Fabrication and Characterization of Nanocomposite Flexible Membranes of PVA and Fe3O4
Abstract
:1. Introduction
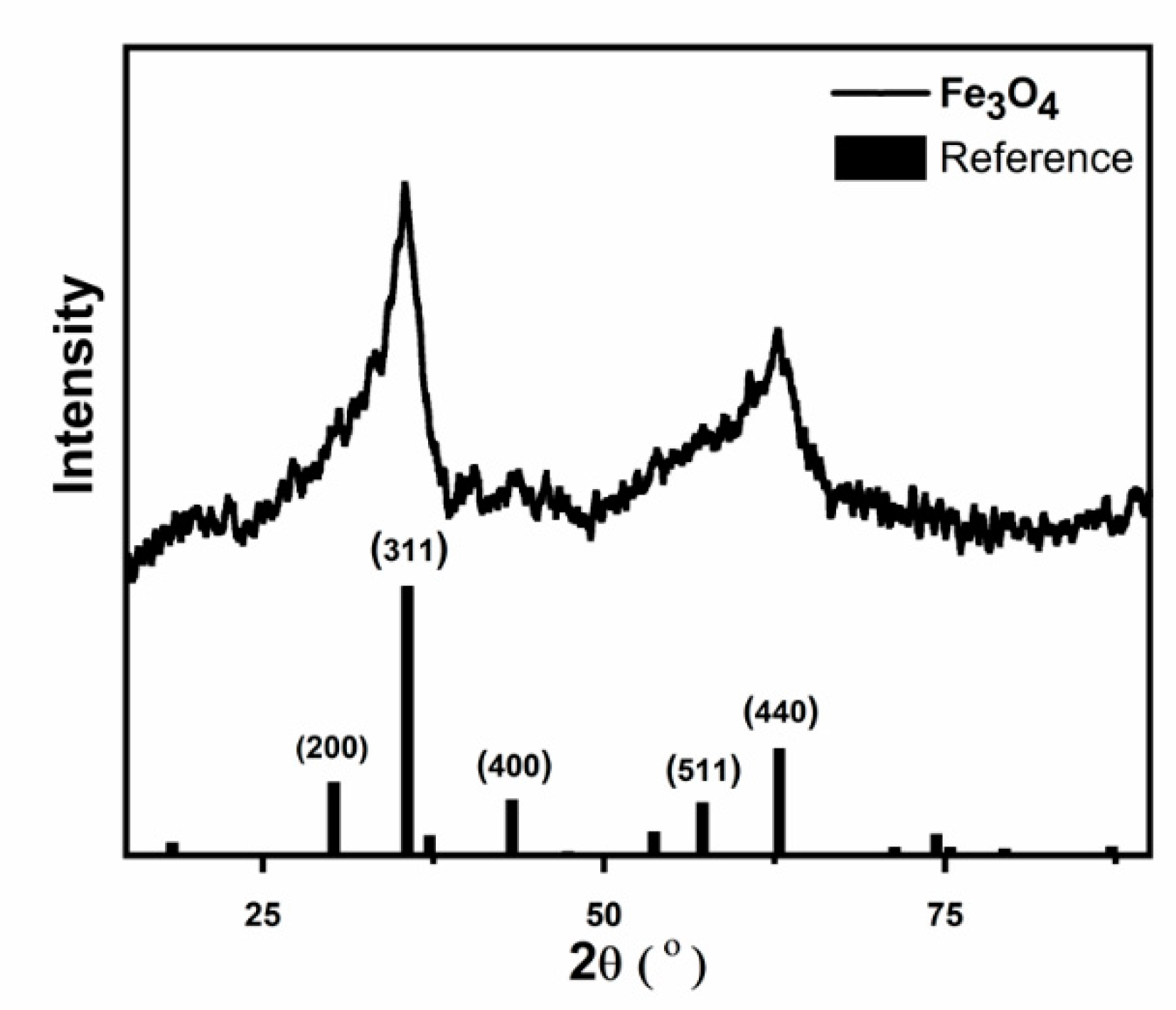
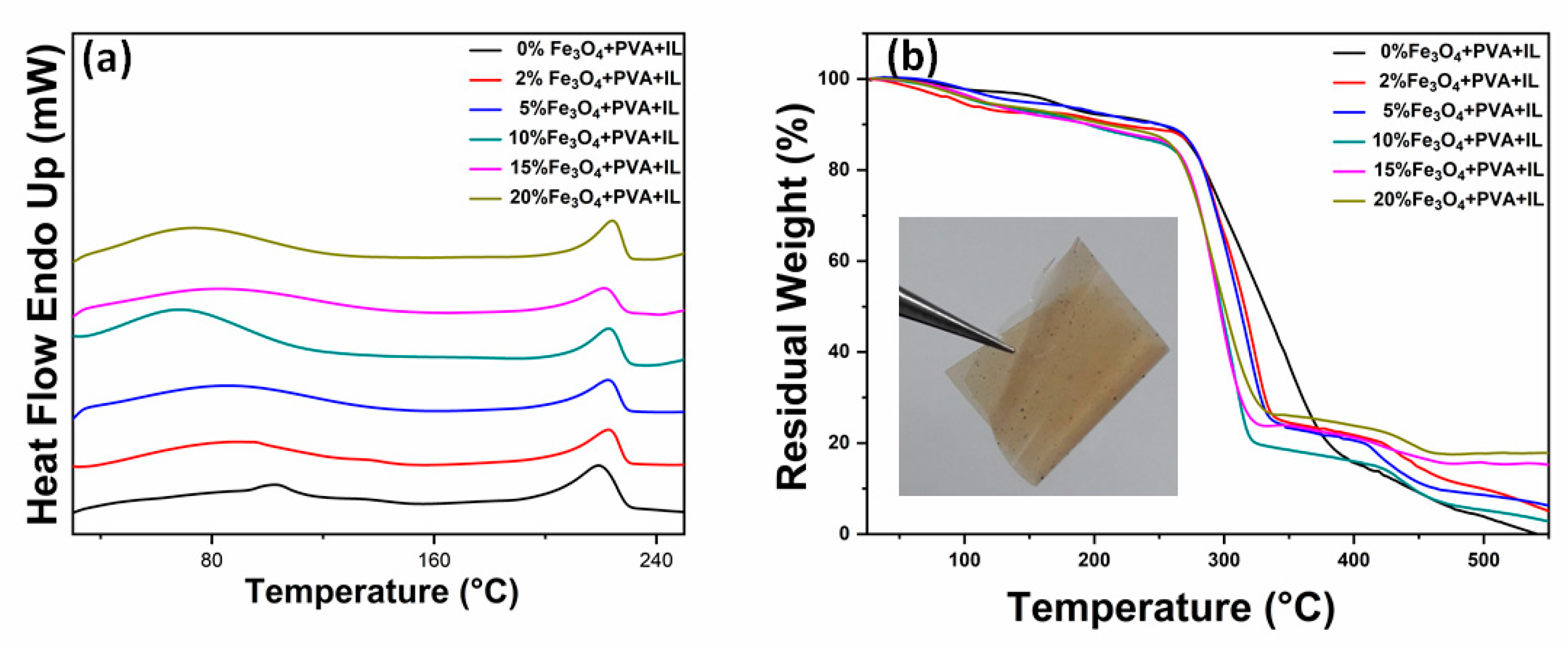
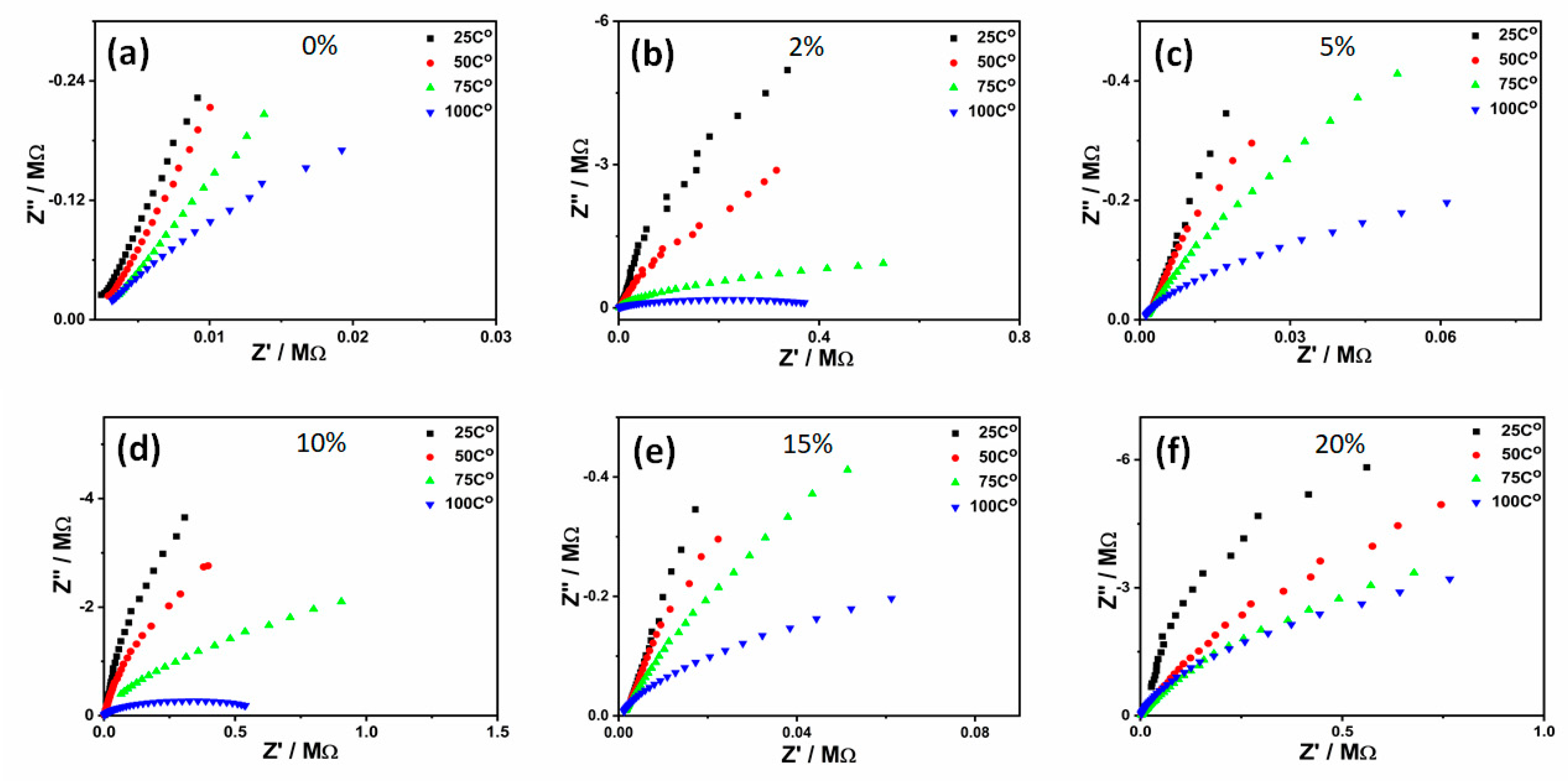
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Synthesis of Nanoparticles
3.3. Membrane Preparation
4. Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Josh, V.; Haik, M.Y.; Ayesh, A.I.; Mohsin, M.A.; Haik, Y. Electrical properties of sorbitol doped PVA-PAA polymer membranes. J. Appl. Polym. Sci. 2012, 128, 3861–3869. [Google Scholar] [CrossRef]
- Finch, C.A. Polyvinyl Alcohol: Properties and Applications; John Wiley: London, UK, 1973; p. 622. [Google Scholar]
- Ayesh, A.I.; Mohsin, M.A.; Haik, M.Y.; Haik, Y. Investigations on electrical properties of poly (vinyl alcohol) doped with 1-methyl-3-n-decyl-imidazolium bromide ionic liquid. Curr. Appl. Phys. 2012, 12, 1223–1228. [Google Scholar] [CrossRef]
- Sharkawy, A.A.; Klitzman, B.; Truskey, G.A.; Reichert, W.M. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma–tissue exchange properties. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 40, 586–597. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly (vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar]
- Hema, M.; Selvasekarapandian, S.; Arunkumar, D.; Sakunthala, A.; Nithya, H. FTIR, XRD and ac impedance spectroscopic study on PVA based polymer electrolyte doped with NH4X (X = Cl, Br, I). J. Non-Cryst. Solids 2009, 355, 84–90. [Google Scholar] [CrossRef]
- Mamunya, Y.P.; Davydenko, V.; Pissis, P.; Lebedev, E. Electrical and thermal conductivity of polymers filled with metal powders. Eur. Polym. J. 2002, 38, 1887–1897. [Google Scholar] [CrossRef]
