Banhahubak-Tang Tablet, a Standardized Medicine Attenuates Allergic Asthma via Inhibition of Janus Kinase 1 (JAK1)/ Signal Transducer and Activator of Transcription 6 (STAT6) Signal Pathway
Abstract
1. Introduction
2. Results
2.1. Effect of BHT on Airway Remodeling in OVA and PM10-Induced Mice
2.2. Effects of BHT on Inflammatory Cells in Bronchoalveolar Lavage Fluid (BALF) of OVA and PM10-Induced Mice
2.3. Effects of BHT on Secretion of Serum Immunoglobulin Levels in OVA and PM10-Induced Mice
2.4. Effects of BHT on JAK1/STAT6 Signal Pathway in OVA + PM10-Induced Mice and PM10-Treated A549
2.5. Effects of BHT on Inflammatory Cytokines in OVA + PM10-Induced Mice and PM10-Treated A549
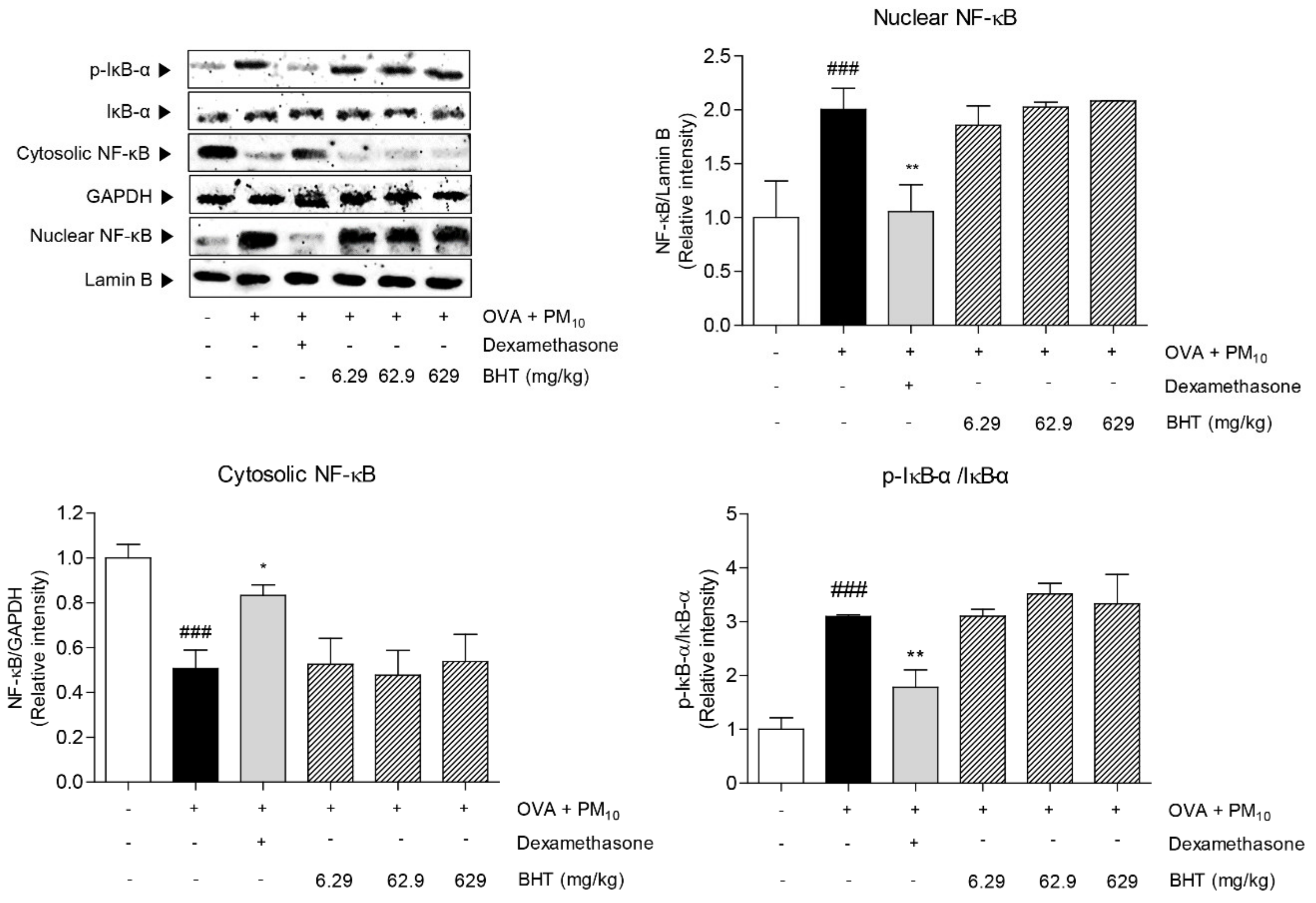
2.6. Effects of BHT on NF-κB Signal Pathway in OVA + PM10-Induced Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of BHT
4.2. Chemicals and Reagents
4.3. Animal Treatment
4.4. Bronchoalveolar Lavage Fluid (BALF) Analysis
4.5. Serum Analysis
4.6. Histopathological Analysis
4.7. Cell Treatment
4.8. Cell Survival Assay
4.9. Nuclear and Cytosolic Protein Fractionation
4.10. Western Blot Analysis
4.11. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.12. High-Performance Liquid Chromatography (HPLC) Analysis of BHT
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Sanchez-Solis, M. Early Lung Function and Future Asthma. Front. Pediatr. 2019, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Croisant, S. Epidemiology of Asthma: Prevalence and Burden of Disease. Adv. Exp. Med. Biol. 2013, 795, 17–29. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kandhare, A.D.; Rojatkar, S.; Bodhankar, S. Ameliorative effects of Artemisia pallens in a murine model of ovalbumin-induced allergic asthma via modulation of biochemical perturbations. Biomed. Pharmacother. 2017, 94, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Gilmour, M.I.; MacNee, W. Asthma and PM10. Respir. Res. 2000, 1, 12–15. [Google Scholar] [CrossRef]
- Reddy, A.P.; Gupta, M.R. Management of Asthma: The Current US and European Guidelines. Adv. Exp. Med. Biol. 2013, 795, 81–103. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Kang, D.; Kim, J.-E. Fine, Ultrafine, and Yellow Dust: Emerging Health Problems in Korea. J. Korean Med Sci. 2014, 29, 621–622. [Google Scholar] [CrossRef]
- Huang, K.-L.; Liu, S.-Y.; Chou, C.C.K.; Lee, Y.-H.; Cheng, T.-J. The effect of size-segregated ambient particulate matter on Th1/Th2-like immune responses in mice. PLoS ONE 2017, 12, e0173158. [Google Scholar] [CrossRef]
- Reddy, A.T.; Lakshmi, S.P.; Reddy, R.C. Murine Model of Allergen Induced Asthma. J. Vis. Exp. 2012. [Google Scholar] [CrossRef]
