Environmental Risk Assessment Resulting from Sediment Contamination with Perfluoroalkyl Substances
Abstract
:1. Introduction
- -
- determination of 17 PFASs concentration in sediment samples collected in the area of the Gulf of Gdansk;
- -
- assessment of sediment ecotoxicity and investigation of the relation between measured ecotoxicity results and PFASs concentrations;
- -
- environmental risk assessment due to the presence of PFASs in sediments and water samples (estimated values based on partitioning behavior of pollutants between sediment and surface water).
2. Results
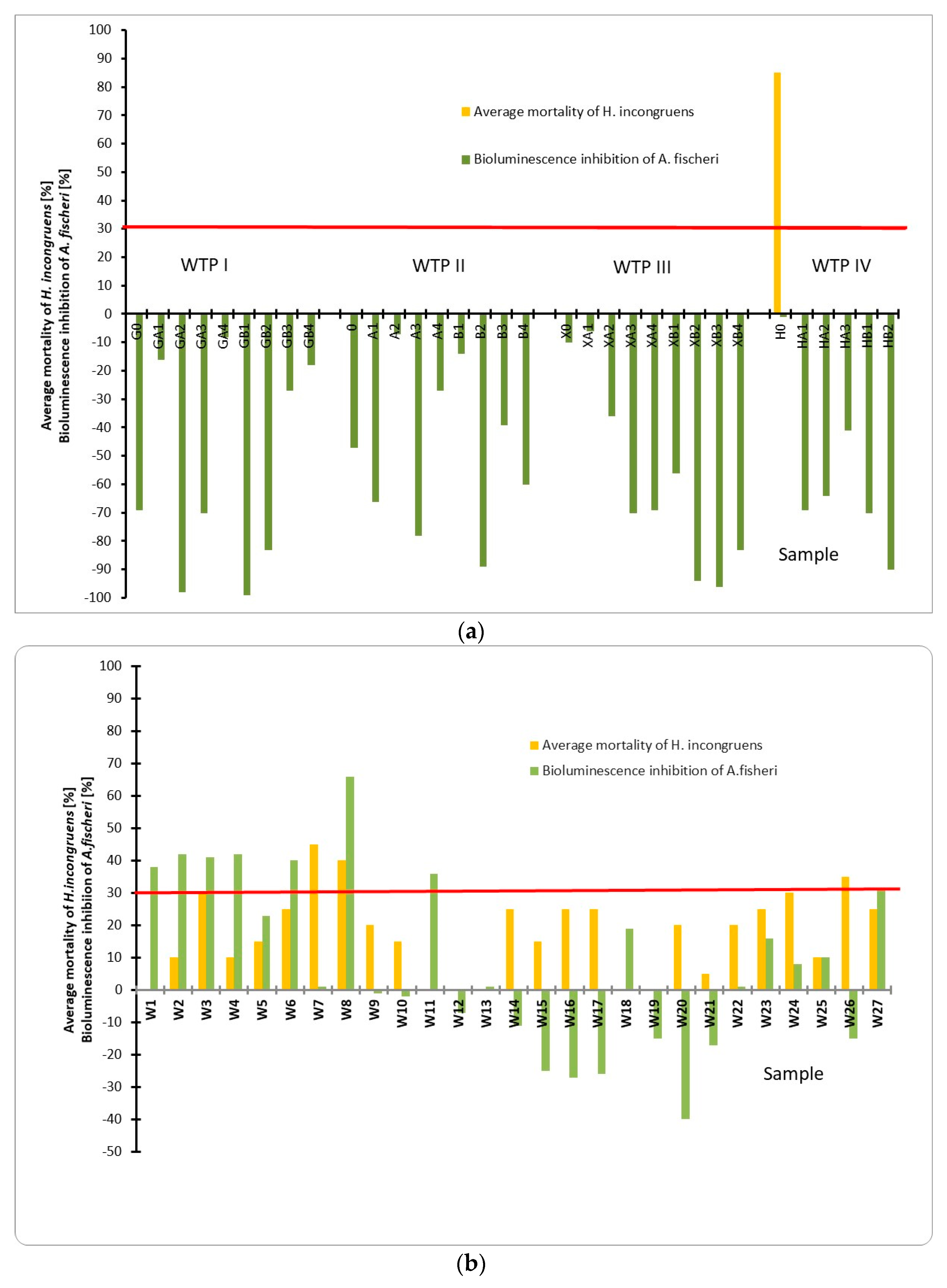
2.1. Toxicity of Sediment Samples