- Baqiya, M.A.; Taufiq, A.; Sunaryono, M.; Sari, P.; Dwihapsari, Y. Development of PVA/Fe3O4 as smart magnetic hydrogels for biomedical applications. Hydrogels. Lond. Intechopen 2018, 159–178. [Google Scholar]
- Nguyen, H.H.; Ta, H.K.T.; Park, S.; Phan, T.B.; Pham, N.K. Resistive switching effect and magnetic properties of iron oxide nanoparticles embedded-polyvinyl alcohol film. Rsc Adv. 2020, 10, 12900–12907. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, Y.; Liang, S.; Liu, H.; Han, B. Hydrogenolysis of glycerol catalyzed by Ru-Cu bimetallic catalysts supported on clay with the aid of ionic liquids. Green Chem. 2009, 11, 1000–1006. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Abu-Hani, A.F.S.; Mahmoud, S.T.; Haik, Y. Selective H2S sensor based on CuO nanoparticles embedded in organic membranes. Sens. Actuators B Chem. 2016, 231, 593–600. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Qadri, S.; Baboo, V.J.; Haik, M.Y.; Haik, Y. Nano-floating gate organic memory devices utilizing Ag-Cu nanoparticles embedded in PVA-PAA-glycerol polymer. Synth. Met. 2013, 183, 24–28. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Ayesh, A.; Qamhieh, N.; Ghamlouche, H.; Thaker, S.; El-Shaer, M. Fabrication of size-selected Pd nanoclusters using a magnetron plasma sputtering source. J. Appl. Phys. 2010, 107, 034317. [Google Scholar] [CrossRef]
- Haik, M.Y.; Ayesh, A.I.; Abdulrehman, T.; Haik, Y. Novel organic memory devices using Au–Pt–Ag nanoparticles as charge storage elements. Mater. Lett. 2014, 124, 67–72. [Google Scholar] [CrossRef]
- Ai, L.; Wang, Y.; Tao, G.; Zhao, P.; Umar, A.; Wang, P.; He, H. Polydopamine-based surface modification of ZnO nanoparticles on sericin/polyvinyl alcohol composite film for antibacterial application. Molecules 2019, 24, 503. [Google Scholar] [CrossRef] [Green Version]
