New Bioactive Peptides Identified from a Tilapia Byproduct Hydrolysate Exerting Effects on DPP-IV Activity and Intestinal Hormones Regulation after Canine Gastrointestinal Simulated Digestion
Abstract
:1. Introduction
2. Results
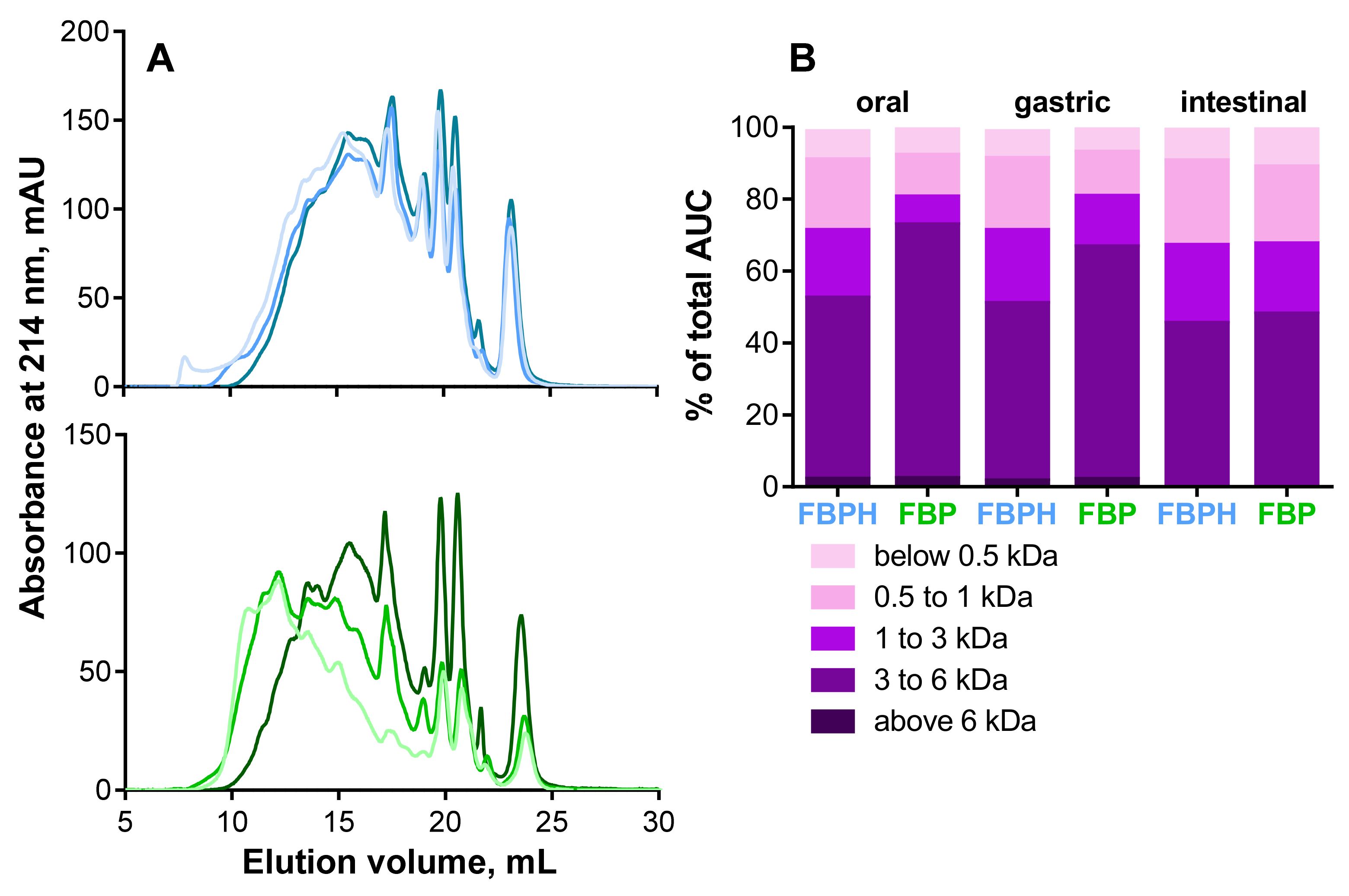
2.1. Peptide Profile Modifications during SGID
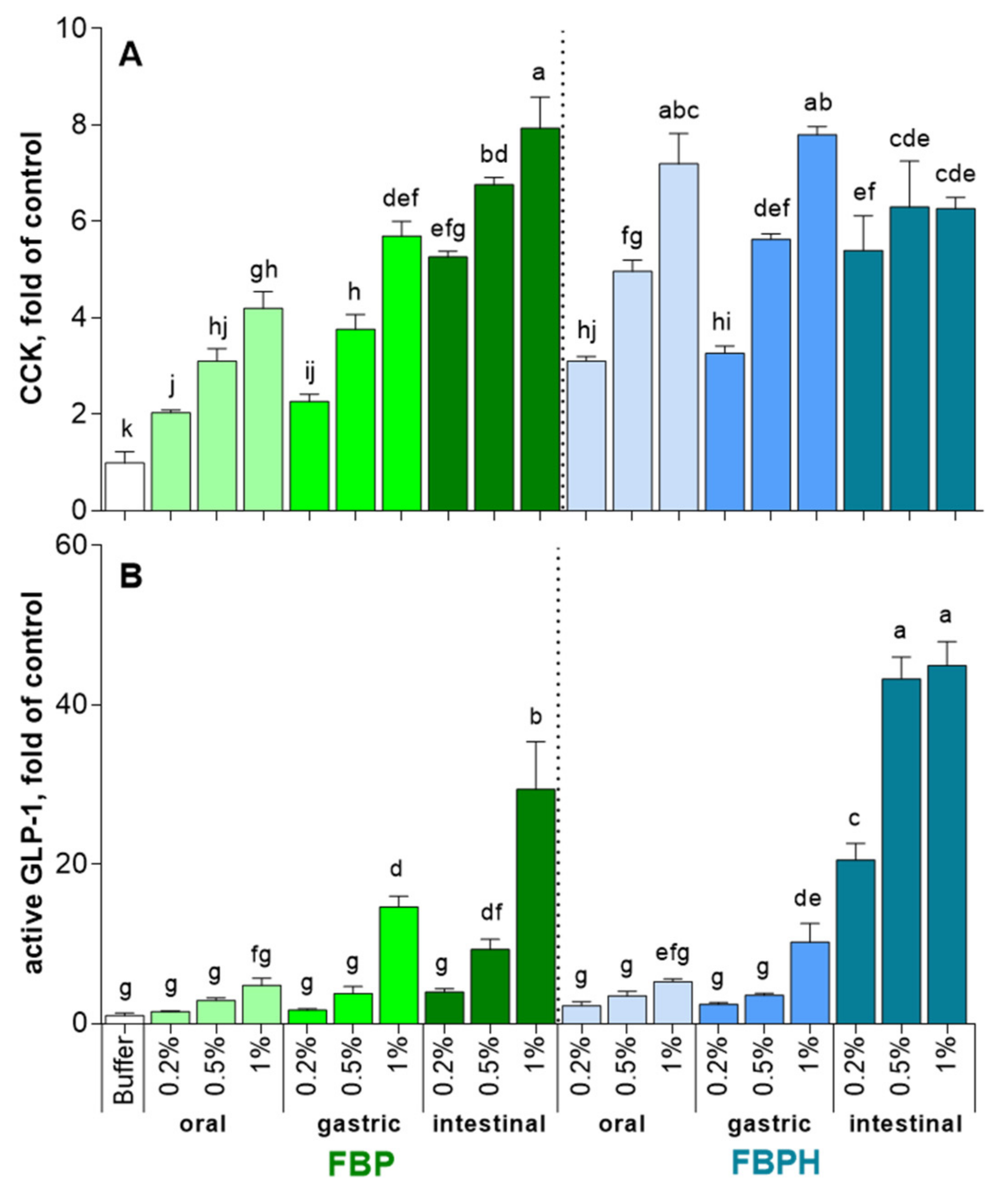
2.2. CCK and GLP-1 Secretion Induced by FBPH and FBP Digests
2.3. Intestinal DPP-IV Inhibition Activity of FBPH and FBP Digests
2.4. CCK and GLP-1 Secretion-Stimulating Peptides Identification
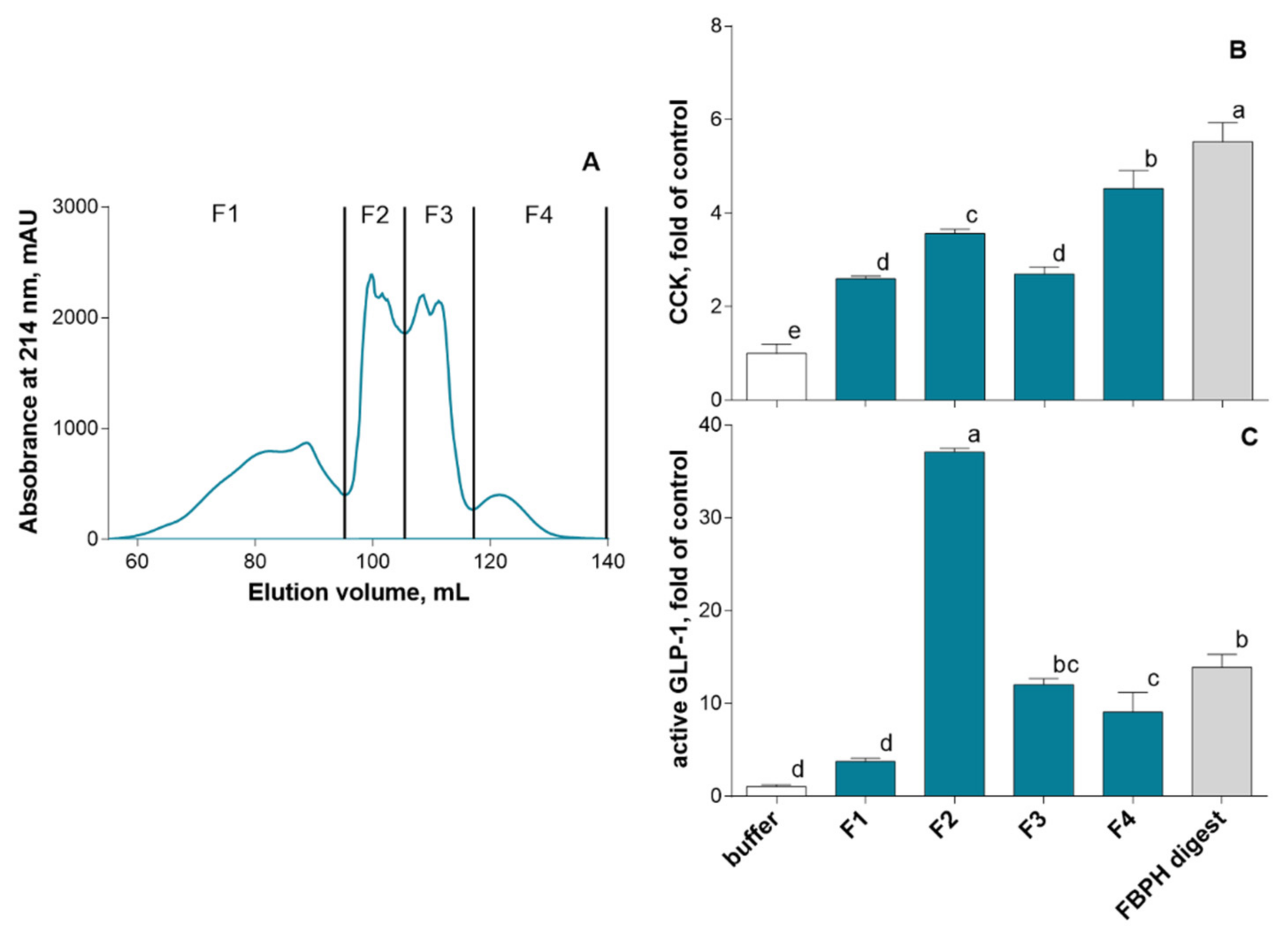
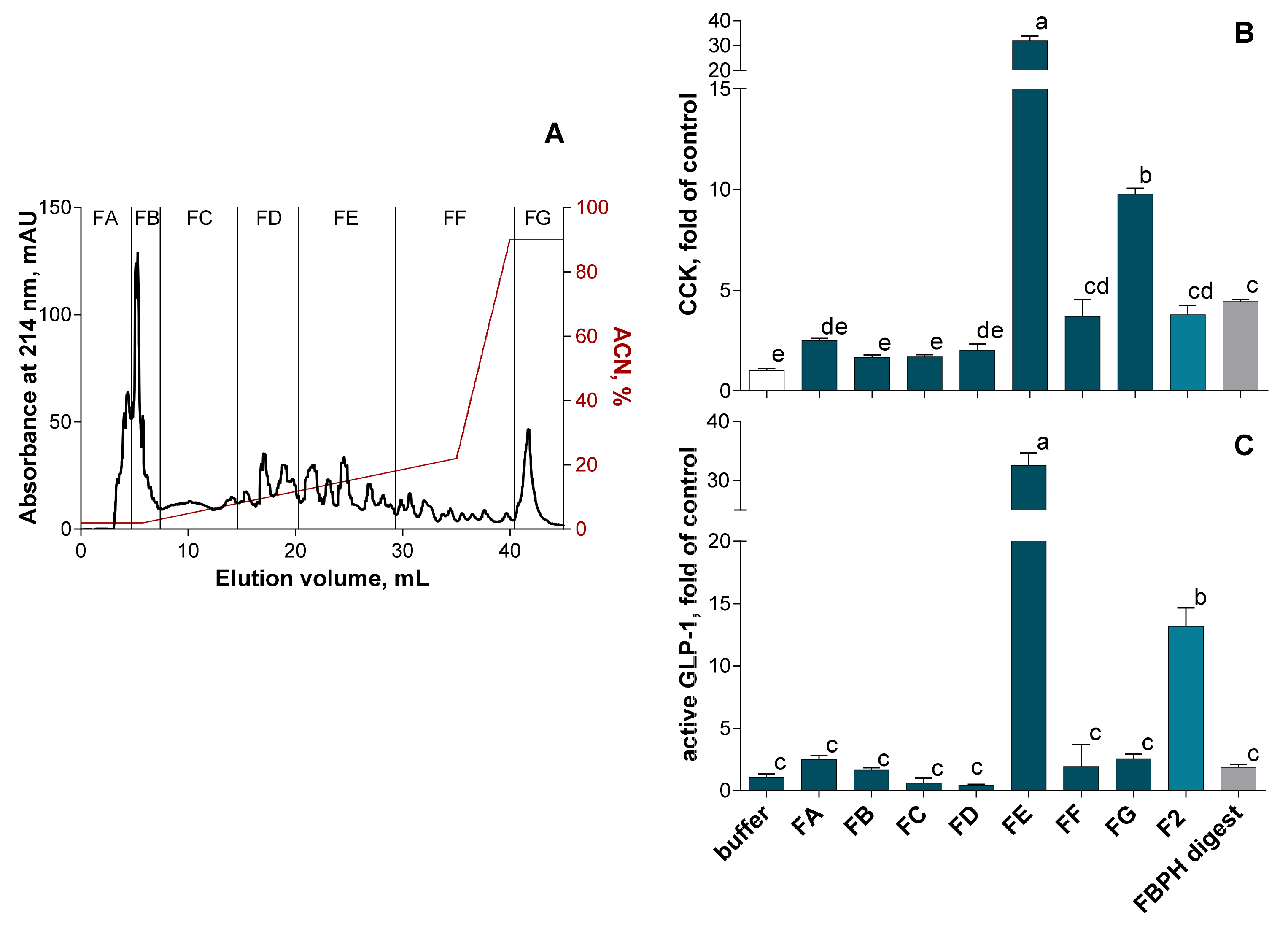
2.4.1. SEC and RP-HPLC Fractionation of FBPH Intestinal Digest
2.4.2. RP-HPLC-MS/MS Peptides Identification in the FE Subfraction
2.4.3. CCK and GLP-1 Secretion Stimulation Induced by Synthetic Peptides from FBPH Intestinal Digest
2.5. Identification of Peptides in the Basolateral Side of the Intestinal Barrier Able to Inhibit In Vitro and In Situ the DPP-IV Activity
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. In Vitro Simulated Canine Gastrointestinal Digestion of FBP and FBPH
4.3. Size Exclusion Chromatography by Fast Protein Liquid Chromatography (SEC-FPLC)
4.4. Cell Culture Conditions
4.5. CCK and GLP-1 Secretion Study
4.6. DPP-IV Activity Assay
4.7. Fractionation of the FBPH Intestinal Digest
4.7.1. SEC-FPLC Fractionation
4.7.2. HPLC Fractionation
4.8. Peptide Sequences Identification in HPLC Fractions
4.8.1. RP-HPLC-MS/MS Analysis of HPLC Fractions
4.8.2. Mass Spectrometry Data Processing
4.9. Intestinal Barrier Passage and Peptide Identification
4.9.1. Transport Study
4.9.2. Peptide Sequences Identification in Apical and Basolateral Supernatant by Mass Spectrometry
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- United Nations; Department of Economic and Social Affairs; Population Division. World Population Prospects Highlights; UN: New York, NY, USA, 2019; ISBN 978-92-1-148316-1. [Google Scholar]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action. In The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandler, M.; Cunningham, S.; Lund, E.M.; Khanna, C.; Naramore, R.; Patel, A.; Day, M.J. Obesity and Associated Comorbidities in People and Companion Animals: A One Health Perspective. J. Comp. Pathol. 2017, 156, 296–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhlund, M.; Palmgren, M.; Holst, B.S. Overweight in Adult Cats: A Cross-Sectional Study. Acta Vet. Scand. 