A Simple Biorefinery Concept to Produce 2G-Lactic Acid from Sugar Beet Pulp (SBP): A High-Value Target Approach to Valorize a Waste Stream
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lactic Acid Fermentation
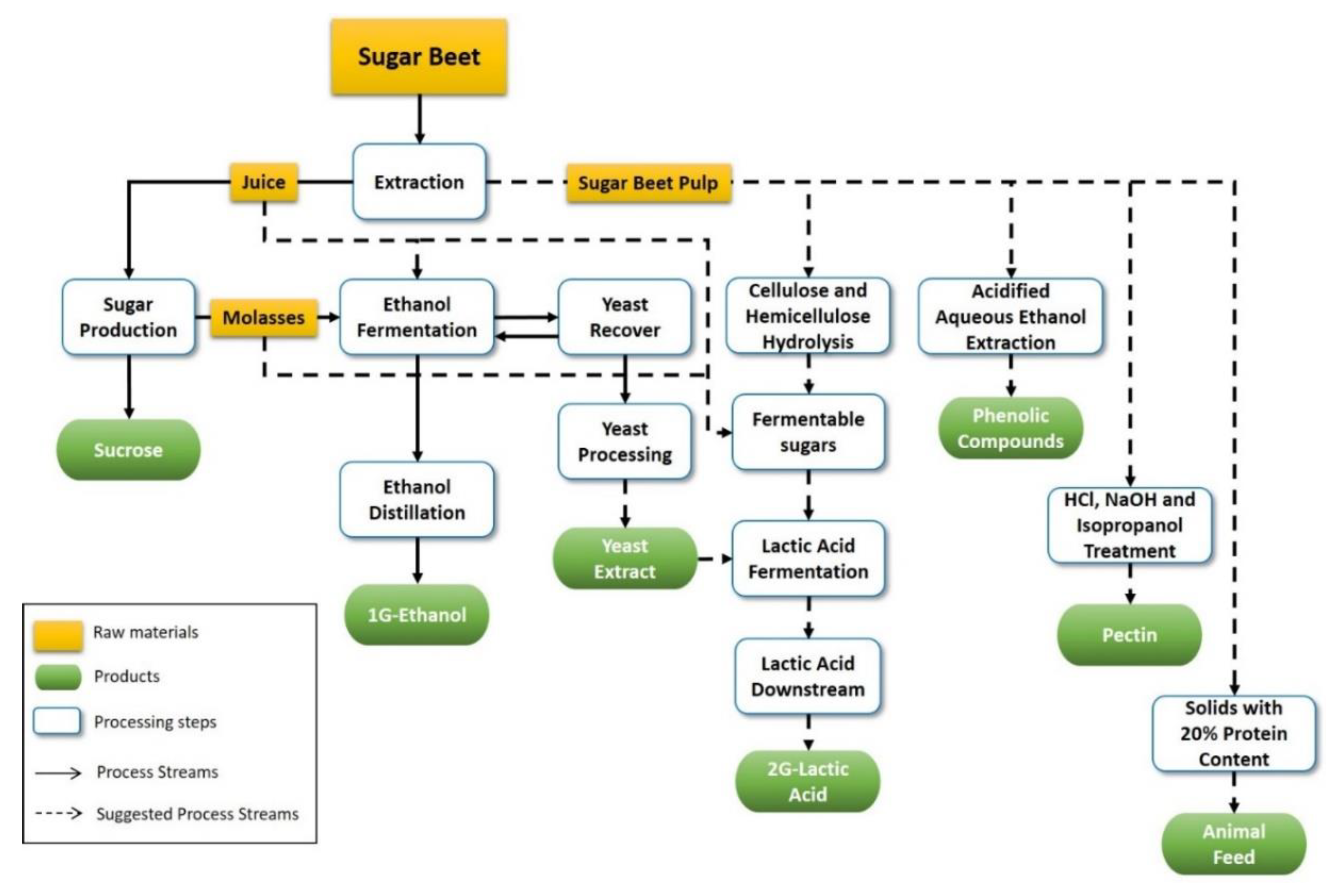
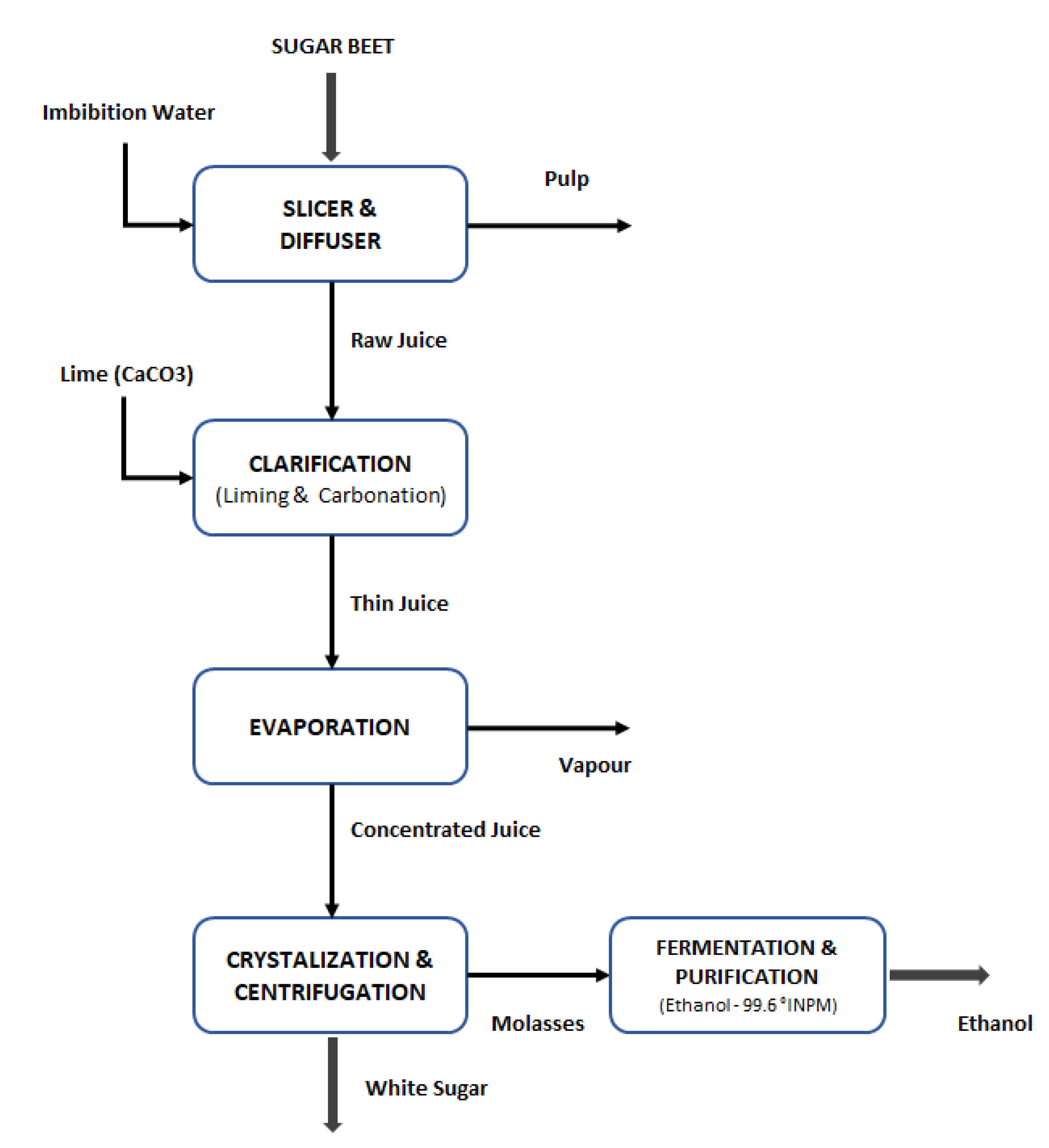
2.2. Biorefinery Proposal Process Design
2.3. Alternative Lactic Acid Downstream
3. Materials and Methods
3.1. Microorganism—Bacillus coagulans A166
3.2. Sugar Beet Pulp
3.3. Sugar Beet Pulp Hydrolysis
- 0.3 mL of Accellerase 1500/g of cellulose
- 0.114 mL of Pectinase L40/L of pre-treated SBP
- 0.429 mL of Protease Fermgen/L of pre-treated SBP
3.4. Fermentation
3.4.1. Batch Fermentation
3.4.2. Continuous Fermentation
3.5. Down-Stream Processing—Electrodialysis
- Decolorization was performed using PUROLITE MN-502 (Purolite, Ratingen, Germany)
- Softening was carried out using PUROLITE S950 acid chelating resin (Purolite).