- Walders, N.; Kopel, S.J.; Koinis-Mitchell, D.; McQuaid, E.L. Patterns of quick-relief and long-term controller medication use in pediatric asthma. J. Pediatr. 2005, 146, 177–182. [Google Scholar] [CrossRef]
- Kanazawa, H. Anticholinergic agents in asthma: chronic bronchodilator therapy, relief of acute severe asthma, reduction of chronic viral inflammation and prevention of airway remodeling. Curr. Opin. Pulm. Med. 2006, 12, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, L.; Zhang, X.; Long, Y.; Zou, F.; Yan, C.; Wei, Z. Protective effects and active ingredients of Salvia miltiorrhiza Bunge extracts on airway responsiveness, inflammation and remodeling in mice with ovalbumin-induced allergic asthma. Phytomedicine 2018, 52, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Prietsch, S.O.M.; Ducharme, F.M. Inhaled corticosteroids in children with persistent asthma: effects on growth. Evidence-Based Child Heal. A Cochrane Rev. J. 2014, 9, 829–930. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, J.; Nelson, H.S. Safety of long-acting ??-agonists in asthma: a review. Curr. Opin. Pulm. Med. 2008, 14, 64–69. [Google Scholar] [CrossRef]
- Vogelberg, C.; Goldstein, S.; Graham, L.; Kaplan, A.; De La Hoz, A.; Hamelmann, E. A comparison of tiotropium, long-acting β2-agonists and leukotriene receptor antagonists on lung function and exacerbations in paediatric patients with asthma. Respir. Res. 2020, 21, 19. [Google Scholar] [CrossRef]
- Oikawa, T.; Ito, G.; Hoshino, T.; Koyama, H.; Hanawa, T. Hangekobokuto (Banxia-houpo-tang), a Kampo Medicine that Treats Functional Dyspepsia. Evidence Based Complement. Altern. Med. 2007, 6, 375–378. [Google Scholar] [CrossRef]
- Lim, S.-H.; Jeong, H.-Y.; Won, H.-Y.; Kim, H.-W.; Choi, C.-W.; Jeong, H.-S.; Kim, Y.-G.; Cho, S. Effects of Banhahubak-tang Extract on Psychological Stress. Korea J. Herbol. 2012, 27, 81–88. [Google Scholar] [CrossRef][Green Version]
- Holgate, S.T. The Airway Epithelium is Central to the Pathogenesis of Asthma. Allergol. Int. 2008, 57, 1–10. [Google Scholar] [CrossRef]
- Song, J.; Kang, J.; Lin, B.; Li, J.; Zhu, Y.; Du, J.; Yang, X.; Xi, Z.; Li, R. Mediating Role of TRPV1 Ion Channels in the Co-exposure to PM2.5 and Formaldehyde of Balb/c Mice Asthma Model. Sci. Rep. 2017, 7, 11926. [Google Scholar] [CrossRef]
- Gandhi, V.D.; Vliagoftis, H. Airway Epithelium Interactions with Aeroallergens: Role of Secreted Cytokines and Chemokines in Innate Immunity. Front. Immunol. 2015, 6, 147. [Google Scholar] [CrossRef]