2018, 60, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, S.; Depoortere, I. Nutrient Sensing in the Gut: New Roads to Therapeutics? Trends Endocrinol. Metab. 2013, 24, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, I.; Akiba, Y.; Kaji, I.; Kaunitz, J.D. Duodenal Luminal Nutrient Sensing. Curr. Opin. Pharmacol. 2014, 19, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Caron, J.; Domenger, D.; Dhulster, P.; Ravallec, R.; Cudennec, B. Protein Digestion-Derived Peptides and the Peripheral Regulation of Food Intake. Front. Endocrinol. 2017, 8. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin Hormones: Their Role in Health and Disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef]
- Engel, M.; Hoffmann, T.; Manhart, S.; Heiser, U.; Chambre, S.; Huber, R.; Demuth, H.-U.; Bode, W. Rigidity and Flexibility of Dipeptidyl Peptidase IV: Crystal Structures of and Docking Experiments with DPIV. J. Mol. Biol. 2006, 355, 768–783. [Google Scholar] [CrossRef]
- Godinho, R.; Mega, C.; Teixeira-de-Lemos, E.; Carvalho, E.; Teixeira, F.; Fernandes, R.; Reis, F. The Place of Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes Therapeutics: A “Me Too” or “the Special One” Antidiabetic Class? J. Diabetes Res. 2015, 2015, 28. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Prospects for the Management of Type 2 Diabetes Using Food Protein-Derived Peptides with Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Activity. Curr. Opin. Food Sci. 2016, 8, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Dale, H.F.; Madsen, L.; Lied, G.A. Fish–Derived Proteins and Their Potential to Improve Human Health. Nutr. Rev. 2019, 77, 572–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cudennec, B.; Ravallec-Plé, R.; Courois, E.; Fouchereau-Peron, M. Peptides from Fish and Crustacean By-Products Hydrolysates Stimulate Cholecystokinin Release in STC-1 Cells. Food Chem. 2008, 111, 970–975. [Google Scholar] [CrossRef]
- Cudennec, B.; Fouchereau-Peron, M.; Ferry, F.; Duclos, E.; Ravallec, R. In Vitro and in Vivo Evidence for a Satiating Effect of Fish Protein Hydrolysate Obtained from Blue Whiting (Micromesistius Poutassou) Muscle. J. Funct. Foods 2012, 4, 271–277. [Google Scholar] [CrossRef]
- Cudennec, B.; Balti, R.; Ravallec, R.; Caron, J.; Bougatef, A.; Dhulster, P.; Nedjar, N. In Vitro Evidence for Gut Hormone Stimulation Release and Dipeptidyl-Peptidase IV Inhibitory Activity of Protein Hydrolysate Obtained from Cuttlefish (Sepia Officinalis) Viscera. Food Res. Int. 2015, 78, 238–245. [Google Scholar] [CrossRef]