- Mono- and bi-polar electrodialysis were carried out with 11 cation exchange membranes Type II and 10 anion exchange membranes Type II (both from Fujifilm, Tilburg, Netherlands)
- Anion and cation exchange chromatographies were performed using a weak anion exchange resin RELITE EXA 133 and a strong cation exchange resin RELITE EXC 08 (both from Resindion S. R. L., Binasco, Italy)
- Concentration was done by vacuum distillation plant (Büchi Labortechnik, Essen, Germany)
3.6. Analytics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations Sugarbeet. Available online: http://www.fao.org/land-water/databases-and-software/crop-information/sugarbeet/en/ (accessed on 1 March 2019).
- Food and Agriculture Organization of the United Nations: Crops. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 20 December 2018).
- Hunt, N. EU Sugar Companies Struggle to Survive as Prices Plunge Post-Quotas. Available online: https://www.reuters.com/article/us-europe-sugar/eu-sugar-companies-struggle-to-survive-as-prices-plunge-post-quotas-idUSKCN1HA1K5 (accessed on 1 March 2019).
- Tomaszewska, J.; Bieliński, D.; Binczarski, M.; Berlowska, J.; Dziugan, P.; Piotrowski, J.; Stanishevsky, A.; Witońska, I.A. Products of sugar beet processing as raw materials for chemicals and biodegradable polymers. RSC Adv. 2018, 8, 3161–3177. [Google Scholar] [CrossRef] [Green Version]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Schneider, R.; Papapostolou, H.; Ladakis, D.; Koutinas, A.; Venus, J. Restructuring the conventional sugar beet industry into a novel biorefinery: Fractionation and bioconversion of sugar beet pulp into succinic acid and value-added coproducts. ACS Sustain. Chem. Eng. 2019, 7, 6569–6579. [Google Scholar] [CrossRef]
- Hamley-Bennett, C.; Lye, G.J.; Leak, D.J. Selective fractionation of sugar beet pulp for release of fermentation and chemical feedstocks; Optimisation of thermo-chemical pre-treatment. Bioresour. Technol. 2016, 209, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Lee, C.; Yu, C.; Cheng, Y.-S.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl. Energy 2013, 105, 1–7. [Google Scholar] [CrossRef]
- Nahar, N.; Pryor, S.W. Enzymatic hydrolysis and fermentation of crushed whole sugar beets. Biomass Bioenerg. 2013, 59, 512–519. [Google Scholar] [CrossRef]
- Habeeb, A.A.M.; Gad, A.E.; EL-Tarabany, A.A.; Mustafa, M.M.; Atta, M.A.A. Using of sugar beet pulp by-product in farm animals feeding. Int. J. Sci. Res. Sci. Technol. 2017, 3, 107–120. [Google Scholar]
- Ziemiński, K.; Kowalska-Wentel, M. Effect of different sugar beet pulp pretreatments on biogas production efficiency. Appl. Biochem. Biotechnol. 2017, 181, 1211–1227. [Google Scholar] [CrossRef] [Green Version]
- Berlowska, J.; Pielech-Przybylska, K.; Balcerek, M.; Cieciura, W.; Borowski, S.; Kregiel, D. Integrated bioethanol fermentation/anaerobic digestion for valorization of sugar beet pulp. Energies 2017, 10, 1255. [Google Scholar] [CrossRef] [Green Version]
- Berłowska, J.; Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Patelski, P.; Dziugan, P.; Kręgiel, D. Simultaneous saccharification and fermentation of sugar beet pulp for efficient bioethanol production. Biomed. Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Günan Yücel, H.; Aksu, Z. Ethanol fermentation characteristics of Pichia stipitis yeast from sugar beet pulp hydrolysate: Use of new detoxification methods. Fuel 2015, 158, 793–799. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, C.; Cheng, Y.-S.; Lee, C.; Simmons, C.W.; Dooley, T.M.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Integrating sugar beet pulp storage, hydrolysis and fermentation for fuel ethanol production. Appl. Energy 2012, 93, 168–175. [Google Scholar] [CrossRef]
