Analysis of a “3-(Naphthalen-1-ylimino)indolin-2-one” Compound and Its Antimicrobial Assessment Using Lipid-Based Self-Nanoemulsifying Formulations
Abstract
:1. Introduction
2. Results and Discussion
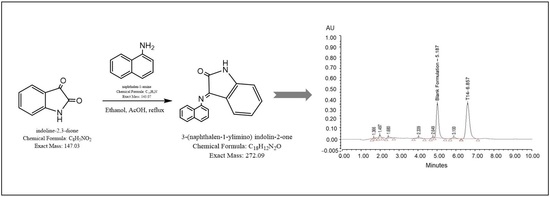
2.1. NMR and Mass Analysis of 3-(Naphthalen-1-ylimino)indolin-2-one (2)
2.2. Optimization of UPLC Peak Separation Conditions
2.3. Linearity and Range
2.4. System Suitability
2.5. Force Degradation Studies
2.5.1. Acid Degradation
2.5.2. Base Degradation
2.5.3. Oxidation
2.5.4. Thermal Degradation
2.6. Accuracy (Recovery) and Precision of “3-(Naphthalen-1-ylimino)indolin-2-one” Compound
2.6.1. Precision
Intra-Day
Inter-Day
2.7. Application
2.8. Antimicrobial Activity Screening
2.8.1. Sample Preparation
2.8.2. Cup-plate Method
2.8.3. Activity Performance
3. Materials and Methods
3.1. Materials
3.2. Experimental Methods
3.2.1. Procedure of “3-(Naphthalen-1-ylimino)indolin-2-one” Synthesis (2)
3.2.2. NMR Analysis of “3-(Naphthalen-1-ylimino)indolin-2-one” (2)
3.2.3. UHPLC Analysis of “3-(Naphthalen-1-ylimino)indolin-2-one” (2)
3.2.4. UPLC Chromatographic Conditions
3.2.5. Analytical Method Development and Optimization
Method Validation
Linearity and Range
Specificity
Accuracy and Precision
Limit of Detection (LOD)and Limit of Quantification (LOQ)
Lipid-Based Self-Nanoemulsifying Formulation Development
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Sonam, V.; Kakkar, R. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. MedChemComm 2019, 10, 351–368. [Google Scholar]
- Ratnamala, P.; Sonawane, R.R.T. The chemistry and synthesis of 1H-indole-2,3-dione (Isatin) and its derivatives. Int. Lett. Chem. Phys. Astron. 2013, 12, 30–36. [Google Scholar]
- Varma, R.S.; Nobles, W.L. Antiviral, antibacterial, and antifungal activities of isatin N-Mannich bases. J. Pharm. Sci. 1975, 64, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.K.; Pandeya, S.N.; Singh, A.; Srivastava, B.K.; Pandey, M. Synthesis and Antimicrobial Activity of Schiff’s and N-Mannich Bases of Isatin and Its Derivatives with 4-Amino-N-Carbamimidoyl Benzene Sulfonamide. Int. J. Pharm. Sci. Drug Res. 2010, 2, 151–154. [Google Scholar]
- Varma, R.S.; Nobles, W.L. Substituted 3-aminomethylbenzoxazoline-2-thiones as potential antibacterial agents. J. Pharm. Sci. 1972, 61, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Adibi, H.; Khodaei, M.M.; Pakravan, P.; Abiri, R. Synthesis, characterization, and in vitro antimicrobial evaluation of hydrazone and bishydrazone derivatives of isatin. Pharm. Chem. J. 2010, 44, 219–227. [Google Scholar] [CrossRef]
- Malawska, B. New anticonvulsant agents. Curr. Top. Med. Chem. 2005, 5, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.S.; Nobles, W.L. Application of 1-(N-beta-hydroxyethyl-4-piperidyl)-3-(4-piperidyl)-propane in the Mannich reaction. I. Substituted beta-aminoketones. J. Pharm. Sci. 1967, 56, 455–459. [Google Scholar] [CrossRef]
- Popp, F.D. Potential anticonvulsants. IX. Some isatin hydrazones and related compounds. J. Heterocycl. Chem. 1984, 21, 1641–1645. [Google Scholar] [CrossRef]
- Premanathan, M.; Radhakrishnan, S.; Kulangiappar, K.; Singaravelu, G.; Thirumalaiarasu, V.; Sivakumar, T.; Kathiresan, K. Antioxidant & anticancer activities of isatin (1H-indole-2,3-dione), isolated from the flowers of Couroupita guianensis Aubl. Indian J. Med. Res. 2012, 136, 822–826. [Google Scholar]