- Nobile, V.; Duclos, E.; Michelotti, A.; Bizzaro, G.; Negro, M.; Soisson, F. Supplementation with a Fish Protein Hydrolysate (Micromesistius Poutassou): Effects on Body Weight, Body Composition, and CCK/GLP-1 Secretion. Food Nutr. Res. 2016, 60, 29857. [Google Scholar] [CrossRef] [Green Version]
- Harnedy, P.A.; Parthsarathy, V.; Mclaughlin, C.M.; Kee, M.B.O.; Allsopp, P.J.; Mcsorley, E.M.; Harte, F.P.M.O.; Fitzgerald, R.J. Blue Whiting (Micromesistius Poutassou ) Muscle Protein Hydrolysate with in Vitro and in Vivo Antidiabetic Properties. J. Funct. Foods 2018, 40, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Parthsarathy, V.; McLaughlin, C.M.; Harnedy, P.A.; Allsopp, P.J.; Crowe, W.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Boarfish (Capros Aper) Protein Hydrolysate Has Potent Insulinotropic and GLP-1 Secretory Activity in Vitro and Acute Glucose Lowering Effects in Mice. Int. J. Food Sci. Technol. 2019, 54, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.H.; Wang, T.Y.; Hung, C.C.; Chen, M.C.; Hsu, K.C. Improvement of Glycemic Control in Streptozotocin-Induced Diabetic Rats by Atlantic Salmon Skin Gelatin Hydrolysate as the Dipeptidyl-Peptidase IV Inhibitor. Food Funct. 2015. [Google Scholar] [CrossRef]
- Huang, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Dipeptidyl-Peptidase IV Inhibitory Activity of Peptides Derived from Tuna Cooking Juice Hydrolysates. Peptides 2012, 35, 114–121. [Google Scholar] [CrossRef]
- Li-chan, E.C.Y.; Hunag, S.; Jao, C.; Ho, K.; Hsu, K. Peptides Derived from Atlantic Salmon Skin Gelatin as Dipeptidyl-Peptidase IV Inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A Standardised Static in Vitro Digestion Method Suitable for Food–an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arndt, M.; Chokshi, H.; Tang, K.; Parrott, N.J.; Reppas, C.; Dressman, J.B. Dissolution Media Simulating the Proximal Canine Gastrointestinal Tract in the Fasted State. Eur. J. Pharm. Biopharm. 2013, 84, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Hatton, G.B.; Yadav, V.; Basit, A.W.; Merchant, H.A. Animal Farm: Considerations in Animal Gastrointestinal Physiology and Relevance to Drug Delivery in Humans. J. Pharm. Sci. 2015, 104, 2747–2776. [Google Scholar] [CrossRef]
- Hervera i Abab, M. Methods for Predicting the Energy Value of Commercial Dog Foods. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2011. [Google Scholar]
