Synthesis and Facile Dearomatization of Highly Electrophilic Nitroisoxazolo[4,3-b]pyridines
Abstract
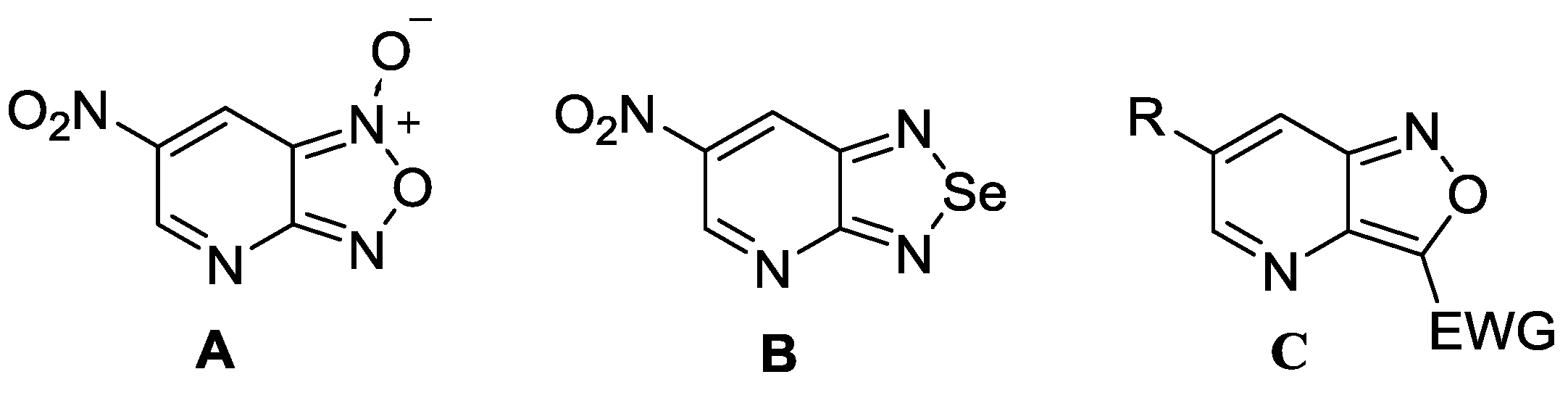
1. Introduction
2. Results and Discussion
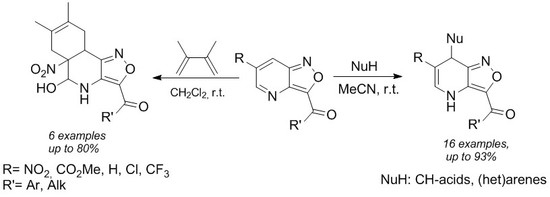
2.1. Synthesis of 6-R-Isoxazolo[4,3-b]pyridines 3a–j
2.2. X-ray of 2a, 2c, 3b
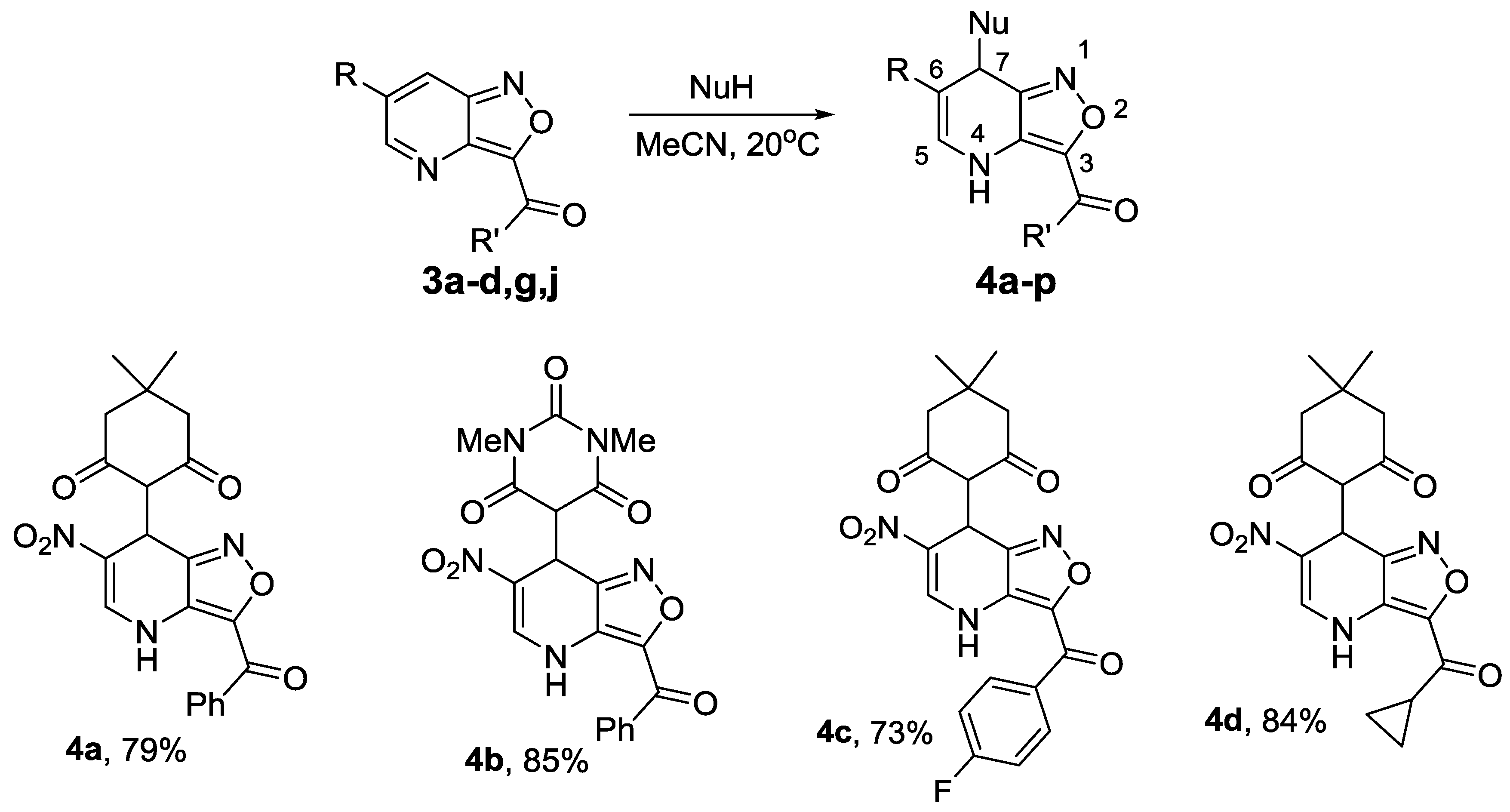
2.3. Nucleophilic Addition to 6-R-Isoxaxolo[4,3-b]pyridines
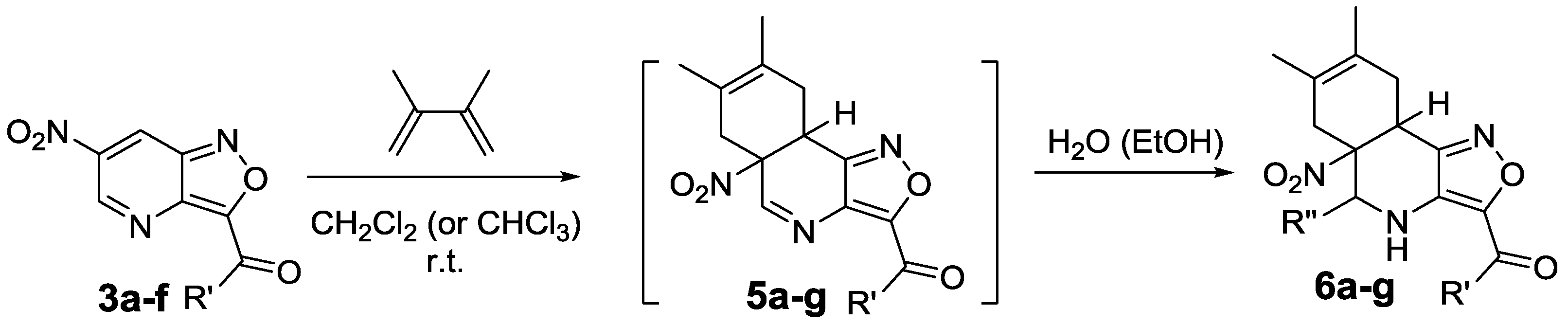
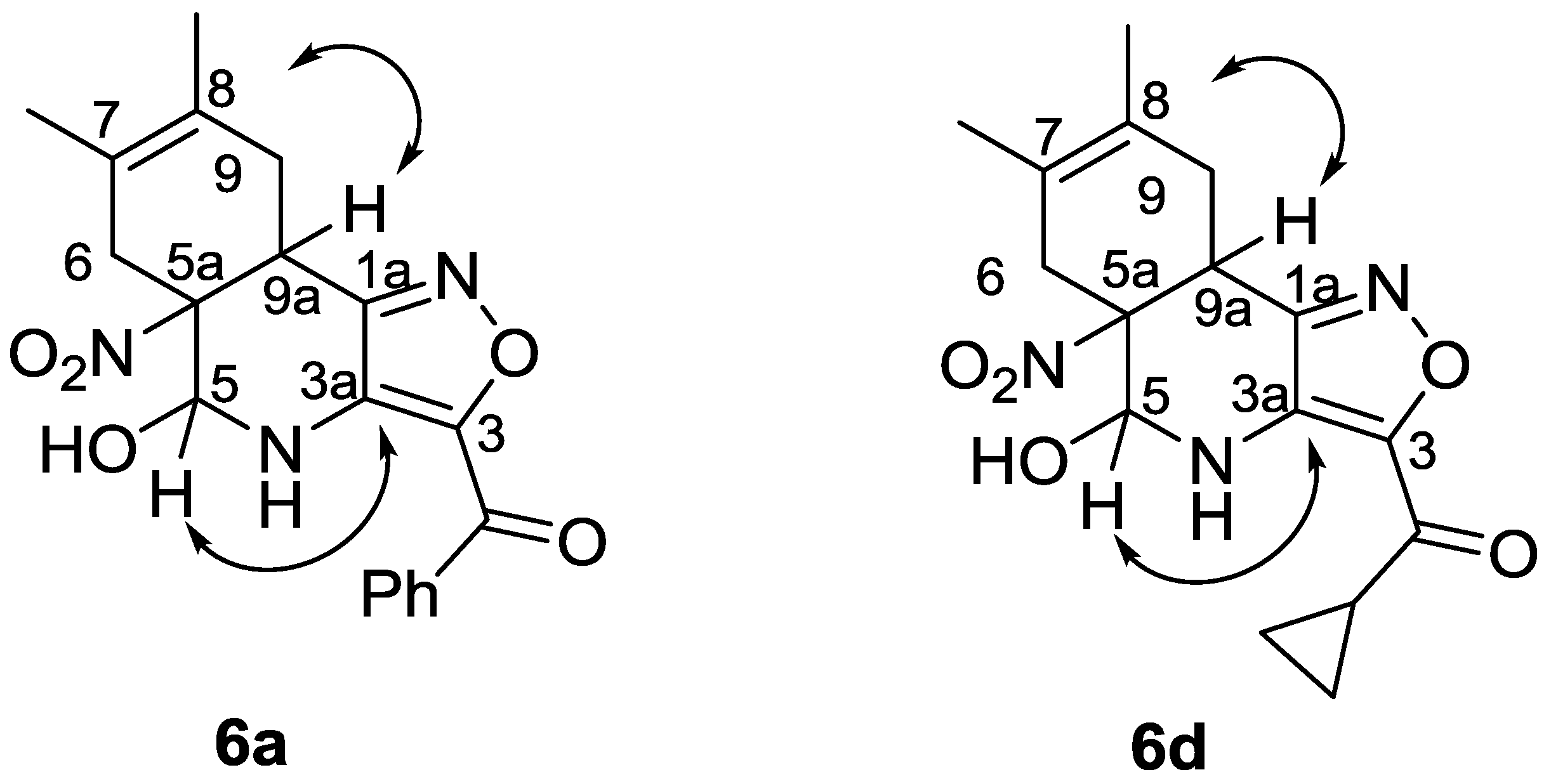
2.4. 6-NO2-Isoxazolo[4,3-b]pyridines in Diels-Alder Reactions
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Synthesis of Compounds 2a–i
4.3. Synthesis of Compounds 3a–i
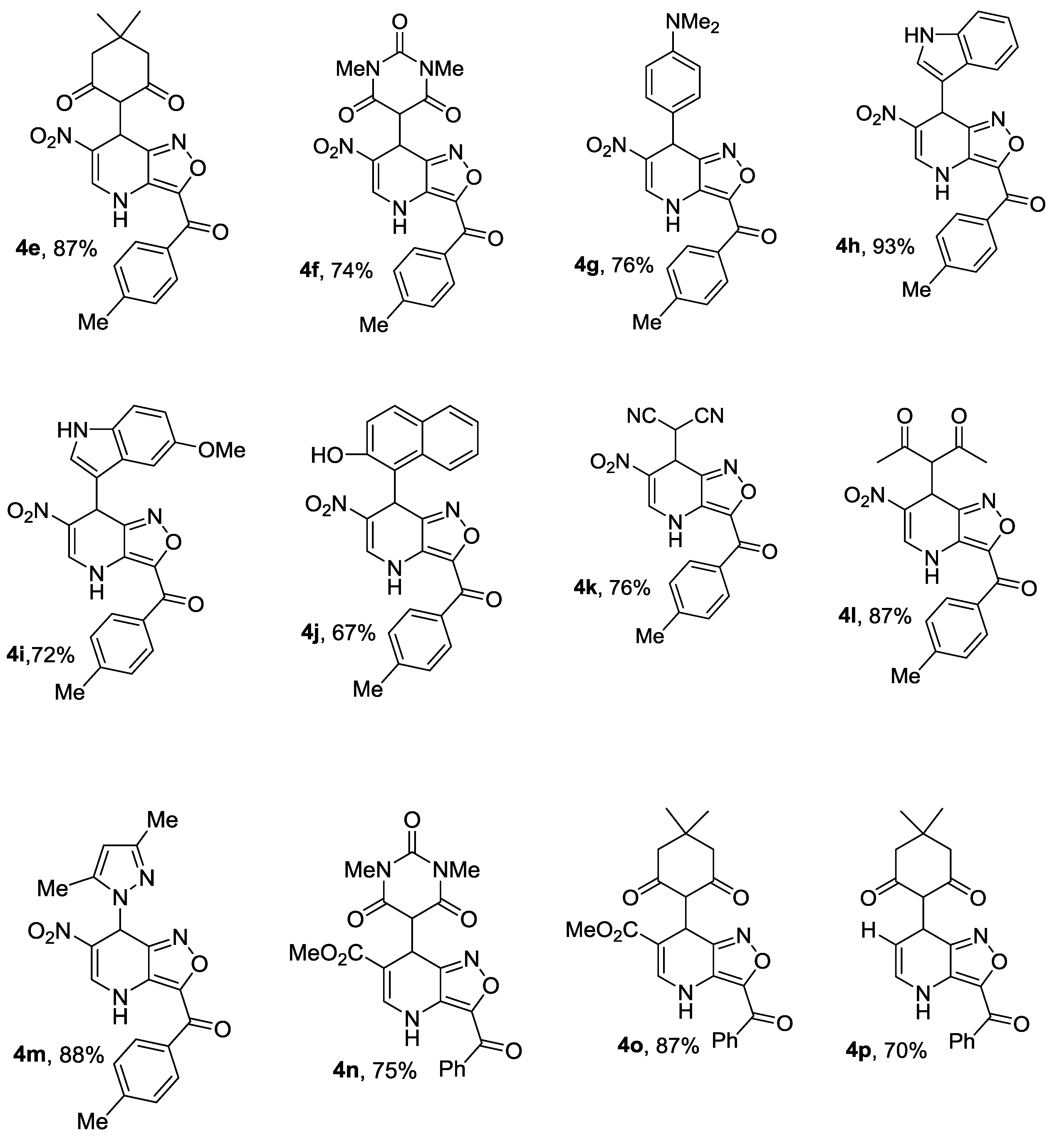
4.4. Synthesis of Compounds 4a–p
4.5. Synthesis of Compounds 6a–g
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nepali, K.; Lee, H.-Y.; Liou, J.-P. Nitro-group-containing drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.; Nishino, S.; Spain, J. Naturally-occurring nitro compounds. Nat. Prod. Rep. 2011, 28, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Le, S.T.; Asahara, H.; Nishiwaki, N. An alternative synthetic approach to 3-alkylated/arylated 5-nitropyridines. J. Org. Chem. 2015, 80, 8856–8858. [Google Scholar] [CrossRef]
- Yang, S.; Yan, J.; Wang, L.; Guo, X.; Li, S.; Wu, G.; Zuo, R.; Huang, X.; Wang, H.; Wang, L.; et al. Nitropyridinyl Ethyleneimine Compound, the Pharmaceutical Composition Containing It, the Preparation Method and Use Thereof. U.S. Patent 2014/0073796 A1, 13 March 2014. [Google Scholar]
- Jhons, B.A.; Spaltenstein, A. HIV Integrase Inhibitors. U.S. Patent WO 2007/09101 A2, 18 January 2007. [Google Scholar]
- Romero, D.L.; Morge, R.A.; Biles, C.; Discovery, synthesis, and bioactivity of bis(heteroaryl)piperazines. 1. a novel class of non-nucleoside HIV-1 reverse transcriptase inhibitors. J. Med. Chem. 1994, 37, 999–1014. [Google Scholar] [CrossRef]