- Tomita, H.; Okazaki, F.; Tamaru, Y. Biomethane production from sugar beet pulp under cocultivation with Clostridium cellulovorans and methanogens. AMB Express 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Bellido, C.; Infante, C.; Coca, M.; González-Benito, G.; Lucas, S.; García-Cubero, M.T. Efficient acetone–butanol–ethanol production by Clostridium beijerinckii from sugar beet pulp. Bioresour. Technol. 2015, 190, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Eş, I.; Mousavi Khaneghah, A.; Barba, F.J.; Saraiva, J.A.; Sant’Ana, A.S.; Hashemi, S.M.B. Recent advancements in lactic acid production - A review. Food Res. Int. 2018, 107, 763–770. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.A.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Venus, J. Membrane technologies for lactic acid separation from fermentation broths derived from renewable resources. Membranes 2018, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sánchez, M.; Coll Lozano, C.; Venus, J. Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J. Clean. Prod. 2020, 247, 119165. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Tadi, S.R.R.; Pavan, A.S.S.; Sivaprakasam, S.; Rajaram, S. Effect of nitrogen sources and neutralizing agents on D-lactic acid production from Kodo millet bran hydrolysate: Comparative study and kinetic analysis. J. Food Sci. Technol. 2019, 57, 915–926. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, A.; Mao, Y.; Wu, B.; He, M.; Hu, W.; Chen, J.; Li, W. Efficient L-lactic acid production from purified sweet sorghum juice coupled with soybean hydrolysate as nitrogen source by Lactobacillus thermophilus A69 strain. J. Chem. Technol. Biotechnol. 2019, 94, 1752–1759. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, P.; Tao, F. L-Lactic acid production by Bacillus coagulans through simultaneous saccharification and fermentation of lignocellulosic corncob residue. Bioresour. Technol. Rep. 2019, 6, 131–137. [Google Scholar] [CrossRef]
- Bahry, H.; Abdalla, R.; Pons, A.; Taha, S.; Vial, C. Optimization of lactic acid production using immobilized Lactobacillus rhamnosus and carob pod waste from the Lebanese food industry. J. Biotechnol. 2019, 306, 81–88. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, I.; Acedos, M.G.; Ladero, M.; Santos, V.E. On the use of resting L. delbrueckii spp. delbrueckii cells for D-lactic acid production from orange peel wastes hydrolysates. Biochem. Eng. J. 2019, 145, 162–169. [Google Scholar] [CrossRef]
- Aulitto, M.; Fusco, S.; Nickel, D.B.; Bartolucci, S.; Contursi, P.; Franzén, C.J. Seed culture pre-adaptation of Bacillus coagulans MA-13 improves lactic acid production in simultaneous saccharification and fermentation. Biotechnol. Biofuels 2019, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Adiletta, G.; Brachi, P.; Riianova, E.; Crescitelli, A.; Miccio, M.; Kostryukova, N. A simplified biorefinery concept for the valorization of sugar beet pulp: Ecofriendly isolation of pectin as a step preceding torrefaction. Waste Biomass Valorization 2019. [Google Scholar] [CrossRef]
- Huang, X.; Li, D.; Wang, L. jun Characterization of pectin extracted from sugar beet pulp under different drying conditions. J. Food Eng. 2017, 211, 1–6. [Google Scholar] [CrossRef]