- Benhangi, H.M.; Ahmadi, S.; Hakimi, M.; Molafilabi, A.; Faraji, H.; Mashkani, B. Protective effects of isatin and its synthetic derivatives against iron, copper and lead toxicity. Toxicol. In Vitro 2019, 54, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Ayman El-Faham, W.N.H.; Wadaan Mohammad, A.M.; Khattab Sherine, N.; Ghabbour Hazem, A.; Fun, H.-K.; Mohammed, R.S. Microwave Synthesis, Characterization, and Antimicrobial Activity of Some Novel Isatin Derivatives. J. Chem. 2015, 2015, 716987. [Google Scholar] [CrossRef] [Green Version]
- Zgórniak-Nowosielska, I.G.A.; Poteć, Z. The antiviral activity of isatin beta-thiosemicarbazone derivatives on vaccinia virus infection in mice. Arch. Immunol. Exp. (Warsz) 1976, 24, 597–601. [Google Scholar]
- Gabriel, D.; Pontes, L.B.; Da Silva, J.S.; Sudo, R.T.; Corrêa, M.B.; Pinto, A.C.; Garden, S.J.; Zapata-Sudo, G. Pharmacological activity of novel 2-hydroxyacetophenone isatin derivatives on cardiac and vascular smooth muscles in rats. J. Cardiovasc. Pharm. 2011, 57, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Abele, E.; Abele, R.; Dzenitis, O.; Lukevics, E. Indole and Isatin Oximes: Synthesis, Reactions, and Biological Activity. (Review). Chem. Heterocycl. Compd. 2003, 39, 3–35. [Google Scholar] [CrossRef]
- Mondal, P.; Jana, S.; Bose, A.; Banerjee, M. Synthesis and Evaluation of 1,3 Di-Substituted Schiff, Mannich Bases and Spiro Isatin Derivatives. J. Young Pharm. 2010, 2, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Javad Azizian, M.K.M.; Omidreza, F.; Nima, R.; Ramin, M. Synthesis, biological activity and docking study of some new isatin Schiff base derivatives. Med. Chem. Res. 2012, 21, 3730–3740. [Google Scholar] [CrossRef]
- Sridhar, S.K.; Saravanan, M.; Ramesh, A. Synthesis and antibacterial screening of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur. J. Med. Chem. 2001, 36, 615–625. [Google Scholar] [CrossRef]
- Ali, M.S.; Ghori, M.; Khatri, A.R. Stability indicating simultaneous determination of domperidone (DP), methylparaben (MP) and propylparaben by high performance liquid chromatography (HPLC). J. Pharm. Biomed. Anal. 2006, 41, 358–365. [Google Scholar] [CrossRef]
- Chen, G.; Meng, M.; Zhang, Y.; Hao, X.-J.; Wang, Y.; Mu, S. Synthesis, Cytoprotective and Anti-Tumor Activities of Isatin Schiff Bases. Lett. Drug Des. Discov. 2015, 12, 802–805. [Google Scholar] [CrossRef]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of self-nanoemulsifying drug delivery system (SNEDDS) for poorly water-soluble talinolol: Preparation, in vitro and in vivo assessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvekhgeimer, M.G.A. Synthesis of heterocyclic compounds by the cyclization of isatin and its derivatives (review). Chem. Heterocycl. Compd. 1996, 32, 249–276. [Google Scholar] [CrossRef]
- Bergman, J.; Lindström, J.; Tilstam, U. The structure and properties of some indolic constituents in Couroupita guianensis aubl. Tetrahedron 1985, 41, 2879–2881. [Google Scholar] [CrossRef]
- Halket, J.M.; Watkins, P.J.; Przyborowska, A.; Goodwin, B.L.; Clow, A.; Glover, V.; Sandler, M. Isatin (indole-2,3-dione) in urine and tissues. Detection and determination by gas chromatography-mass spectrometry. J. Chromatogr. 1991, 562, 279–287. [Google Scholar] [CrossRef]