- Korolev, S.P.; Kondrashina, O.V.; Druzhilovsky, D.S.; Starosotnikov, A.M.; Dutov, M.D.; Bastrakov, M.A.; Dalinger, I.L.; Filimonov, D.A.; Shevelev, S.A.; Poroikov, V.V.; et al. Structural-Functional Analysis of 2,1,3-Benzoxadiazoles and Their N-oxides as HIV-1 Integrase Inhibitors. Acta Nat. 2013, 5, 63–72. [Google Scholar] [CrossRef]
- Kaufman, H.A. Process for Producing Certain Amino Substituted Nitro Pyridines. U.S. Patent 3470172 A, 30 September 1969. [Google Scholar]
- Zhang, Q.; Zuo, J.; Chen, X.-L.; Yu, W. Synthesis and application of nitro-pyridine derivatives. Chem. Res. Appl. 2002, 14, 383–386. [Google Scholar]
- Gao, H.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef]
- Gryl, M.; Seidler, T.; Wojnarska, J.; Stadnicka, K.; Matulková, I.; Němec, I.; Němec, P. Co-crystals of 2-amino-5-nitropyridine barbital with extreme birefringence and large second harmonic generation effect. Chem. Eur. J. 2018, 24, 8727–8731. [Google Scholar] [CrossRef]
- Terrier, F. Nucleophilic Aromatic Displacement; Feuer, H., Ed.; VCH: New York, NY, USA, 1991; pp. 18–138. [Google Scholar]
- Chupakhin, O.N.; Charushin, V.N. Recent advances in the field of nucleophilic aromatic substitution of hydrogen. Tetrahedron Lett. 2016, 57, 2665–2672. [Google Scholar] [CrossRef]
- Makosza, M. Reactions of nucleophiles with nitroarenes: Multifacial and versatile electrophiles. Chem. Eur. J. 2014, 20, 5536–5545. [Google Scholar] [CrossRef]
- Makosza, M. Nucleophilic substitution of hydrogen in electron-deficient arenes, a general process of great practical value. Chem. Soc. Rev. 2010, 38, 2855–2868. [Google Scholar] [CrossRef] [PubMed]