2.2. Determination of PFASs in Sediment Samples
2.3. Environmental Risk Assessment of PFASs
3. Discussion
4. Materials and Methods
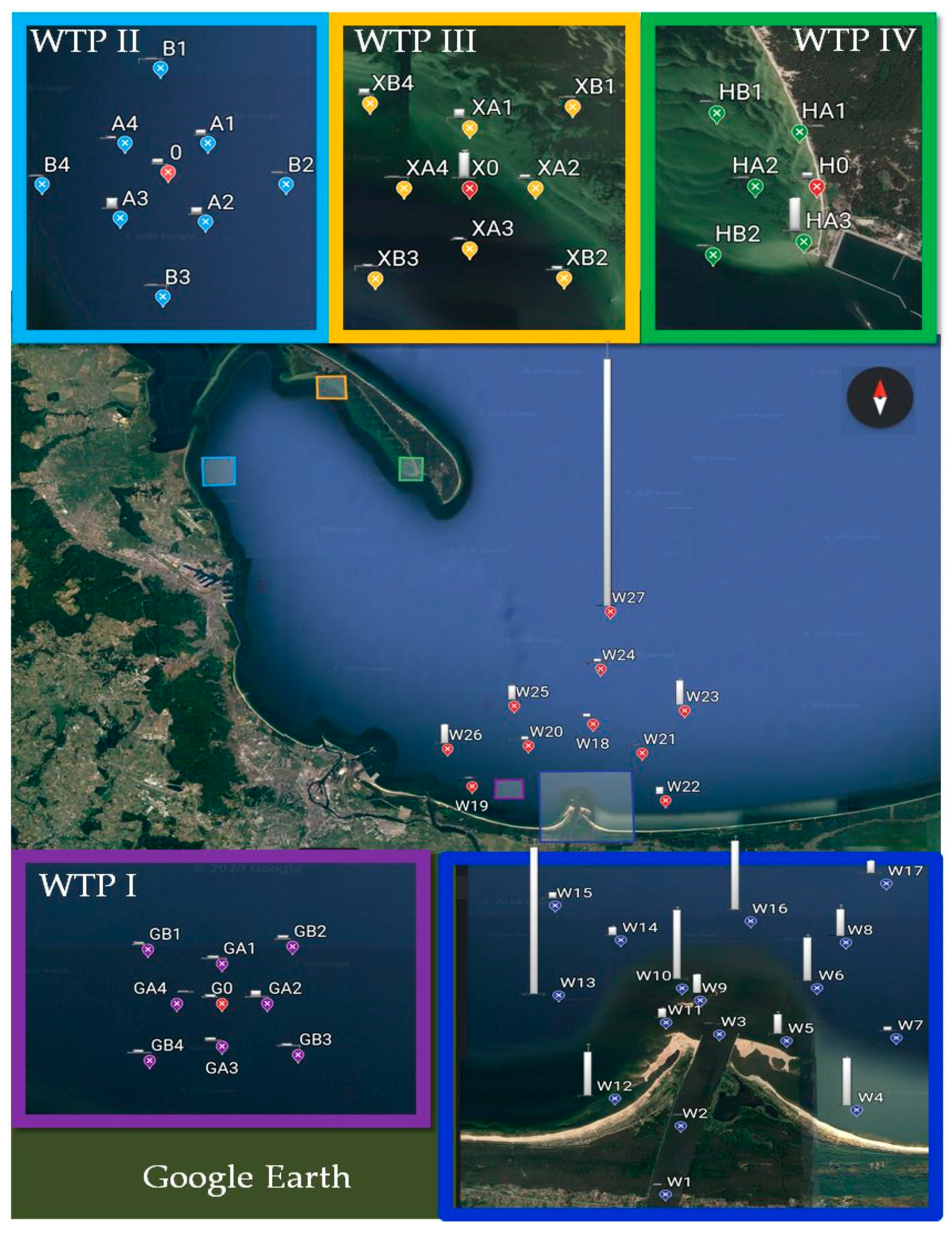
4.1. Research Area Characteristic
4.2. Sample Collection
4.3. Analytical Procedure
4.3.1. Toxicity Test
4.3.2. Chromatographic Analysis
4.3.3. Environmental Risk Assessment
- ○
- 0.01 ≤ RQ < 0.1 -> “low risk”
- ○
- 0.1 ≤ RQ < 1 -> “medium risk”
- ○
- 1 ≤ RQ -> “high risk”
5. Conclusions
- -
- Concentrations of Σ17PFSAs varied between 0.00403 ± 0.0073 and 40.6 ± 2.5 ng/g d.w. for samples taken around the discharge collectors and between 0.509 ± 0.017 and 614 ± 46 ng/g d.w. for samples taken at the Vistula estuary.