- Caron, J.; Domenger, D.; Belguesmia, Y.; Kouach, M.; Lesage, J.; Goossens, J.-F.; Dhulster, P.; Ravallec, R.; Cudennec, B. Protein Digestion and Energy Homeostasis: How Generated Peptides May Impact Intestinal Hormones? Food Res. Int. 2016, 88, 310–318. [Google Scholar] [CrossRef]
- Chaudhari, D.D.; Singh, R.; Mallappa, R.H.; Rokana, N.; Kaushik, J.K.; Bajaj, R.; Batish, V.K.; Grover, S. Evaluation of Casein & Whey Protein Hydrolysates as Well as Milk Fermentates from Lactobacillus Helveticus for Expression of Gut Hormones. Indian J. Med. Res. 2017, 146, 409–419. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Atlantic Salmon (Salmo Salar) Co-Product-Derived Protein Hydrolysates: A Source of Antidiabetic Peptides. Food Res. Int. 2018, 106, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-Y.; Hsieh, C.-H.; Hung, C.-C.; Jao, C.-L.; Chen, M.-C.; Hsu, K.-C. Fish Skin Gelatin Hydrolysates as Dipeptidyl Peptidase IV Inhibitors and Glucagon-like Peptide-1 Stimulators Improve Glycaemic Control in Diabetic Rats: A Comparison between Warm- and Cold-Water Fish. J. Funct. Foods 2015, 19, 330–340. [Google Scholar] [CrossRef]
- Harnedy-Rothwell, P.A.; McLaughlin, C.M.; O’Keeffe, M.B.; Le Gouic, A.V.; Allsopp, P.J.; McSorley, E.M.; Sharkey, S.; Whooley, J.; McGovern, B.; O’Harte, F.P.M.; et al. Identification and Characterisation of Peptides from a Boarfish (Capros Aper) Protein Hydrolysate Displaying in Vitro Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory and Insulinotropic Activity. Food Res. Int. 2020, 131, 108989. [Google Scholar] [CrossRef]
- Guo, L.; Harnedy, P.A.; Zhang, L.; Li, B.; Zhang, Z.; Hou, H.; Zhao, X.; FitzGerald, R.J. In Vitro Assessment of the Multifunctional Bioactive Potential of Alaska Pollock Skin Collagen Following Simulated Gastrointestinal Digestion. J. Sci. Food Agric. 2015, 95, 1514–1520. [Google Scholar] [CrossRef]
- Caron, J.; Domenger, D.; Dhulster, P.; Ravallec, R.; Cudennec, B. Using Caco-2 Cells as Novel Identi Fi Cation Tool for Food-Derived DPP-IV Inhibitors. Food Res. Int. 2017, 92, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gagnon, J.; Nair, S.; Sha, S. Herring Milt Protein Hydrolysate Improves Insulin Resistance in High-Fat-Diet-Induced Obese Male C57BL/6J Mice. Mar. Drugs 2019, 17, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, W.; McLaughlin, C.M.; Allsopp, P.J.; Slevin, M.M.; Harnedy, P.A.; Cassidy, Y.; Baird, J.; Devaney, M.; Fitzgerald, R.J.; O’Harte, F.P.M.; et al. The Effect of Boarfish Protein Hydrolysate on Postprandial Glycaemic Response and Satiety in Healthy Adults. Proc. Nutr. Soc. 2018, 77. [Google Scholar] [CrossRef] [Green Version]