- Terrier, F.; Millot, F.; Norris, W.P. Meisenheimer complexes: A kinetic study of water and hydroxide ion attacks on 4,6-dinitrobenzofuroxan in aqueous solution. J. Am. Chem. Soc. 1976, 98, 5883–5890. [Google Scholar] [CrossRef]
- Buncel, E.; Crampton, M.R.; Strauss, M.J.; Terrier, F. Electron-Deficient Aromatic and Heteroaromatic Base Interactions; Elsevier: Amsterdam, The Netherlands, 1984; pp. 166–296. [Google Scholar]
- Buncel, E.; Dust, J.; Terrier, F. Rationalizing the regioselectivity in polynitroarene anionic. sigma.-adduct formation. Relevance to nucleophilic aromatic substitution. Chem. Rev. 1995, 95, 2261–2280. [Google Scholar] [CrossRef]
- Spear, R.J.; Norris, W.P.; Read, R.W. Direct (uncatalysed) formation of Meisenheimer complexes from primary, secondary and tertiary arylamines. Tetrahedron Lett. 1983, 24, 1555–1558. [Google Scholar] [CrossRef]
- Terrier, F.; Chatrousse, A.P.; Soudais, Y.; Hlaibi, M. Methanol attack on highly electrophilic 4,6-dinitrobenzofurazan and 4,6-dinitrobenzofuroxan derivatives. A kinetic study. J. Org. Chem. 1984, 49, 4176–4181. [Google Scholar] [CrossRef]
- Strauss, M.J.; Renfrow, R.A.; Buncel, E. Ambident aniline reactivity in meisenheimer complex formation. J. Am. Chem. Soc. 1983, 105, 2473–2474. [Google Scholar] [CrossRef]
- Buncel, E.; Renfrow, R.A.; Strauss, M.J. Ambident nucleophilic reactivity in. sigma.-complex formations. 6. Reactivity-selectivity relationships in reactions of ambident nucleophiles with the superelectrophiles 4,6-dinitrobenzofuroxan and 4,6-dinitro-2-(2,4,6-trinitrophenyl) benzotriazole 1-oxide. J. Org. Chem. 1987, 52, 488–495. [Google Scholar] [CrossRef]
- Strauss, M.J.; De Fusco, A.; Terrier, F. Sulfur base complexes of 4,6-dinitrobenzofuroxan. Isolation of cysteine complex; Support for cellular thiol addition as a primary step in the in vitro inhibition of nucleic acid synthesis by nitrobenzofuroxans. Tetrahedron Lett. 1981, 22, 1945–1948. [Google Scholar] [CrossRef]