- -
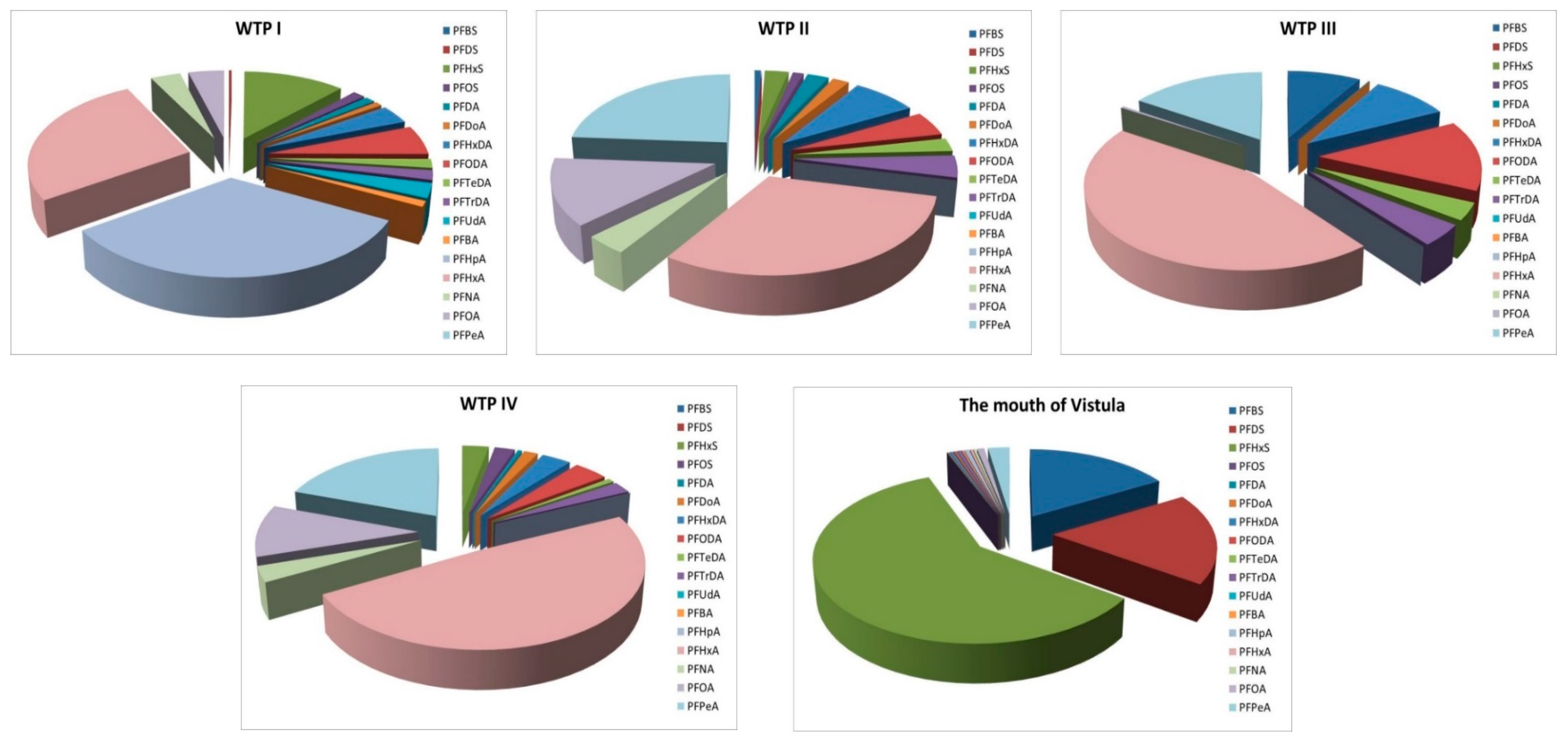
- Percentage composition of the PFASs depended on the sampling location; in the case of samples collected around the wastewater treatment plant outlets, the percentage composition is similar, while it differs for samples collected at the mouth of the Vistula.
- -
- In the case of samples taken around the discharge collectors, where long-chain PFASs were expected, the largest percentage composition was observed for PFHxA (C6), PFPeA (C5), PFHpA (C7), and PFOA (C8); those compounds can be emitted with treated wastewater.
- -
- PFHxS, PFDS, and PFBS dominated in the case of samples collected at the mouth of the Vistula, which is consistent with the studies of other authors on river sediments.
- -
- No toxic effect was observed on the indicator organisms for most of the samples. In several samples, a significant increase in the luminescence of the Aliivibrio fischeri bacteria was observed, which may indicate the presence of the phenomenon of hormesis.
- -
- No relationship was observed between ecotoxicity and the content of compounds from the PFASs group.
- -
- The environmental risks resulting from all PFASs seemed to be negligible, indicating low or medium (six sampling points of Vistula’s aquatic phase in the River) risk from PFASs to aquatic organisms.
- -
- For all sediment samples, RQ values were lower than 0.01.
