Crystalline Peroxosolvates: Nature of the Coformer, Hydrogen-Bonded Networks and Clusters, Intermolecular Interactions
Abstract
:1. Introduction
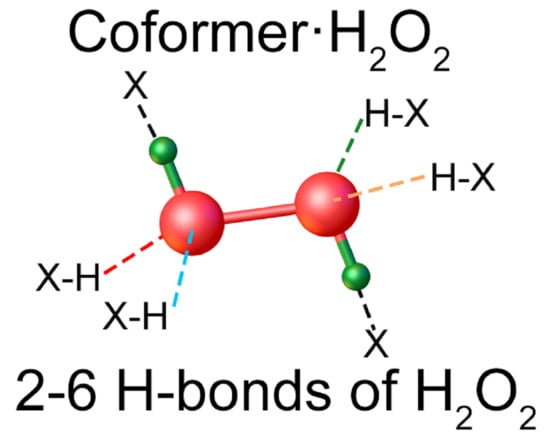
2. Chemical Composition of Crystalline Peroxosolvates
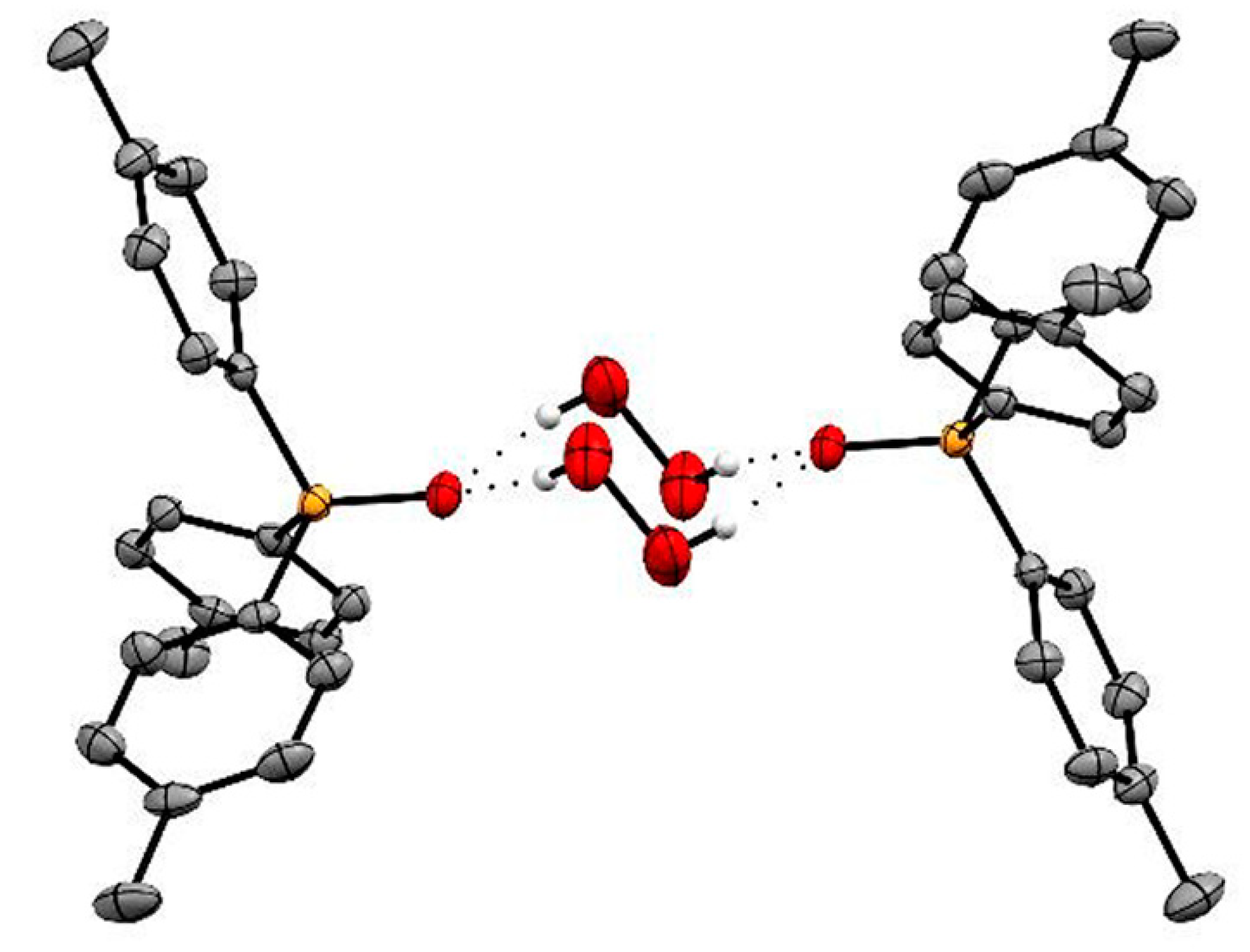
3. Dimensions and Topology of Peroxide Clusters in the Crystalline Phase
4. The Energies of Intermolecular Interactions of H2O2 in Organic Crystals: Calculations by the Kohn–Sham Methods with Periodic Boundary Conditions
5. Examples of H-bond Networks: Average Distances, Types of Coordination
6. Trends and Prospects
6.1. High-Energy Substances
6.2. Mixed Pharmaceutical Forms. Antiseptic and Analgesic Effect
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tanatar, S. Percarbonate. Ber. Dtsch. Chem. Ges. 1899, 32, 1544–1546. [Google Scholar] [CrossRef]
- Tanatar, S. Double Compounds of Hydrogen Peroxide with Organic Substances. J. Russ. Phys. Chem. Soc. 1906, 40L, 376–380. [Google Scholar]
- Jakob, H.; Leininger, S.; Lehmann, T.; Jacobi, S.; Gutewort, S. Peroxo Compounds, Inorganic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 1–33. [Google Scholar]
- Goti, A.; Cardona, F. Hydrogen Peroxide in Green Oxidation Reactions: Recent Catalytic Processes. In Green Chemical Reactions; Springer: Dordrecht, The Netherlands, 2008; pp. 191–212. [Google Scholar]
- Jones, C.W. Applications of Hydrogen Peroxide and Derivatives; Royal Society of Chemistry: Cambridge, UK, 1999; pp. 1–264. [Google Scholar]
- Schumb, W.C.; Satterfield, C.N.; Wentworth, R.L. Hydrogen peroxide; Reinhold Publishing Corporation: New York, NY, USA, 1955; pp. 1–759. [Google Scholar]