- Norris, W.P.; Spear, R.J.; Read, R.W. Explosive Meisenheimer complexes formed by addition of nucleophilic reagents to 4,6-dinitrobenzofurazan 1-oxide. Aust. J. Chem. 1983, 36, 297–309. [Google Scholar] [CrossRef]
- Terrier, F.; Halle’, J.C.; Pouet, M.J.; Simonnin, M.P. The proton sponge as nucleophile. J. Org. Chem. 1986, 51, 410–411. [Google Scholar] [CrossRef]
- Halle´, J.C.; Simonnin, M.P.; Pouet, M.J.; Terrier, F. Nucleophilic displacement of hydrogen in 4,6-dinitro-benzofuroxan and benzofurazan. Synthesis of some novel 7-substituted 4,6-dinitro-benzofuroxans and- benzofurazans. Tetrahedron Lett. 1985, 26, 1307–1310. [Google Scholar] [CrossRef]
- Terrier, F.; Halle´, J.C.; Pouet, M.J.; Simonnin, M.P. Nonconventional electrophilic heteroaromatic substitutions: Ring vs. side-chain reactivity of 2,5-dimethyl five-membered ring heterocycles toward electron-deficient aromatics. J. Org. Chem. 1984, 49, 4363–4367. [Google Scholar] [CrossRef]
- Terrier, F.; Kizilian, E.; Halle´, J.C.; Buncel, E. 4,6-dinitrobenzofuroxan: A stronger electrophile than the p-nitrobenzenediazonium cation and proton. J. Am. Chem. Soc. 1992, 114, 1740–1742. [Google Scholar] [CrossRef]
- Terrier, F.; Pouet, M.J.; Halle´, J.C.; Hunt, S.; Jones, J.R.; Buncel, E. Electrophilic heteroaromatic substitutions: Reactions of 5-X-substituted indoles with 4,6-dinitrobenzofuroxan. J. Chem. Soc. Perkin Trans. 1993, 2, 16651–16672. [Google Scholar] [CrossRef]
- Terrier, F.; Pouet, M.J.; Halle´, J.C.; Kizilian, E.; Buncel, E. Electrophilic aromatic substitutions: Reactions of hydroxyl- and methoxy-substituted benzenes with 4.6-dinitrobenzofuroxan: Kinetics and mechanism. J. Phys. Org. Chem. 1998, 11, 707–714. [Google Scholar] [CrossRef]