- Alamri, R.G.; Kazi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K. Development and validation of bioanalytical UHPLC-UV method for simultaneous analysis of unchanged fenofibrate and its metabolite fenofibric acid in rat plasma: Application to pharmacokinetics. Saudi Pharm. J. 2017, 25, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Alghazi, M.; Alanazi, F.; Mohsin, K.; Siddiqui, N.A.; Shakeel, F.; Haq, N. Simultaneous separation of antihyperlipidemic drugs by green ultrahigh-performance liquid chromatography–diode array detector method: Improving the health of liquid chromatography. J. Food Drug Anal. 2017, 25, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Kazi, M.; Alhajri, A.; AlShehri, S.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.M.; Altamimi, M.A.; Alanazi, F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef]








| Parameter | “3-(Naphthalen-1-ylimino)indolin-2-one” |
|---|---|
| Concentration range | 5–100 PPM |
| Intercept (a) | 6933.932 |
| Slope (b) | 28,067 |
| Correlation coefficient (R) | 0.9998 |
| RT a | ~6.8 min |
| λmax | 234 nm |
| Relative standard deviation of slope | 0.8% |
| Limit of detection (LOD) b | 2.470493 μg/mL |
| Limit of quantification (LOQ) c | 0.815263 μg/mL |
| Assay Type | Amount (μg/mL) | Precision (%) | Accuracy (%) | Drug | |
|---|---|---|---|---|---|
| Added | Found ± SD | ||||
| Inter-day | 5 | 4.98 ± 0.03 | 0.503 | 99.63 | “3-(Naphthalen-1-ylimino)indolin-2-one” |
| 10 | 9.97 ± 0.06 | 0.563 | 99.67 | ||
| 100 | 99.32 ± 1.06 | 1.07 | 99.32 | ||
| Intra-day | 5 | 4.97 ± 0.019 | 0.392 | 99.47 | |
| 10 | 9.97 ± 0.059 | 0.598 | 99.71 | ||
| 100 | 99.32 ± 1.25 | 1.246 | 99.32 | ||
| Real Sample | Manufacturer | Amount Claimed | Found (mg/mL) ± SD | % of Labelled Claim |
|---|---|---|---|---|
| BSO/I988/KrEL (35/15/50, %w/w) | In house, Pharmacy lab, King Saud University | 5.00 mg | 4.91 ± 0.28 (mg) | 98.20 |
| N | Compound | Zone of Inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | E. coli | P. aeruginosa | A. baumannii | Mycobacterium | C. albicans | Asp. niger | ||
| 1 | Isatin | 22 | 18 | 30 | 16 | 22 | NZ | 15 | NZ |
| 2 | Compound (2) | 18 | 15 | 12 | 15 | 20 | NZ | 10 | 25 |
| 3 | Isatin-F | 12 | 10 | 20 | NZ | 15 | NZ | 10 | 13 |
| 4 | Compound (2)-F | 13 | 12 | 23 | NZ | 12 | NZ | 12 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali Syed, S.; Bari, A.; Aldughaim, M.S.; Rashid, M.A.; Shariare, M.H.; Kazi, M. Analysis of a “3-(Naphthalen-1-ylimino)indolin-2-one” Compound and Its Antimicrobial Assessment Using Lipid-Based Self-Nanoemulsifying Formulations. Molecules 2021, 26, 15. https://doi.org/10.3390/molecules26010015
Ali Syed S, Bari A, Aldughaim MS, Rashid MA, Shariare MH, Kazi M. Analysis of a “3-(Naphthalen-1-ylimino)indolin-2-one” Compound and Its Antimicrobial Assessment Using Lipid-Based Self-Nanoemulsifying Formulations. Molecules. 2021; 26(1):15. https://doi.org/10.3390/molecules26010015
Chicago/Turabian StyleAli Syed, Saeed, Ahmed Bari, Mohammed S. Aldughaim, Md Abdur Rashid, Mohammad Hossain Shariare, and Mohsin Kazi. 2021. "Analysis of a “3-(Naphthalen-1-ylimino)indolin-2-one” Compound and Its Antimicrobial Assessment Using Lipid-Based Self-Nanoemulsifying Formulations" Molecules 26, no. 1: 15. https://doi.org/10.3390/molecules26010015