- Dale, H.F.; Jensen, C.; Hausken, T.; Lied, E.; Hatlebakk, J.G.; Brønstad, I.; Lihaug Hoff, D.A.; Lied, G.A. Effect of a Cod Protein Hydrolysate on Postprandial Glucose Metabolism in Healthy Subjects: A Double-Blind Cross-over Trial. J. Nutr. Sci. 2018, 7, e33. [Google Scholar] [CrossRef] [Green Version]
- Santos-Hernández, M.; Miralles, B.; Amigo, L.; Recio, I. Intestinal Signaling of Proteins and Digestion-Derived Products Relevant to Satiety. J. Agric. Food Chem. 2018, 66, 10123–10131. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Lee, M.; Shiu, A.L.; Yo, S.J.; Hallden, G.; Aponte, G.W. GPR93 Activation by Protein Hydrolysate Induces CCK Transcription and Secretion in STC-1 Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1366–G1375. [Google Scholar] [CrossRef]
- Nakajima, S.; Hira, T.; Hara, H. Calcium-Sensing Receptor Mediates Dietary Peptide-Induced CCK Secretion in Enteroendocrine STC-1 Cells. Mol. Nutr. Food Res. 2012, 56, 753–760. [Google Scholar] [CrossRef]
- Darcel, N.P.; Liou, A.P.; Tome, D.; Raybould, H.E. Activation of Vagal Afferents in the Rat Duodenum by Protein Digests Requires PepT1. J. Nutr. 2005, 135, 1491–1495. [Google Scholar] [CrossRef] [Green Version]
- Caron, J.; Cudennec, B.; Domenger, D.; Belguesmia, Y.; Flahaut, C.; Kouach, M.; Lesage, J.; Goossens, J.-F.; Dhulster, P.; Ravallec, R. Simulated GI Digestion of Dietary Protein: Release of New Bioactive Peptides Involved in Gut Hormone Secretion. Food Res. Int. 2016, 89, 382–390. [Google Scholar] [CrossRef]
- De Graaf, C.; Blom, W.A.; Smeets, P.A.; Stafleu, A.; Hendriks, H.F. Biomarkers of Satiation and Satiety. Am. J. Clin. Nutr. 2004, 79, 946–961. [Google Scholar] [CrossRef] [Green Version]
- Domenger, D.; Caron, J.; Belguesmia, Y.; Lesage, J.; Dhulster, P.; Ravallec, R.; Cudennec, B. Bioactivities of Hemorphins Released from Bovine Haemoglobin Gastrointestinal Digestion: Dual Effects on Intestinal Hormones and DPP-IV Regulations. J. Funct. Foods 2017, 36, 9–17. [Google Scholar] [CrossRef]