- -
- However, it is necessary to evaluate the environmental risk using new toxicity data if they become available or if accessibility of long term analytical data on pollutants concentration in the environment will increase.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Boisvert, G.; Sonne, C.; Rigét, F.F.; Dietz, R.; Letcher, R.J. Bioaccumulation and biomagnification of perfluoroalkyl acids and precursors in East Greenland polar bears and their ringed seal prey. Environ. Pollut. 2019, 252, 1335–1343. [Google Scholar] [CrossRef]
- Gallen, C.; Eaglesham, G.; Drage, D.; Nguyen, T.H.; Mueller, J. A mass estimate of perfluoroalkyl substance (PFAS) release from Australian wastewater treatment plants. Chemosphere 2018, 208, 975–983. [Google Scholar] [CrossRef]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- Shen, A.; Lee, S.; Ra, K.; Suk, D.; Moon, H.-B. Historical trends of perfluoroalkyl substances (PFASs) in dated sediments from semi-enclosed bays of Korea. Mar. Pollut. Bull. 2018, 128, 287–294. [Google Scholar] [CrossRef]
- UNEP. SC-4/17: Listing of Perfluorooctane Sulfonic Acid, Its Salts and Perfluorooctane Sulfonyl Fluoride; United Nations Environment Programme, UNEP: Nairobi, Kenya, 2019. [Google Scholar]
- EFSA. Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Zhang, C.; Yan, H.; Li, F.; Zhou, Q. Occurrence and fate of perfluorinated acids in two wastewater treatment plants in Shanghai, China. Environ. Sci. Pollut. Res. 2015, 22, 1804–1811. [Google Scholar] [CrossRef]
- Kibambe, M.G.; Momba, M.; Daso, A.; Coetzee, M.A. Evaluation of the efficiency of selected wastewater treatment processes in removing selected perfluoroalkyl substances (PFASs). J. Environ. Manag. 2020, 255, 109945. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cho, Y.-J.; Lee, M.; Lee, B.-D. Detection and Treatment Methods for Perfluorinated Compounds in Wastewater Treatment Plants. Appl. Sci. 2019, 9, 2500. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, H.; Deng, W. Perfluorooctane sulfonate (PFOS) distribution and effect factors in the water and sediment of the Yellow River Estuary, China. Environ. Monit. Assess. 2013, 185, 8517–8524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xie, Z.; Tang, J.; Zhang, G.; Ebinghaus, R. Spatial distribution of perfluoroalkyl acids in surface sediments of the German Bight, North Sea. Sci. Total. Environ. 2015, 511, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; You, C. Sediment–water distribution of perfluorooctane sulfonate (PFOS) in Yangtze River Estuary. Environ. Pollut. 2010, 158, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Huo, S.; Xi, B.; Hu, S.; Zhang, J.; He, Z. Spatial distribution and source apportionment of PFASs in surface sediments from five lake regions, China. Sci. Rep. 2016, 6, 22674. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, C.; Yu, Y.; Han, J. Sorption of perfluorooctane sulfonate (PFOS) on marine sediments. Mar. Pollut. Bull. 2012, 64, 902–906. [Google Scholar] [CrossRef]
- He, X.; Li, A.; Wangae, S.; Chen, H.; Yang, Z. Perfluorinated substance assessment in sediments of a large-scale reservoir in Danjiangkou, China. Environ. Monit. Assess. 2018, 190, 66. [Google Scholar] [CrossRef]
- Falandysz, J.; Rostkowski, P.; Jarzyńska, G.; Falandysz, J.J.; Taniyasu, S.; Yamashita, N. Determination of perfluorinated alkylated substances in sediments and sediment core from the Gulf of Gdańsk, Baltic Sea. J. Environ. Sci. Health Part A 2012, 47, 428–434. [Google Scholar] [CrossRef]
- Joerss, H.; Apel, C.; Ebinghaus, R. Emerging per- and polyfluoroalkyl substances (PFASs) in surface water and sediment of the North and Baltic Seas. Sci. Total. Environ. 2019, 686, 360–369. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, C.; Zhou, Q.; Yang, S. Occurrence of perfluorinated alkyl substances in sediment from estuarine and coastal areas of the East China Sea. Environ. Sci. Pollut. Res. 2014, 22, 1662–1669. [Google Scholar] [CrossRef]
- Wang, S.; Ma, L.; Chen, C.; Li, Y.; Wu, Y.; Liu, Y.; Dou, Z.; Yamazaki, E.; Yamashita, N.; Lin, B.-L.; et al. Occurrence and partitioning behavior of per- and polyfluoroalkyl substances (PFASs) in water and sediment from the Jiulong Estuary-Xiamen Bay, China. Chemosphere 2020, 238, 124578. [Google Scholar] [CrossRef]
- Gebbink, W.A.; Bignert, A.; Berger, U. Perfluoroalkyl Acids (PFAAs) and Selected Precursors in the Baltic Sea Environment: Do Precursors Play a Role in Food Web Accumulation of PFAAs? Environ. Sci. Technol. 2016, 50, 6354–6362. [Google Scholar] [CrossRef]
- Asaoka, S.; Yoshiki, R.; Haga, Y.; Matsumura, C.; Umehara, A.; Takeda, K. Spatial Distribution of Perfluorinated Organic Compounds in Surface Marine Sediments from the Seto Inland Sea, Japan. J. Water Environ. Technol. 2020, 18, 226–237. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, J.-J.; Rodenburg, L.A.; Cai, M.; Wu, Z.; Ke, H.; Chitsaz, M. Perfluoroalkyl substances in sediments from the Bering Sea to the western Arctic: Source and pathway analysis. Environ. Int. 2020, 139, 105699. [Google Scholar] [CrossRef]