- Sung, J.-E.; Lee, H.-A.; Kim, J.-E.; Yun, W.-B.; An, B.-S.; Yang, S.-Y.; Kim, D.-S.; Lee, C.-Y.; Lee, H.-S.; Bae, C.-J.; et al. Saponin-enriched extract of Asparagus cochinchinensis alleviates airway inflammation and remodeling in ovalbumin-induced asthma model. Int. J. Mol. Med. 2017, 40, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.F. Airway goblet cell hyperplasia in asthma: hypersecretory and anti-inflammatory? Clin. Exp. Allergy 2002, 32, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Koerner-Rettberg, C.; Doths, S.; Stroet, A.; Schwarze, J. Reduced Lung Function in a Chronic Asthma Model Is Associated with Prolonged Inflammation, but Independent of Peribronchial Fibrosis. PLoS ONE 2008, 3, e1575. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Meng, Y.; Adcock, I.M.; Yao, X. Role of inflammatory cells in airway remodeling in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chen, H.-B.; Wang, Y.; Lin, J.-H.; Hu, Y.; Fang, Y.-R. Changes in interleukin-17 and transforming growth factor beta 1 levels in serum and bronchoalveolar lavage fluid and their clinical significance among children with asthma. Transl. Pediatr. 2013, 2, 154–159. [Google Scholar] [PubMed]
- Zhang, R.; Kubo, M.; Murakami, I.; Setiawan, H.; Takemoto, K.; Inoue, K.; Fujikura, Y.; Ogino, K. L-Arginine administration attenuates airway inflammation by altering L-arginine metabolism in an NC/Nga mouse model of asthma. J. Clin. Biochem. Nutr. 2015, 56, 201–207. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; A Layhadi, J.; Rondon, C.; Shamji, M. Mucosal IgE immune responses in respiratory diseases. Curr. Opin. Pharmacol. 2019, 46, 100–107. [Google Scholar] [CrossRef]
- Bao, Z.; Zhang, P.; Yao, Y.; Lu, G.; Tong, Z.; Yan, B.; Tu, L.; Yang, G.; Zhou, J. Deguelin Attenuates Allergic Airway Inflammation via Inhibition of NF-κb Pathway in Mice. Int. J. Boil. Sci. 2017, 13, 492–504. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.E. The Role of Immunoglobulin E in Allergy and Asthma. Am. J. Respir. Crit. Care Med. 2001, 164, 1–5. [Google Scholar] [CrossRef]
- Pernis, A.B.; Rothman, P.B. JAK-STAT signaling in asthma. J. Clin. Investig. 2002, 109, 1279–1283. [Google Scholar] [CrossRef]
- Tomita, K.; Caramori, G.; Ito, K.; Sano, H.; Lim, S.; Oates, T.; Cosío, B.G.; Chung, K.F.; Tohda, Y.; Barnes, P.; et al. STAT6 expression in T cells, alveolar macrophages and bronchial biopsies of normal and asthmatic subjects. J. Inflamm. 2012, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.; Strehlow, I. Cytokines and STAT signaling. HIV-1: Mol. Boil. Pathog. 2000, 47, 113–174. [Google Scholar]
- Chung, K.F.; Barnes, P.J. Cytokines in asthma. Thorax 1999, 54, 825–857. [Google Scholar] [CrossRef] [PubMed]
- Corren, J. Role of Interleukin-13 in Asthma. Curr. Allergy Asthma Rep. 2013, 13, 415–420. [Google Scholar] [CrossRef]
