., Iglesias, B. A., Alves, E., Araki, K., & Garcia, C. R. S. (2020). Porphyrin Derivative Nanoformulations for Therapy and Antiparasitic Agents. Molecules, 25(9), 2080. https://doi.org/10.3390/molecules25092080