- Nishi, T.; Hara, H.; Asano, K.; Tomita, F. The Soybean β-Conglycinin β 51-63 Fragment Suppresses Appetite by Stimulating Cholecystokinin Release in Rats. J. Nutr. 2003, 133, 2537–2542. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.; Chen, W.; Addepalli, R.; Colgrave, M.; Singh, T.; Tran, C.; Day, L. In Vitro Transport and Satiety of a Beta-Lactoglobulin Dipeptide and Beta-Casomorphin-7 and Its Metabolites. Food Funct. 2014, 5, 2706–2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulipano, G.; Faggi, L.; Cacciamali, A.; Caroli, A.M. Whey Protein-Derived Peptide Sensing by Enteroendocrine Cells Compared with Osteoblast-like Cells: Role of Peptide Length and Peptide Composition, Focussing on Products of β-Lactoglobulin Hydrolysis. Int. Dairy J. 2017, 72, 55–62. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Amigo, L.; Recio, I. Induction of CCK and GLP-1 Release in Enteroendocrine Cells by Egg White Peptides Generated during Gastrointestinal Digestion. Food Chem. 2020, 329, 127188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chandra, R.; Samsa, L.A.; Gooch, B.; Fee, B.E.; Cook, J.M.; Vigna, S.R.; Grant, A.O.; Liddle, R.A. Amino Acids Stimulate Cholecystokinin Release through the Ca2+-Sensing Receptor. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G528–G537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, A.P.; Sei, Y.; Zhao, X.; Feng, J.; Lu, X.; Thomas, C.; Pechhold, S.; Raybould, H.E.; Wank, S.A. The Extracellular Calcium-Sensing Receptor Is Required for Cholecystokinin Secretion in Response to l-Phenylalanine in Acutely Isolated Intestinal I Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G538–G546. [Google Scholar] [CrossRef] [Green Version]
- Stagsted, J.; Zhou, J.; Jessen, R.; Torngaard Hansen, E. Dietary Peptides. U.S. Patent 16/062,429, 27 December 2018. [Google Scholar]
- Le Nevé, B.; Daniel, H. Selected Tetrapeptides Lead to a GLP-1 Release from the Human Enteroendocrine Cell Line NCI-H716. Regul. Pept. 2011, 167, 14–20. [Google Scholar] [CrossRef]
- Komatsu, Y.; Wada, Y.; Izumi, H.; Shimizu, T.; Takeda, Y.; Hira, T.; Hara, H. Casein Materials Show Different Digestion Patterns Using an in Vitro Gastrointestinal Model and Different Release of Glucagon-like Peptide-1 by Enteroendocrine GLUTag Cells. Food Chem. 2019, 277, 423–431. [Google Scholar] [CrossRef]