- Barnes, P. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef]
- Hoshino, A.; Tsuji, T.; Matsuzaki, J.; Jinushi, T.; Ashino, S.; Teramura, T.; Chamoto, K.; Tanaka, Y.; Asakura, Y.; Sakurai, T.; et al. STAT6-mediated signaling in Th2-dependent allergic asthma: critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. Int. Immunol. 2004, 16, 1497–1505. [Google Scholar] [CrossRef]
- E Nocker, R.; Schoonbrood, D.F.; A Van De Graaf, E.; E Hack, C.; Lutter, R.; Jansen, H.M.; Out, T.A. Interleukin-8 in airway inflammation in patients with asthma and chronic obstructive pulmonary disease. Int. Arch. Allergy Immunol. 1996, 109, 183–191. [Google Scholar] [CrossRef]
- Schuliga, M. NF-kappaB Signaling in Chronic Inflammatory Airway Disease. Biomol. 2015, 5, 1266–1283. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Chen, B.; Zhuo, Q.; Bao, C.; Lin, L. Propofol inhibits NF-κB activation to ameliorate airway inflammation in ovalbumin (OVA)-induced allergic asthma mice. Int. Immunopharmacol. 2017, 51, 158–164. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Shin, I.-S.; Jeon, W.-Y.; Lim, H.-S.; Kim, J.-H.; Ha, H. Pinellia ternataBreitenbach attenuates ovalbumin-induced allergic airway inflammation and mucus secretion in a murine model of asthma. Immunopharmacol. Immunotoxicol. 2013, 35, 410–418. [Google Scholar] [CrossRef]
- Shin, T.Y.; Kim, D.-K.; Chae, B.S.; Lee, E.J. Antiallergic action of Magnolia officinalis on immediate hypersensitivity reaction. Arch. Pharmacal Res. 2001, 24, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.; Businga, T.R.; Kline, J.N.; Bishop, G. Anti-inflammatory effects of the neurotransmitter agonist Honokiol in a mouse model of allergic asthma. J. Immunol. 2010, 185, 5586–5597. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Han, L.; Lv, R.; Ling, L. Magnolol exerts anti-asthmatic effects by regulating Janus kinase-signal transduction and activation of transcription and Notch signaling pathways and modulating Th1/Th2/Th17 cytokines in ovalbumin-sensitized asthmatic mice. Korean J. Physiol. Pharmacol. 2019, 23, 251–261. [Google Scholar] [CrossRef]
- Liang, Z.; Nie, H.; Xu, Y.; Peng, J.; Zeng, Y.; Wei, Y.; Wen, X.; Qiu, J.; Zhong, W.; Deng, X.; et al. Therapeutic effects of rosmarinic acid on airway responses in a murine model of asthma. Int. Immunopharmacol. 2016, 41, 90–97. [Google Scholar] [CrossRef]