- Churakov, A.V.; Sladkevich, S.; Lev, O.; Tripol’skaya, T.A.; Prikhodchenko, P.V. Cesium Hydroperoxostannate: First Complete Structural Characterization of a Homoleptic Hydroperoxocomplex. Inorg. Chem. 2010, 49, 4762–4764. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Medvedev, A.G.; Tripol’skaya, T.A.; Popov, V.S.; Mokrushin, A.S.; Krut’ko, D.P.; Prikhodchenko, P.V.; Lev, O. H2O2 Induced Formation of Graded Composition Sodium-doped Tin Dioxide and Template-free Synthesis of Yolk–shell SnO2 Particles and their Sensing Application. Dalton Trans. 2017, 46, 16171–16179. [Google Scholar] [CrossRef] [PubMed]
- Wolanov, Y.; Lev, O.; Churakov, A.V.; Medvedev, A.G.; Novotortsev, V.M.; Prikhodchenko, P.V. Preparation of Pure Hydrogen Peroxide and Anhydrous Peroxide Solutions from Crystalline Serine Perhydrate. Tetrahedron 2010, 66, 5130–5133. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Mikhaylov, A.A.; Churakov, A.V.; Vener, M.V.; Tripol’skaya, T.A.; Cohen, S.; Lev, O.; Prikhodchenko, P.V. Potassium, Cesium, and Ammonium Peroxogermanates with Inorganic Hexanuclear Peroxo Bridged Germanium Anion Isolated from Aqueous Solution. Inorg. Chem. 2015, 54, 8058–8065. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane Transport of Hydrogen Peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Churakov, A.V.; Grishanov, D.A.; Medvedev, A.G.; Mikhaylov, A.A.; Tripol’skaya, T.A.; Vener, M.V.; Navasardyan, M.A.; Lev, O.; Prikhodchenko, P.V. Cyclic Dipeptide Peroxosolvates: First Direct Evidence for Hydrogen Bonding Between Hydrogen Peroxide and a Peptide Backbone. CrystEngComm 2019, 21, 4961–4968. [Google Scholar] [CrossRef]
- Cambridge Structural Database. Ver. 5.41. 2020. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 1 December 2020).