- Abu-Hani, A.F.S.; Awwad, F.; Greish, Y.E.; Ayesh, A.I.; Mahmoud, S.T. Design, fabrication, and characterization of low-power gas sensors based on organic-inorganic nano-composite. Org. Electron. 2017, 42, 284–292. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Lee, W.; Oh, Y.-S.; Lee, J.-K. Magnetic nanoparticles as a catalyst vehicle for simple and easy recycling. New J. Chem. 2003, 27, 227–229. [Google Scholar] [CrossRef]
- Faria, V.W.; Oliveira, D.G.; Kurz, M.H.; Gonçalves, F.F.; Scheeren, C.W.; Rosa, G.R. Palladium nanoparticles supported in a polymeric membrane: An efficient phosphine-free “green” catalyst for Suzuki–Miyaura reactions in water. Rsc Adv. 2014, 4, 13446–13452. [Google Scholar] [CrossRef]
- Ghanbari, D.; Salavati-Niasari, M.; Ghasemi-Kooch, M. A sonochemical method for synthesis of Fe3O4 nanoparticles and thermal stable PVA-based magnetic nanocomposite. J. Ind. Eng. Chem. 2014, 20, 3970–3974. [Google Scholar] [CrossRef]
- Ardiyanti, H.; Suharyadi, E.; Kato, T.; Iwata, S. Crystal structures and magnetic properties of magnetite (Fe3O4)/polyvinyl alcohol (PVA) ribbon. Aip Conf. Proc. 2016, 1725, 020007. [Google Scholar]
- Makhluf, S.B.D.; Abu-Mukh, R.; Rubinstein, S.; Breitbart, H.; Gedanken, A. Modified PVA-Fe3O4 nanoparticles as protein carriers into sperm cells. Small 2008, 4, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.; Zawodzinski, T.; Wilson, M.; Gottesfeld, S. Characterization of polymer electrolyte fuel cells using AC impedance spectroscopy. J. Electrochem. Soc. 1996, 143, 587–599. [Google Scholar] [CrossRef]
- Fakher, S.; Nejm, R.; Ayesh, A.; Al-Ghaferi, A.; Zeze, D.; Mabrook, M. Single-walled carbon-nanotubes-based organic memory structures. Molecules 2016, 21, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahir, T.M. Impedance spectroscopy: Theory, experiment, and applications. J. Am. Chem. Soc. 2005, 127, 12431. [Google Scholar] [CrossRef]