- Townsend, E.A.; Zhang, Y.; Xu, C.; Wakita, R.; Emala, C.W. Active Components of Ginger Potentiate β-Agonist–Induced Relaxation of Airway Smooth Muscle by Modulating Cytoskeletal Regulatory Proteins. Am. J. Respir. Cell Mol. Boil. 2013, 50, 115–124. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Uddandrao, V.V.S.; Ponnusamy, P.; Balakrishnan, S.; Brahmanaidu, P.; Vadivukkarasi, S.; Ganapathy, S.; Zaoni, L.; Zhongxi, L.; et al. Asthma-Alleviating Potential of 6-Gingerol: Effect on Cytokines, Related mRNA and c-Myc, and NFAT1 Expression in Ovalbumin-Sensitized Asthma in Rats. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 41–50. [Google Scholar] [CrossRef]
- Ninave, P.B.; Patil, S.D. Antiasthmatic potential of Zizyphus jujuba Mill and Jujuboside B.—Possible role in the treatment of asthma. Respir. Physiol. Neurobiol. 2019, 260, 28–36. [Google Scholar] [CrossRef]
Sample Availability:Banhahubak-tang tablet is commercially available. |





| Gene | Forward Primer (5′→3′) | Reverse Primer (3′→5′) |
|---|---|---|
| TNF-α | TACTGAACTTCGGGGTGATTGGTCC | CAGCCTTGTCCCTTGAAGAGAACC |
| IL-1β | CAGGATGAGGACATGAGCACC | CTCTGCACACTCAAACTCCAC |
| IL-6 | CGGAGAGGAGACTTCACAGAGGA | GGAGAGCATTGGAAATTGGGG |
| IL-8 | TGTGGGAGGCTGTGTTTGTA | TGTGGGAGGCTGTGTTTGTA |
| IL-17A | TCCAGAAGGCCCTCAGACTA | AGCATCTTCTCGACCCTGAA |
| IL-4 | ATGGGTCTCAACCCCCAGC | GCTCTTTACGCTTTCCAGGAAGTC |
| IL-5 | ATGATCGTGCCTCTGTGCCTGGAGC | CTGTTTTTCCTGGAGTAAACTGGGG |
| IL-13 | ACCACGGTCATTGCTCTCA | GTGTCT CGGACATGCAAGCT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, Y.K.; Jin, S.C.; Kim, M.H.; Choi, L.Y.; Lee, Y.-B.; Yang, W.M. Banhahubak-Tang Tablet, a Standardized Medicine Attenuates Allergic Asthma via Inhibition of Janus Kinase 1 (JAK1)/ Signal Transducer and Activator of Transcription 6 (STAT6) Signal Pathway. Molecules 2020, 25, 2206. https://doi.org/10.3390/molecules25092206
Nam YK, Jin SC, Kim MH, Choi LY, Lee Y-B, Yang WM. Banhahubak-Tang Tablet, a Standardized Medicine Attenuates Allergic Asthma via Inhibition of Janus Kinase 1 (JAK1)/ Signal Transducer and Activator of Transcription 6 (STAT6) Signal Pathway. Molecules. 2020; 25(9):2206. https://doi.org/10.3390/molecules25092206
Chicago/Turabian StyleNam, Yeon Kyung, Seong Chul Jin, Mi Hye Kim, La Yoon Choi, Yong-Bok Lee, and Woong Mo Yang. 2020. "Banhahubak-Tang Tablet, a Standardized Medicine Attenuates Allergic Asthma via Inhibition of Janus Kinase 1 (JAK1)/ Signal Transducer and Activator of Transcription 6 (STAT6) Signal Pathway" Molecules 25, no. 9: 2206. https://doi.org/10.3390/molecules25092206
APA StyleNam, Y. K., Jin, S. C., Kim, M. H., Choi, L. Y., Lee, Y.-B., & Yang, W. M. (2020). Banhahubak-Tang Tablet, a Standardized Medicine Attenuates Allergic Asthma via Inhibition of Janus Kinase 1 (JAK1)/ Signal Transducer and Activator of Transcription 6 (STAT6) Signal Pathway. Molecules, 25(9), 2206. https://doi.org/10.3390/molecules25092206