- Kind, J.; Niclas, H. Preparation of 5,7-dinitrobenzofurazan-4-yl aryl diketones via meisenheimer complexes. J. Synth. Commun. 1993, 23, 1569–1576. [Google Scholar] [CrossRef]
- Lakhdar, S.; Goumont, R.; Boubaker, T.; Mokhtari, M.; Terrier, F. Nitrobenzoxadiazoles and related heterocycles: A relationship between aromaticity, superelectrophilicity and pericyclic reactivity. Org. Biomol. Chem. 2006, 4, 1910–1919. [Google Scholar] [CrossRef]
- Terrier, F.; Simonnin, M.P.; Pouet, M.J.; Strauss, M.J. Reactivity of carbon acids toward 4,6-dinitrobenzofuroxan. Studies of keto-enol equilibriums and diastereoisomerism in carbon-bonded anionic sigma. Complexes. J. Org. Chem. 1981, 46, 3537–3543. [Google Scholar] [CrossRef]
- Terrier, F.; Sebban, M.; Goumont, R.; Halle´, J.C.; Moutiers, G.; Cangelosi, I.; Buncel, E. Dual behavior of 4-aza-6-nitrobenzofuroxan. A powerful electrophile in hydration and σ-complex formation and a potential dienophile or heterodiene in diels-alder type reactions. J. Org. Chem. 2000, 65, 7391–7398. [Google Scholar] [CrossRef]
- Remennikov, G.Y.; Pirozhenko, V.V.; Vdovenko, S.I.; Kravchenko, S.A. σ-Complexes in the pyrimidine series. 13. Reaction of 7- and 5-methoxyfuroxano-[3,4-d]pyrimidines with some C-nucleophiles. Chem. Heterocycl. Compd. 1998, 34, 104–110. [Google Scholar] [CrossRef]
- Wertjes, W.C.; Southgate, E.H.; Sarlah, D. Recent advances in chemical dearomatization of nonactivated arenes. Chem. Soc. Rev. 2018, 47, 7996–8017. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.L.; Iglesias, M.J.; Fernandez, I.; Andujar Sanchez, C.M.; Gomez, G.R. Nucleophilic dearomatizing (DNAr) reactions of aromatic, C,H-Systems. A mature paradigm in organic synthesis. Chem. Rev. 2007, 107, 1580–1691. [Google Scholar] [CrossRef] [PubMed]