- Bai, X.; Son, Y. Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci. Total. Environ. 2021, 751, 141622. [Google Scholar] [CrossRef] [PubMed]
- Kahkashan, S.; Wang, X.; Chen, J.; Bai, Y.; Ya, M.; Wu, Y.; Cai, Y.; Wang, S.; Saleem, M.; Aftab, J.; et al. Concentration, distribution and sources of perfluoroalkyl substances and organochlorine pesticides in surface sediments of the northern Bering Sea, Chukchi Sea and adjacent Arctic Ocean. Chemosphere 2019, 235, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Im, J.-K.; Kang, Y.-M.; Jung, S.-Y.; Kho, Y.L.; Zoh, K.-D. Wastewater treatment plants (WWTPs)-derived national discharge loads of perfluorinated compounds (PFCs). J. Hazard. Mater. 2012, 201, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.; Berger, U.; McLachlan, M.S. Mass Balance of Perfluoroalkyl Acids in the Baltic Sea. Environ. Sci. Technol. 2013, 47, 4088–4095. [Google Scholar] [CrossRef]
- Ritter, S.K. FLUOROCHEMICALS GO SHORT. Chem. Eng. News Arch. 2010, 88, 12–17. [Google Scholar] [CrossRef]
- Wu, J.; Junaid, M.; Wang, Z.; Sun, W.; Xu, N. Spatiotemporal distribution, sources and ecological risks of perfluorinated compounds (PFCs) in the Guanlan River from the rapidly urbanizing areas of Shenzhen, China. Chemosphere 2020, 245, 125637. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef]
- Buszewski, B.; Buszewska, T.; Chmarzyński, A.; Kowalkowski, T.; Kowalska, J.; Kosobucki, P.; Zbytniewski, R.; Namieśnik, J.; Kot–Wasik, A.; Pacyna, J.; et al. The present condition of the Vistula river catchment area and its impact on the Baltic Sea coastal zone. Reg. Environ. Chang. 2005, 5, 97–110. [Google Scholar] [CrossRef]
- Becanova, J.; Melymuk, L.; Vojta, Š.; Komprdová, K.; Klánová, K. Screening for perfluoroalkyl acids in consumer products, building materials and wastes. Chemosphere 2016, 164, 322–329. [Google Scholar] [CrossRef]
- Lam, N.H.; Cho, C.-R.; Kannan, K.; Cho, H.-S. A nationwide survey of perfluorinated alkyl substances in waters, sediment and biota collected from aquatic environment in Vietnam: Distributions and bioconcentration profiles. J. Hazard. Mater. 2017, 323, 116–127. [Google Scholar] [CrossRef]
- Xiao, F.; Halbach, T.R.; Simcik, M.F.; Gulliver, J.S. Input characterization of perfluoroalkyl substances in wastewater treatment plants: Source discrimination by exploratory data analysis. Water Res. 2012, 46, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- NGI. PFBS in the Environment: Monitoring and Physical-Chemical Data Related to the Environmental Distribution of Perfluorobutanesulfonic Acid; Norwegian Environmental Agency Report M-1122; Norwegian Geotechnical Institute: Oslo, Norway, October 2018. [Google Scholar]
- Norwegian Environment Agency. Investigation of Sources to PFBSs in the Environment, Norwegian Environment; Agency Report M-759; COWI: Lyngby, Denmark, 2017. [Google Scholar]
- Filipovic, M.; Laudon, H.; McLachlan, M.S.; Berger, U. Mass Balance of Perfluorinated Alkyl Acids in a Pristine Boreal Catchment. Environ. Sci. Technol. 2015, 49, 12127–12135. [Google Scholar] [CrossRef] [PubMed]
- Kruk-Dowgiałło, L. (Ed.) Summary Report of the Analysis of Available Data and Conducted a Natural inventory—Vistula Estuary; PLB 220004; Maritime Institute: Gdansk, Poland, 2013. [Google Scholar]
- Matciak, M.; Nowacki, J. The Vistula river discharge front—surface observations. Oceanologia 1995, 37, 75–88. [Google Scholar]
- You, C.; Jia, C.; Pan, G. Effect of salinity and sediment characteristics on the sorption and desorption of perfluorooctane sulfonate at sediment-water interface. Environ. Pollut. 2010, 158, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ren, G.; Ma, X.; Ding, Y.; Hui, Y.; Qin, P.; Xu, Z.; Gu, X.; Yuan, F.; Liu, Y.; et al. Perfluoroalkyl substances in the Lingang hybrid constructed wetland, Tianjin, China: Occurrence, distribution characteristics, and ecological risks. Environ. Sci. Pollut. Res. 2020, 27, 38580–38590. [Google Scholar] [CrossRef]
- Valsecchi, S.; Conti, D.; Crebelli, R.; Polesello, S.; Rusconi, M.; Mazzoni, M.; Preziosi, E.; Carere, M.; Lucentini, L.; Ferretti, E.; et al. Deriving environmental quality standards for perfluorooctanoic acid (PFOA) and related short chain perfluorinated alkyl acids. J. Hazard. Mater. 2017, 323, 84–98. [Google Scholar] [CrossRef]
- Ankley, G.T.; Cureton, P.; Houde, R. Assessing the Ecological Risks of Per- and Polyfluoroalkyl Substances: Current State-of-the Science and a Proposed Path Forward. Environ. Toxicol. Chem. 2020. [Google Scholar] [CrossRef]
- European Commission. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union L 2013, 226, 1–17. [Google Scholar]
- Zodrow, J.; Frenchmeyer, M.; Dally, K.; Osborn, E.; Anderson, P.; Divine, C. Per- and Polyfluoroalkyl Substances Ecological Risk-Based Screening Levels. SERDP Contract Report ER18-1653. 2020. Available online: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/ER18-1653 (accessed on 28 October 2020).