- Diakogiannaki, E.; Pais, R.; Tolhurst, G.; Parker, H.E.; Horscroft, J.; Rauscher, B.; Zietek, T.; Daniel, H.; Gribble, F.M.; Reimann, F. Oligopeptides Stimulate Glucagon-like Peptide-1 Secretion in Mice through Proton-Coupled Uptake and the Calcium-Sensing Receptor. Diabetologia 2013, 56, 2688–2696. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Cheng, J.; Wu, H. Discovery of Food-Derived Dipeptidyl Peptidase IV Inhibitory Peptides: A Review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenger, D.; Cudennec, B.; Kouach, M.; Touche, V.; Landry, C.; Lesage, J.; Gosselet, F.; Lestavel, S.; Goossens, J.-F.; Dhulster, P.; et al. Food-Derived Hemorphins Cross Intestinal and Blood–Brain Barriers In Vitro. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, I.M.E.; Chen, X.-M.; Kitts, D.D.; Li-Chan, E.C.Y. Investigation into the Bioavailability of Milk Protein-Derived Peptides with Dipeptidyl-Peptidase IV Inhibitory Activity Using Caco-2 Cell Monolayers. Food Funct. 2017, 8, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundquist, P.; Artursson, P. Oral Absorption of Peptides and Nanoparticles across the Human Intestine: Opportunities, Limitations and Studies in Human Tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef]
- Shimizu, M. Food-Derived Peptides and Intestinal Functions. Biofactors 2004, 21, 43–47. [Google Scholar] [CrossRef]
- Shimizu, M. Interaction between Food Substances and the Intestinal Epithelium. Biosci. Biotechnol. Biochem. 2010, 74, 232–241. [Google Scholar] [CrossRef] [Green Version]
- De Cicco, M.; Mamone, G.; Di Stasio, L.; Ferranti, P.; Addeo, F.; Picariello, G. Hidden “Digestome”: Current Analytical Approaches Provide Incomplete Peptide Inventories of Food Digests. J. Agric. Food Chem. 2019, 67, 7775–7782. [Google Scholar] [CrossRef]



| ID Mode | Sequence | RT | m/z | 10−logP | ppm | Identification |
| Database | SAGPQGPIGPR | 27.11 | 518.78 | 46.51 | 0.6 | Collagen type I |
| DVSGGYDE | 20.69 | 841.33 | 41.49 | 6.2 | Collagen type I | |
| HIHVNGA | 16.85 | 747.39 | 38.12 | −1.5 | Collagen type 10 | |
| VAPEEHPT | 17.03 | 879.42 | 36.41 | 3.5 | Alpha-actin | |
| AGPQGPIGPR | 24.51 | 475.26 | 35.37 | 0.0 | Collagen type I | |
| GATGPAGAV | 23.75 | 700.36 | 32.09 | 0.2 | Collagen type I Alpha-actin | |
| EAPLNPK | 17.44 | 768.42 | 31 | −4.6 | Alpha-actin | |
| PEEHPT | 16.74 | 709.32 | 27.74 | 8.3 | Alpha-actin | |
| ID Mode | Sequence | RT | m/z | ALC | ppm | |
| De novo | LGVDE | 21.54 | 532.26 | 98 | 2.6 | nd |
| VVEP | 19.09 | 443.24 | 96 | −10.4 | nd | |
| LTDY | 20.97 | 511.25 | 96 | 15.1 | nd | |
| PSLVH | 17.43 | 552.30 | 96 | −33.1 | nd | |
| ELLK | 16.65 | 502.31 | 95 | −31.5 | nd | |
| LGME | 21.28 | 449.21 | 95 | 6.9 | nd | |
| DLVDK | 17.39 | 589.32 | 95 | 10.6 | nd | |