- Prandi, B.; Baldassarre, S.; Babbar, N.; Bancalari, E.; Vandezande, P.; Hermans, D.; Bruggeman, G.; Gatti, M.; Elst, K.; Sforza, S. Pectin oligosaccharides from sugar beet pulp: Molecular characterization and potential prebiotic activity. Food Funct. 2018, 9, 1557–1569. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Hassanien, M.F.R.; Mahmoud, A.; Sarhan, M.A.; Smetanska, I. Phenolics extracted from potato, sugar beet, and sesame processing by-products. Int. J. Food Prop. 2013, 16, 1148–1168. [Google Scholar] [CrossRef]
- Krajnc, D.; Mele, M.; Glavič, P. Improving the economic and environmental performances of the beet sugar industry in Slovenia: Increasing fuel efficiency and using by-products for ethanol. J. Clean. Prod. 2007, 15, 1240–1252. [Google Scholar] [CrossRef]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Status and perspectives in bioethanol production from sugar beet. In Bioethanol Production from Food Crops; Elsevier Academic Press: Cambridge, MA, USA, 2019; pp. 61–79. ISBN 9780128137666. [Google Scholar]
- Van Zeist, W.J.; Marinussen, M.; Broekema, R.; Groen, E.; Kool, A.; Dolman, M.; Blonk, H. LCI Data for the Calculation Tool Feedprint for Greenhouse Gas Emissions of Feed Production and Utilization - Animal Products; Blonk Consultants: Gouda, The Netherlands, 2012. [Google Scholar]
- Lorenz, F. Optimized standard of sugar manufacturing from beet-first calculations. Sugar Ind. 2010, 135, 21–28. [Google Scholar] [CrossRef]
- Dorst, E. Maintenance in the sugar industry: Suiker Unie. Zuckerindustrie 2003, 3, 163–165. [Google Scholar]
- Bonomi, A.; Cavalett, O.; Pereira da Cunha, M.; Lima, M.A.P. Virtual biorefinery. In Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-26043-3. [Google Scholar]
- Udachan, I.S.; Sahoo, A.K. A study of parameters affecting the solvent extraction of lactic acid from fermentation broth. Braz. J. Chem. Eng. 2014, 31, 821–827. [Google Scholar] [CrossRef] [Green Version]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Pleissner, D.; Neu, A.-K.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Bioresour. Technol. 2016, 218, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Neu, A.; Pleissner, D.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative utilization of coffee mucilage using Bacillus coagulans and investigation of down-stream processing of fermentation broth for optically pure L(+)-lactic acid production. Bioresour. Technol. 2016, 211, 398–405. [Google Scholar] [CrossRef]
- Pleissner, D.; Schneider, R.; Venus, J.; Koch, T. Separation of lactic acid and recovery of salt-ions from fermentation broth. J. Chem. Technol. Biotechnol. 2017, 92, 504–511. [Google Scholar] [CrossRef]
- Alves De Oliveira, R.; Alexandri, M.; Komesu, A.; Venus, J.; Vaz Rossell, C.E.; Maciel Filho, R. Current advances in separation and purification of second-generation lactic acid. Sep. Purif. Rev. 2019. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, G.; Sun, X.; Jin, B. Recovery of lactic acid from kitchen garbage fermentation broth by four-compartment configuration electrodialyzer. Process. Biochem. 2006, 41, 152–158. [Google Scholar] [CrossRef]
- Saxena, A.; Gohil, G.S.; Shahi, V.K. Electrochemical membrane reactor: Single-step separation and ion substitution for the recovery of lactic acid from lactate salts. Ind. Eng. Chem. Res. 2007, 46, 1270–1276. [Google Scholar] [CrossRef]
- Biddy, M.J.; Scarlata, C.; Kinchin, C. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential; NREL/TP-5100-65509, Technical report for the National Renewable Energy Lab; National Renewable Energy Lab: Golden, CO, USA, 2016.