- Bastrakov, M.A.; Nikol’skiy, V.V.; Starosotnikov, A.M.; Fedyanin, I.V.; Shevelev, S.A.; Knyazev, D.A. Reactions of 3-R-5-nitropyridines with nucleophiles: Nucleophilic substitution vs. conjugate addition. Tetrahedron 2019, 75, 130659. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bastrakov, M.A.; Starosotnikov, A.M.; Glukhov, I.V.; Lysov, K.A.; Rakitin, O.A.; Shevelev, S.A. 4.6-dinitrobenzo[c]isothiazole: Synthesis and 1,3-dipolar cycloaddition to azomethine ylide. Mendeleev Commun. 2010, 20, 353–354. [Google Scholar] [CrossRef]
- Bastrakov, M.A.; Leonov, A.I.; Starosotnikov, A.M.; Fedyanin, I.V.; Shevelev, S.A. 8-R-5,7-dinitroquinolines in [3 + 2]-cycloaddition reactions with N-methylazomethine ylide. Russ. Chem. Bull. Int. Ed. 2013, 62, 1052–1059. [Google Scholar] [CrossRef]
- Bastrakov, M.A.; Starosotnikov, A.M.; Fedyanin, I.V.; Kachala, V.V.; Shevelev, S.A. 5-nitro-7,8-furoxanoquinoline: A new type of fused nitroarenes possessing Diels-Alder reactivity. Mendeleev Commun. 2014, 24, 203–205. [Google Scholar] [CrossRef]
- Bastrakov, M.A.; Starosotnikov, A.M.; Pavlov, A.A.; Dalinger, I.L.; Shevelev, S.A. Synthesis of novel polycyclic heterosystems from 5-nitro[1,2,5]selenadiazolo[3,4-e]benzofuroxans. Chem. Heterocycl. Compd. 2016, 52, 690–693. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Bastrakov, M.A.; Pavlov, A.A.; Fedyanin, I.V.; Dalinger, I.L.; Shevelev, S.A. Synthesis of novel polycyclic heterosystems based on 5-nitro[1,2,5]thiadiazolo[3,4-e]benzofuroxan. Mendeleev Commun. 2016, 26, 217–219. [Google Scholar] [CrossRef]