- Conder, J.; Arblaster, J.; Larson, E.; Brown, J.; Higgins, C. Guidance for Assessing the Ecological Risks of PFASs to Threatened and Endangered Species at Aqueous Film Forming Foam-Impacted Sites. SERDP Contract Report ER18-1614. 2020. Available online: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/ER18-1614 (accessed on 28 October 2020).
- Norwegian Pollution Control Authority. Screening of polyfluorinted compounds at four fire training facilities in Norway. TA‐2444/2008. 2008. Available online: https://www.miljodirektoratet.no/globalassets/publikasjoner/klif2/publikasjoner/2444/ta2444.pdf (accessed on 28 October 2020).
- Kudłak, B.; Rogowska, J.; Wolska, L.; Kałas, M.; Łęczyński, L.; Namieśnik, J. Toxicity assessment of sediments associated with the wreck of s/s Stuttgart in the Gulf of Gdańsk (Poland). J. Environ. Monit. 2012, 14, 1230–1235. [Google Scholar] [CrossRef]
- ISO. 11348:2—Water Quality—Determination of The Inhibitory Effect of Water Samples on the Light Emission of V. Fischeri (Luminescent Bacteria Test)—Part 2: Method Using Liquid-Dried Bacteria; ISO: London, UK, 2002. [Google Scholar]
- Cieszynska-Semenowicz, M.; Rogowska, J.; Ratajczyk, W.; Ratajczyk, J.; Wolska, L. Toxicity studies of elemental sulfur in marine sediments. Int. J. Sediment Res. 2018, 33, 191–197. [Google Scholar] [CrossRef]
- ISO. 14371—Water Quality—Determination of Freshwater Sediment Toxicity to Heterocypris Incongruens (Crustacea, Ostracoda); ISO: London, UK, 2012. [Google Scholar]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Gałęzowska, G.; Rogowska, J.; Olkowska, E.; Wolska, L. Determination of 17 Perfluoroalkyl Substances in Sediments Using Automated Solid Phase Extraction and Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry. Chromatographia 2020, 83, 975–983. [Google Scholar] [CrossRef]
- U.S. EPA. Technical Overview of Ecological Risk Assessment—Analysis Phase: Ecological Effects Characterization. Available online: https://www.epa.gov/risk (accessed on 28 October 2020).
- Liu, J.; Lu, G.; Yang, H.; Dang, T.; Yan, Z. Ecological impact assessment of 110 micropollutants in the Yarlung Tsangpo River on the Tibetan Plateau. J. Environ. Manag. 2020, 262, 110291. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health and Consumer Protection. European Commission Technical Guidance Document in Support of Commission Directive 93//67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substance, Part II. EUR 20418 EN/2; European Commission Joint Research Centre: Ispra, Italy, 2003; pp. 100–118. [Google Scholar]
- Van Vlaardingen, P.L.A.; Verbruggen, E.M.J. Guidance for the Derivation of Environmental Risk Limits within the Framework of International and National Environmental Quality Standards Netherlands (INS) Revision; National Institute for Public Health and the Environment: Utrecht, The Netherlands, 2007. [Google Scholar]
- Zhu, Z.; Wang, T.; Wang, P.; Lu, Y.; Giesy, J.P. Perfluoroalkyl and polyfluoroalkyl substances in sediments from South Bohai coastal watersheds, China. Mar. Pollut. Bull. 2014, 85, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Zhang, B.-B.; Zhao, Y.-G.; Wu, Q. Occurrence, spatiotemporal distribution, and ecological risks of steroids in a large shallow Chinese lake, Lake Taihu. Sci. Total. Environ. 2016, 68–79. [Google Scholar] [CrossRef] [PubMed]
- EPI Suite™—Estimation Program Interface—2000–2019 U.S. Environmental Protection Agency for EPI Suite™ and All Component Programs Except for BioHCWIN and KOAWIN. Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 28 October 2020).