- Armstrong, R.; Dickinson, T.; Willis, P. The AC impedance of powdered and sintered solid ionic conductors. J. Electroanal. Chem. Interfacial Electrochem. 1974, 53, 389–405. [Google Scholar] [CrossRef]
- Chamakh, M.; Ayesh, A.I.; Gharaibeh, M.F. Fabrication and characterization of flexible ruthenium oxide-loaded polyaniline/poly (vinyl alcohol) nanofibers. J. Appl. Polym. Sci. 2020, 137, 49125. [Google Scholar] [CrossRef]
- Shaheen, A.; Haija, M.A.; Chamakh, M.; Assayed, G.A.; Banat, F.; Ayesh, A.I. Fabrication and characterization of poly (vinyl alcohol)-Glycerol-Spinel ferrites flexible membranes. J. Appl. Polym. Sci. 2020, 137, 48821. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Salah, B.; Al-Sulaiti, L.A. Production and characterization of flexible semiconducting polymer-nanoparticle composites for x-ray sensors. Radiat. Phys. Chem. 2020, 167, 108233. [Google Scholar] [CrossRef]
- Al-Sulaiti, L.A.; Salah, B.; Ayesh, A.I. Investigation of flexible polymer-Tl2O3 nanocomposites for x-ray detector applications. Appl. Surf. Sci. 2019, 489, 351–357. [Google Scholar] [CrossRef]
- Solano, E.; Frontera, C.; Puig, T.; Obradors, X.; Ricart, S.; Ros, J. Neutron and X-ray diffraction study of ferrite nanocrystals obtained by microwave-assisted growth. A structural comparison with the thermal synthetic route. J. Appl. Cryst. 2014, 47, 414–420. [Google Scholar]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4568–4574. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.-S.; Lee, Y.H.; Musa, A.; Salmah, A.A.; Zamri, I. Use of Fe3O4 nanoparticles for enhancement of biosensor response to the herbicide 2, 4-dichlorophenoxyacetic acid. Sensors 2008, 8, 5775–5791. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, D.; Wei, S.; Wang, Z.; Karki, A.B.; Li, Y.; Bernazzani, P.; Young, D.P.; Gomes, J.; Cocke, D.L. Effects of iron oxide nanoparticles on polyvinyl alcohol: Interfacial layer and bulk nanocomposites thin film. J. Nanoparticle Res. 2010, 12, 2415–2426. [Google Scholar] [CrossRef]
- Kumar, N.R.; Crasta, V.; Praveen, B.; Shreeprakash, B.; Viju, F. Micro structural studies of PVA doped with metal oxide nanocomposites films. Aip Conf. Proc. 2014, 1591, 493–495. [Google Scholar]
- Sabarudin, A.; Wahid, R.; Nalle, F.; Shobirin, R.; Santjojo, D. Designed structure and magnetic characteristic studies of magnetic iron oxide (Fe3O4) nanoparticles coated by polyvinyl alcohol and polyvinyl alcohol-linked with glutaraldehyde. Rasayan J. Chem. 2017, 10, 1261–1270. [Google Scholar]
- Hashim, A.; Habeeb, M.A.; Hadi, A.; Jebur, Q.M.; Hadi, W. Fabrication of novel (PVA-PEG-CMC-Fe3O4) magnetic nanocomposites for piezoelectric applications. Sens. Lett. 2017, 15, 998–1002. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. Part. A 2007, 80, 333–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds in this study are available from the authors. |



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salah, B.; Ayesh, A.I. Fabrication and Characterization of Nanocomposite Flexible Membranes of PVA and Fe3O4. Molecules 2021, 26, 121. https://doi.org/10.3390/molecules26010121
Salah B, Ayesh AI. Fabrication and Characterization of Nanocomposite Flexible Membranes of PVA and Fe3O4. Molecules. 2021; 26(1):121. https://doi.org/10.3390/molecules26010121
Chicago/Turabian StyleSalah, Belal, and Ahmad I. Ayesh. 2021. "Fabrication and Characterization of Nanocomposite Flexible Membranes of PVA and Fe3O4" Molecules 26, no. 1: 121. https://doi.org/10.3390/molecules26010121