- Wee, Y.; Yun, J.; Lee, Y.Y.; Zeng, A.; Ryu, H. Recovery of lactic acid by repeated batch electrodialysis and lactic acid production using electrodialysis wastewater. J. Biosci. Bioeng. 2005, 99, 104–108. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.A.; Komesu, A.; Vaz Rossell, C.E.; Wolf Maciel, M.R.; Maciel Filho, R. A study of the residual fermentation sugars influence on an alternative downstream process for first and second-generation lactic acid. Sustain. Chem. Pharm. 2020, 15, 100206. [Google Scholar] [CrossRef]
- De Oliveira, R.A.; Komesu, A.; Vaz Rossell, C.E.; Wolf Maciel, M.R.; Maciel Filho, R. Concentrating second-generation lactic acid from sugarcane bagasse via hybrid short path evaporation: Operational challenges. Sep. Purif. Technol. 2019, 209, 26–31. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Ankom Technology. Acid Detergent Fiber in Feeds Filter Bag Technique (for A200 and A200I); Ankom Technology: Macedon, NY, USA, 2017; Available online: https://www.ankom.com/sites/default/files/document-files/Method_5_ADF_A200.pdf (accessed on 1 March 2019).
- Engineering and Analysis Division, Method 1687—Total Kjeldahl Nitrogen in Water and Biosolids by Automated Colorimetry with Preliminary Distillation/Digestion; EPA-821-R-01-004, Method 1687 of the U.S. Environmental Protection Agency; Washington, DC, USA, 2001. Available online: https://www.epa.gov/sites/production/files/2015-10/documents/method_1687_draft_2001.pdf (accessed on 1 March 2019).
Sample Availability: Samples of the compounds are not available from the authors. |




| Fermentation Mode | Time | Total Sugars* | Non-Consumed Sugars* | Lactic Acid Produced | Yield | Average Productivity | Max Productivity | µP Max | |
|---|---|---|---|---|---|---|---|---|---|
| h | g | g | g | g/g | g L−1h−1 | g L−1h−1 | h−1 | ||
| B1 | Batch, yeast extract | 24 | 41.05 | 5.03 | 29.90 | 0.83 | 1.25 | 12.48 | - |
| B2 | Batch, protease | 24 | 41.39 | 1.47 | 29.14 | 0.73 | 1.21 | 14.67 | - |
| C1 | Continuous, protease, no cell retention, DR = 0.10 | 54 | 1158.58 | 379.13 | 296.19 | 0.38 | 0.27 | 9.63 | - |
| C2 | Continuous, protease, cell retention, DR = 0.10 | 54 | 739.23 | 145.83 | 450.98 | 0.76 | 0.56 | 11.60 | 1.09 |
| C3 | Continuous, protease, cell retention, DR = 0.15 | 54 | 1322.45 | 254.10 | 779.90 | 0.73 | 0.57 | 14.25 | 1.03 |
| C4 | Continuous, protease, cell retention, DR = 0.20 | 54 | 1406.58 | 268.51 | 887.69 | 0.78 | 0.60 | 16.21 | 1.05 |
| C5 | Continuous, protease, cell retention, DR = 0.30 | 54 | 2116.42 | 707.21 | 972.36 | 0.69 | 0.38 | 9.69 | - |
| C6 | Continuous, protease, cell retention, DR = 0.10 and 0.15 | 150 | 3916.91 | 0.00 | 2781.01 | 0.71 | 0.21 | 18.06 | 1.45 |
| Krajnc et al. [33] | Lorenz [36]; Zeist et al. [35] | Dorst [37]; Zeist et al. [35] | This Work | |
|---|---|---|---|---|
| Granulated Sugar (kg product·tonne−1 sugar beet) | 137 | 160 | 167 | 155 |