| EVLSQ | 21.84 | 575.29 | 95 | −23.8 | nd | |
| LKPT | 20.48 | 235.67 | 92 | −9.1 | nd | |
| DSKPGSL | 17.48 | 703.37 | 92 | 6.1 | nd | |
| LLMMK | 17.19 | 318.18 | 90 | −26.9 | nd | |
| LEL | 18.83 | 374.23 | 80 | −1.6 | nd |
| ID Mode | Sequence | RT | m/z | ALC | 10−logP | In Vitro IC50 (µM) | In Situ IC50 (µM) | Identification |
|---|---|---|---|---|---|---|---|---|
| Database | VAPEEHPT | 15.47 | 440.21 | 28.54 | −3.9 | 409 | 2268 | Alpha-actin |
| De novo | DLDL | 26.88 | 475.24 | 98 | −6.4 | nd | 763 | nd |
| De novo | PDLV | 20.77 | 443.24 | 89 | −12.7 | nd | nd | nd |
| De novo | MDLP | 26.87 | 475.24 | 87 | 31 | nd | 605 | nd |
| De novo | VDAGAP | 16.52 | 529.26 | 84 | 0.7 | nd | nd | nd |
| De novo | EDYT | 27.16 | 264.10 | 84 | −13.5 | nd | nd | nd |
| De novo | VADTMEVV | 16.06 | 440.21 | 82 | 4.3 | 603 | 1130 | nd |
| De novo | DPLV | 22.33 | 443.25 | 81 | −8.9 | 698 | nd | nd |
| De novo | EDTY | 27.63 | 264.10 | 81 | −14.2 | nd | nd | nd |
| De novo | CSSGK | 27.77 | 481.21 | 80 | 6.2 | nd | nd | nd |
| De novo | FAMD | 13.51 | 483.19 | 80 | −1.4 | 775 | 862 | nd |
| De novo | CSSGGY | 29.88 | 573.20 | 80 | 6.4 | nd | 2160 | nd |
| De novo | GPFPLLV | 42.46 | 742.45 | 80 | 2.0 | 263 | 456 | nf |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theysgeur, S.; Cudennec, B.; Deracinois, B.; Perrin, C.; Guiller, I.; Lepoudère, A.; Flahaut, C.; Ravallec, R. New Bioactive Peptides Identified from a Tilapia Byproduct Hydrolysate Exerting Effects on DPP-IV Activity and Intestinal Hormones Regulation after Canine Gastrointestinal Simulated Digestion. Molecules 2021, 26, 136. https://doi.org/10.3390/molecules26010136
Theysgeur S, Cudennec B, Deracinois B, Perrin C, Guiller I, Lepoudère A, Flahaut C, Ravallec R. New Bioactive Peptides Identified from a Tilapia Byproduct Hydrolysate Exerting Effects on DPP-IV Activity and Intestinal Hormones Regulation after Canine Gastrointestinal Simulated Digestion. Molecules. 2021; 26(1):136. https://doi.org/10.3390/molecules26010136
Chicago/Turabian StyleTheysgeur, Sandy, Benoit Cudennec, Barbara Deracinois, Claire Perrin, Isabelle Guiller, Anne Lepoudère, Christophe Flahaut, and Rozenn Ravallec. 2021. "New Bioactive Peptides Identified from a Tilapia Byproduct Hydrolysate Exerting Effects on DPP-IV Activity and Intestinal Hormones Regulation after Canine Gastrointestinal Simulated Digestion" Molecules 26, no. 1: 136. https://doi.org/10.3390/molecules26010136