- Bastrakov, M.A.; Kucherova, A.Y.; Fedorenko, A.K.; Starosotnikov, A.M.; Fedyanin, I.V.; Dalinger, I.L.; Shevelev, S.A. Dearomatization of 3,5-dinitropyridines—An atom-efficient approach to fused 3-nitropyrrolidines. Arkivoc 2017. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Shkaev, D.V.; Bastrakov, M.A.; Fedyanin, I.V.; Shevelev, S.A.; Dalinger, I.L. Nucleophilic dearomatization of 4-aza-6-nitrobenzofuroxan by CH acids in the synthesis of pharmacology-oriented compounds. Beilstein J. Org. Chem. 2017, 13, 2854–2861. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Shkaev, D.V.; Bastrakov, M.A.; Shevelev, S.A.; Dalinger, I.L. Dearomatization of oxa- or selenadiazolopyridines with neutral nucleophiles as an efficient approach to pharmacologically relevant nitrogen compounds. Mendeleev Commun. 2018, 28, 638–640. [Google Scholar] [CrossRef]
- Bastrakov, M.A.; Fedorenko, A.K.; Starosotnikov, A.M.; Kachala, V.V.; Shevelev, S.A. Dearomative (3 + 2) cycloaddition of 2-substituted 3,5-dinitropyridines and N-methyl azomethine ylide. Chem. Heterocycl. Compd. 2019, 55, 72–77. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Il’kov, K.V.; Bastrakov, M.A.; Fedyanin, I.V.; Kokorekin, V.A. Mild and efficient addition of carbon nucleophiles to condensed pyridines: Influence of structure and limits of applicability. Chem. Heterocycl. Comp. 2020, 56, 92–100. [Google Scholar] [CrossRef]
- Cikotiene, I. Intramolecular iodine-mediated oxygen transfer from nitro groups to C ≡ C Bonds. Eur. J. Org. Chem. 2012, 27662–27773. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Motherwell, W.D.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. Retrieval of crystallographically-derived molecular geometry information. J. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef]
- SAINT; Version 8.34A; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystalstructure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


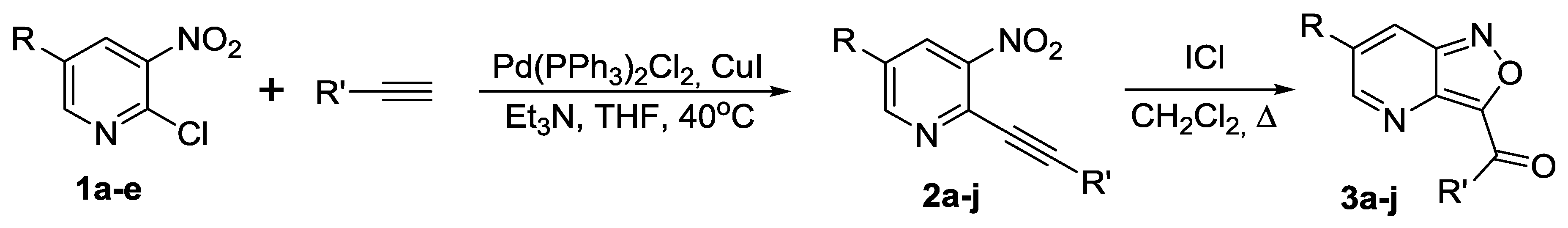

| Compound 1 | R | R′ | Product 2, Yield (%) | Product 3, Yield (%) |