| Lime Fertilizer (CaCO3) (kg product·tonne−1 sugar beet) | 51 | 8 | 31 | 30 |
| Molasses (kg product·tonne−1 sugar beet) | 29 | 30 | 39 | 33 |
| Sugar beet pulp (dry) (kg product·tonne−1 sugar beet) | 45 | 60 | 26 | 44 |
| Sugar beet pulp (wet) (kg product·tonne−1 sugar beet) | - | - | 118 | 198 |
| Beet tops and tails (kg product·tonne−1 sugar beet) | 11 | 10 | 9 | 10 |
| Beet soil (kg product·tonne−1 sugar beet) | 98 | 40 | 4 | 47 |
| Sugar content (%) | - | - | - | 17.1 |
| Pulp moisture (%) | - | - | - | 78.0 |
| Sugar extraction efficiency (%) | - | - | - | 98.0 |
| Raw Juice—Water soluble solids concentration (°Brix) | - | - | - | 14 |
| Concentrated Juice—WSS concentration (°Brix) | - | - | - | 65 |
| Fermentation Yield (g/g) | - | - | - | 0.46 |
| Distillation efficiency (%) | - | - | - | 99.0 |
| Dehydration efficiency (%) | - | - | - | 98.0 |
| Anhydrous ethanol specification (%) | - | - | - | 99.6 |
| Step | Stream | Vol | Residual Sugars | Lactic Acid | Acetic Acid | N kjel Total | P Total | SO42− | Na+ | Other Anions * | Other Cations ** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (L) | (g/L) | (g/L) | (g/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | ||
| Fermentation Broth from C2, C3, and C4 Fermentations | |||||||||||
| Microfiltration (fermentation) | permeate | 62.90 | 9.88 | 29.84 | nd | 506 | 41 | 998 | 12,718 | 4062 | 1075 |
| Nanofiltration | permeate | 62.90 | 9.29 | 25.88 | nd | 110 | 10 | 383 | 10,361 | 3852 | 744 |
| retentate | 6.20 | 12.62 | 25.63 | nd | 2545 | 192 | 4049 | 15,116 | 1244 | 2363 | |
| Decolorization1 | 87.90 | 9.14 | 19.11 | nd | 60 | 15 | 279 | 5993 | 2780 | 206 | |
| Softening | 96.10 | 14.01 | 17.07 | nd | 55 | 14 | 251 | 7258 | 2415 | 99 | |
| Monopolar electrodialysis | concentrate | 17.40 | 0.00 | 89.86 | 8.50 | 125 | 21 | 1319 | 35,414 | 13,530 | 503 |
| dilute | 87.90 | 15.76 | 0.83 | 0.86 | 75 | 7 | 5 | 361 | 24 | 9 | |
| Bipolar electrodialysis | acid | 13.50 | 0.00 | 110.30 | nd | 64 | 22 | 1666 | 2460 | 16,990 | 158 |
| base | 22.80 | 1.67 | nd | nd | 9 | <4 | nd | 27,100 | 195 | 393 | |
| salt | 9.45 | 0.00 | 1.05 | nd | 36 | 7 | nd | 299 | 108 | 103 | |
| Decolorization2 | 18.20 | 0.00 | 80.15 | nd | 47 | 14 | 1113 | 1497 | 11,607 | 139 | |
| Anion exchanger | 38.50 | 0.00 | 20.92 | nd | nd | 1 | 5 | 664 | 28 | 9 | |
| Cation exchanger | 48.50 | 0.00 | 17.81 | nd | nd | 7 | 5 | 13 | 13 | 1 | |
| Vacuum distillation | concentrate | 1.10 | 0.00 | 788.80 | 13.15 | 126 | - | 308 | 697 | 665 | 10 |
| condensate | 47.10 | 0.00 | nd | 2.83 | nd | - | 0 | 0 | 1 | 0 | |
| Fermentation Broth from C6 Fermentation | |||||||||||
| Microfiltration (fermentation) | permeate | 88.00 | 0.00 | 31.66 | nd | 509 | 509 | 1808 | 12,819 | 4522 | 1329 |
| Nanofiltration | permeate | 88.50 | 0.00 | 28.73 | nd | 141 | 141 | 789 | 10,881 | 4342 | 1007 |