- Hoke, R.A.; Bouchelle, L.D.; Ferrell, B.D.; Buck, R.C. Comparative acute freshwater hazard assessment and preliminary PNEC development for eight fluorinated acids. Chemosphere 2012, 87, 725–733. [Google Scholar] [CrossRef]
- Guo, R.; Liu, X.; Liu, J.; Liu, Y.; Qiao, X.; Ma, M.; Zheng, B.; Zhao, X. Occurrence, partition and environmental risk assessment of per- and polyfluoroalkyl substances in water and sediment from the Baiyangdian Lake. China Sci. Rep. 2020, 10, 4691. [Google Scholar] [CrossRef]
- Navarro, I.; de la Torre, A.; Sanz, P.; Martinez, M.D. Perfluoroalkyl acids (PFAAs): Distribution, trends and aquatic ecological risk assessment in surface water from Tagus River basin (Spain). Environ. Pollut. 2020, 256, 113511. [Google Scholar] [CrossRef]
- Analysis of the Risks Arising from the Industrial Use of Perfuorooctanoic Acid (PFOA) and Ammonium Perfluorooctanoate (APFO) and from Their Use in Consumer Articles. Evaluation of the Risk Reduction Measures for Potential Restrictions on the Manufacture, Placing on the Market and Use of PFOA and APFO; FINAL REPORT (20.12.2008–20.10.2009); RPS Advies B.V.: Delft, The Netherlands, 2010.
- Mhadhbi, L.; Rial, D.; Perez, S.; Beiras, R. Ecological risk assessment of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in marine environment using Isochrysis galbana, Paracentrotus lividus, Siriella armata and Psetta maxima. J. Environ. Monit. 2012, 14, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Technical Report, No. 92—Soil and Sediment Risk Assessment of Organic Chemicals; European Centre for Ecotoxicology and Toxicology of Chemicals: Brussels, Belgium, 2004.
- Von der Trenck, K.T.; Konietzka, R.; Biegel-Engler, A.; Brodsky, J.; Hädicke, A.; Quadflieg, A.; Stockerl, R.; Stahl, T. Significance thresholds for the assessment of contaminated groundwater: Perfluorinated and polyfluorinated chemicals. Environ. Sci. Eur. 2018, 30, 19. [Google Scholar] [CrossRef]
- Qi, P.; Wang, Y.; Mu, J.L.; Wang, J. Aquatic predicted no-effect concentration derivation for perfluorooctane sulfonic acid. Environ. Toxicol. Chem. 2011, 30, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Hernando, M.D.; Mezcua, M.; Alba, A.R.F.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]


| WTP I—WTP IV | PFBA | PFPeA | PFHxA | PFHpA | PFOA | PFNA | PFDA | PFUdA | PFDoA | PFTrDA | PFTeDA | PFHxDA | PFODA | PFBS | PFDS | PFHxS | PFOS |
| PFPeA | −0.095 | ||||||||||||||||
| PFHxA | −0.084 | 0.887 | |||||||||||||||
| PFHpA | 0.011 | −0.130 | −0.094 | ||||||||||||||
| PFOA | −0.102 | 0.319 | 0.086 | −0.027 | |||||||||||||
| PFNA | 0.124 | 0.717 | 0.595 | −0.080 | 0.465 | ||||||||||||
| PFDA | 0.082 | 0.412 | 0.083 | −0.074 | 0.551 | 0.540 | |||||||||||
| PFUdA | 0.311 | −0.152 | −0.091 | 0.107 | −0.100 | 0.050 | 0.028 | ||||||||||
| PFDoA | −0.037 | 0.744 | 0.614 | −0.090 | 0.450 | 0.952 | 0.573 | −0.066 | |||||||||
| PFTrDA | −0.073 | 0.713 | 0.658 | −0.113 | 0.285 | 0.407 | 0.388 | −0.159 | 0.449 | ||||||||
| PFTeDA | −0.065 | 0.712 | 0.739 | −0.003 | 0.105 | 0.198 | 0.263 | −0.101 | 0.226 | 0.696 | |||||||
| PFHxDA | −0.100 | 0.639 | 0.724 | −0.088 | 0.141 | 0.323 | 0.162 | −0.189 | 0.343 | 0.870 | 0.774 | ||||||
| PFODA | −0.032 | 0.149 | 0.164 | −0.189 | 0.168 | 0.069 | 0.062 | −0.118 | 0.082 | 0.337 | 0.182 | 0.474 | |||||