|---|---|---|---|---|
| 1a | NO2 | Ph | 2a, 72 | 3a, 85 |
| 1a | NO2 | 4-Me-C6H4 | 2b, 63 | 3b, 87 |
| 1a | NO2 | 4-F-C6H4 | 2c, 61 | 3c, 72 |
| 1a | NO2 | c-C3H5 | 2d, 84 | 3d, 74 |
| 1a | NO2 | c-C5H9 | 2e, 82 | 3e, 71 * |
| 1a | NO2 | n-C5H11 | 2f, 35 | 3f, 80 * |
| 1b | CO2Me | Ph | 2g, 76 | 3g, 65 |
| 1c | CF3 | Ph | 2h, 42 | 3h, 73 |
| 1d | Cl | Ph | 2i, 40 | 3i, 60 |
| 1e | H | Ph | 2j, 82 | 3j, 80 |
| Compound 3 | R′ | R″ | Product 6, Yield (%) |
|---|---|---|---|
| 3a | Ph | OH | 6a, 74 |
| 3b | 4-Me-C6H4 | OH | 6b, 80 |
| 3c | 4-F-C6H4 | OH | 6c, 73 |
| 3d | c-C3H5 | OH | 6d, 80 |
| 3e | c-C5H9 | OH | 6e, 51 |
| 3f | n-C5H11 | OH | 6f, 35 |
| 3b | 4-Me-C6H4 | OEt | 6g, 42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastrakov, M.A.; Fedorenko, A.K.; Starosotnikov, A.M.; Fedyanin, I.V.; Kokorekin, V.A. Synthesis and Facile Dearomatization of Highly Electrophilic Nitroisoxazolo[4,3-b]pyridines. Molecules 2020, 25, 2194. https://doi.org/10.3390/molecules25092194
Bastrakov MA, Fedorenko AK, Starosotnikov AM, Fedyanin IV, Kokorekin VA. Synthesis and Facile Dearomatization of Highly Electrophilic Nitroisoxazolo[4,3-b]pyridines. Molecules. 2020; 25(9):2194. https://doi.org/10.3390/molecules25092194
Chicago/Turabian StyleBastrakov, Maxim A., Alexey K. Fedorenko, Alexey M. Starosotnikov, Ivan V. Fedyanin, and Vladimir A. Kokorekin. 2020. "Synthesis and Facile Dearomatization of Highly Electrophilic Nitroisoxazolo[4,3-b]pyridines" Molecules 25, no. 9: 2194. https://doi.org/10.3390/molecules25092194
APA StyleBastrakov, M. A., Fedorenko, A. K., Starosotnikov, A. M., Fedyanin, I. V., & Kokorekin, V. A. (2020). Synthesis and Facile Dearomatization of Highly Electrophilic Nitroisoxazolo[4,3-b]pyridines. Molecules, 25(9), 2194. https://doi.org/10.3390/molecules25092194