| retentate | 7.50 | 0.00 | 25.33 | nd | 2975 | 2975 | 7672 | 14,445 | 1264 | 3038 | |
| Softening | 99.50 | 2.38 | 27.02 | nd | 75 | 4 | 702 | 10,934 | 3935 | 333 | |
| Monopolar electrodialysis | concentrate | 22.45 | 0.00 | 107.80 | 9.37 | 167 | 27 | 2208 | 41,486 | 17,310 | 1394 |
| dilute | 83.35 | 3.02 | 0.85 | nd | 118 | 11 | 11 | 408 | 40 | 33 | |
| Bipolar electrodialysis | acid | 20.70 | 0.00 | 114.90 | 9.80 | 108 | 23 | 2891 | 7714 | 18,423 | 357 |
| base | 31.70 | 0.00 | 0.50 | 0.64 | 17 | 7 | 32 | 26,777 | 253 | 757 | |
| salt | 10.05 | 0.00 | 0.82 | 0.56 | 58 | 14 | 16 | 597 | 21 | 43 | |
| Decolorization | 1 | 26.50 | 0.00 | 87.00 | 7.70 | 41 | 19 | 2395 | 5349 | 14,195 | 154 |
| 2 | 40.00 | 0.00 | 55.43 | 5.26 | 23 | 24 | 1534 | 2725 | 8978 | 61 | |
| Anion exchanger1 | 78.75 | 0.00 | 15.73 | 2.48 | 17 | <4 | 3 | 1522 | 60 | 33 | |
| Cation exchanger1 | 83.20 | 0.00 | 14.59 | nd | 4 | <3 | 3 | 2 | 58 | 1 | |
| Anion exchanger2 | 83.20 | 0.00 | 13.10 | 1.94 | nd | 6 | 2 | 2 | 25 | 1 | |
| Vacuum distillation1 | concentrate | 17.60 | 0.00 | 56.75 | 3.30 | 18 | 5 | 7 | 11 | 107 | 4 |
| condensate | 46.00 | 0.00 | 0.11 | 0.98 | 1 | <4 | 1 | 1 | 1 | 2 | |
| Cation exchanger2 | 20.40 | 0.00 | 59.12 | 4.58 | - | - | 8 | 12 | 97 | 9 | |
| Anion exchanger3 | 20.40 | 0.00 | 58.71 | 4.52 | - | - | 10 | 13 | 25 | 8 | |
| Vacuum distillation2 | concentrate | 1.35 | 0.00 | 819.70 | 40.00 | 193 | 12 | 26 | 90 | 328 | 43 |
| condensate | 19.00 | 0.00 | nd | 7.30 | 9 | 405 | 1 | 1 | 0 | 1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves de Oliveira, R.; Schneider, R.; Hoss Lunelli, B.; Vaz Rossell, C.E.; Maciel Filho, R.; Venus, J. A Simple Biorefinery Concept to Produce 2G-Lactic Acid from Sugar Beet Pulp (SBP): A High-Value Target Approach to Valorize a Waste Stream. Molecules 2020, 25, 2113. https://doi.org/10.3390/molecules25092113
Alves de Oliveira R, Schneider R, Hoss Lunelli B, Vaz Rossell CE, Maciel Filho R, Venus J. A Simple Biorefinery Concept to Produce 2G-Lactic Acid from Sugar Beet Pulp (SBP): A High-Value Target Approach to Valorize a Waste Stream. Molecules. 2020; 25(9):2113. https://doi.org/10.3390/molecules25092113
Chicago/Turabian StyleAlves de Oliveira, Regiane, Roland Schneider, Betânia Hoss Lunelli, Carlos Eduardo Vaz Rossell, Rubens Maciel Filho, and Joachim Venus. 2020. "A Simple Biorefinery Concept to Produce 2G-Lactic Acid from Sugar Beet Pulp (SBP): A High-Value Target Approach to Valorize a Waste Stream" Molecules 25, no. 9: 2113. https://doi.org/10.3390/molecules25092113
APA StyleAlves de Oliveira, R., Schneider, R., Hoss Lunelli, B., Vaz Rossell, C. E., Maciel Filho, R., & Venus, J. (2020). A Simple Biorefinery Concept to Produce 2G-Lactic Acid from Sugar Beet Pulp (SBP): A High-Value Target Approach to Valorize a Waste Stream. Molecules, 25(9), 2113. https://doi.org/10.3390/molecules25092113