| PFBS | −0.072 | −0.097 | −0.108 | −0.097 | −0.109 | −0.101 | −0.082 | −0.119 | −0.086 | 0.417 | −0.117 | 0.275 | 0.500 | ||||
| PFDS | −0.045 | −0.066 | −0.068 | 0.873 | 0.034 | −0.066 | −0.053 | −0.071 | −0.059 | −0.019 | 0.068 | 0.032 | −0.117 | −0.050 | |||
| PFHxS | −0.081 | 0.381 | 0.296 | 0.675 | 0.297 | 0.483 | 0.363 | −0.089 | 0.524 | 0.286 | 0.216 | 0.238 | −0.037 | −0.090 | 0.798 | ||
| PFOS | 0.029 | 0.724 | 0.649 | −0.074 | 0.370 | 0.923 | 0.538 | 0.049 | 0.959 | 0.473 | 0.242 | 0.373 | 0.114 | −0.078 | −0.049 | 0.534 | |
| ΣPFASs | −0.091 | 0.942 | 0.949 | −0.009 | 0.295 | 0.698 | 0.297 | −0.121 | 0.721 | 0.778 | 0.745 | 0.779 | 0.279 | 0.006 | 0.045 | 0.462 | 0.736 |
| VISTULA | PFBA | PFPeA | PFHxA | PFHpA | PFOA | PFNA | PFDA | PFUdA | PFDoA | PFTrDA | PFTeDA | PFHxDA | PFODA | PFBS | PFDS | PFHxS | PFOS |
| PFPeA | −0.182 | ||||||||||||||||
| PFHxA | −0.123 | −0.047 | |||||||||||||||
| PFHpA | −0.069 | −0.131 | 0.933 | ||||||||||||||
| PFOA | −0.066 | −0.149 | 0.953 | 0.991 | |||||||||||||
| PFNA | −0.090 | −0.153 | 0.946 | 0.984 | 0.991 | ||||||||||||
| PFDA | −0.142 | 0.051 | −0.106 | −0.191 | −0.183 | −0.135 | |||||||||||
| PFUdA | −0.083 | −0.208 | 0.065 | −0.022 | 0.000 | 0.038 | 0.433 | ||||||||||
| PFDoA | −0.069 | −0.180 | 0.870 | 0.835 | 0.854 | 0.855 | 0.140 | 0.082 | |||||||||
| PFTrDA | −0.084 | −0.226 | 0.627 | 0.518 | 0.543 | 0.585 | −0.032 | 0.389 | 0.548 | ||||||||
| PFTeDA | −0.102 | −0.115 | 0.808 | 0.733 | 0.755 | 0.762 | 0.188 | 0.137 | 0.967 | 0.533 | |||||||
| PFHxDA | −0.105 | −0.041 | 0.845 | 0.761 | 0.784 | 0.789 | 0.198 | 0.196 | 0.947 | 0.490 | 0.974 | ||||||
| PFODA | −0.078 | 0.252 | 0.119 | −0.094 | −0.065 | −0.046 | 0.590 | 0.374 | 0.304 | 0.032 | 0.479 | 0.533 | |||||
| PFBS | 0.731 | −0.272 | −0.123 | −0.079 | −0.077 | −0.091 | 0.048 | 0.354 | −0.086 | −0.014 | −0.071 | −0.066 | 0.065 | ||||
| PFDS | −0.087 | −0.105 | 0.037 | −0.045 | −0.039 | −0.019 | 0.549 | 0.512 | 0.383 | 0.057 | 0.512 | 0.499 | 0.741 | 0.129 | |||
| PFHxS | −0.108 | 0.223 | 0.100 | −0.050 | −0.030 | −0.029 | 0.388 | 0.619 | −0.029 | −0.067 | 0.036 | 0.189 | 0.576 | 0.189 | 0.301 | ||
| PFOS | −0.058 | 0.205 | −0.102 | 0.048 | −0.004 | 0.080 | 0.231 | −0.065 | −0.026 | −0.062 | 0.021 | 0.022 | 0.049 | −0.118 | 0.019 | −0.081 | |
| ΣPFASs | 0.029 | 0.109 | 0.115 | −0.023 | −0.004 | 0.000 | 0.476 | 0.718 | 0.110 | −0.013 | 0.200 | 0.324 | 0.699 | 0.381 | 0.554 | 0.939 | −0.079 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałęzowska, G.; Rogowska, J.; Olkowska, E.; Ratajczyk, W.; Wolska, L. Environmental Risk Assessment Resulting from Sediment Contamination with Perfluoroalkyl Substances. Molecules 2021, 26, 116. https://doi.org/10.3390/molecules26010116
Gałęzowska G, Rogowska J, Olkowska E, Ratajczyk W, Wolska L. Environmental Risk Assessment Resulting from Sediment Contamination with Perfluoroalkyl Substances. Molecules. 2021; 26(1):116. https://doi.org/10.3390/molecules26010116
Chicago/Turabian StyleGałęzowska, Grażyna, Justyna Rogowska, Ewa Olkowska, Wojciech Ratajczyk, and Lidia Wolska. 2021. "Environmental Risk Assessment Resulting from Sediment Contamination with Perfluoroalkyl Substances" Molecules 26, no. 1: 116. https://doi.org/10.3390/molecules26010116