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Miller, E.W.; Tulyathan, O.; Isacoff, E.Y.; Chang, C.J. Molecular Imaging of Hydrogen Peroxide Produced for Cell Signaling. Nat. Chem. Biol. 2007, 3, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Encrenaz, T.; Greathouse, T.K.; Richter, M.J.; Bézard, B.; Fouchet, T.; Lefèvre, F.; Montmessin, F.; Forget, F.; Lebonnois, S.; Atreya, S.K. Simultaneous Mapping of H2O and H2O2 on Mars from Infrared High-resolution Imaging Spectroscopy. Icarus 2008, 195, 547–556. [Google Scholar] [CrossRef]
- Sies, H. Role of Metabolic H2O2 Generation: Redox Signaling and Oxidative Stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.R.; Yang, S. Hydrogen Peroxide: A Signaling Messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen Peroxide as a Signal Controlling Plant Programmed Cell Death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 Mediates Hydrogen Peroxide Uptake to Regulate Downstream Intracellular Signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [Green Version]
- Chauvigné, F.; Boj, M.; Finn, R.N.; Cerdà, J. Mitochondrial Aquaporin-8-mediated Hydrogen Peroxide Transport is Essential for Teleost Spermatozoon Motility. Sci. Rep. 2015, 5, 7789. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Moller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific Aquaporins Facilitate the Diffusion of Hydrogen Peroxide Across Membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated Transmembrane Diffusion of Hydrogen Peroxide. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Chernyshov, I.Y.; Vener, M.V.; Prikhodchenko, P.V.; Medvedev, A.G.; Lev, O.; Churakov, A.V. Peroxosolvates: Formation Criteria, H2O2 Hydrogen Bonding, and Isomorphism with the Corresponding Hydrates. Cryst. Growth Des. 2017, 17, 214–220. [Google Scholar] [CrossRef]
- Churakov, A.V.; Grishanov, D.A.; Medvedev, A.G.; Mikhaylov, A.A.; Vener, M.V.; Navasardyan, M.A.; Tripol’skaya, T.A.; Lev, O.; Prikhodchenko, P.V. Stabilization of Hydrogen Peroxide by Hydrogen Bonding in the Crystal Structure of 2-aminobenzimidazole Perhydrate. CrystEngComm 2020, 22, 2866–2872. [Google Scholar] [CrossRef]
- Fritchie, C.J.; McMullan, R.K. Neutron Diffraction Study of the 1:1 Urea:Hydrogen Peroxide Complex at 81 K. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1981, 37, 1086–1091. [Google Scholar] [CrossRef]
- Churakov, A.V.; Prikhodchenko, P.V.; Lev, O.; Medvedev, A.G.; Tripol’skaya, T.A.; Vener, M.V. A Model Proton-Transfer System in the Condensed Phase: NH4+OOH−, a Crystal with Short Intermolecular H-bonds. J. Chem. Phys. 2010, 133, 164506–164515. [Google Scholar] [CrossRef] [PubMed]
- Wolanov, Y.; Prikhodchenko, P.V.; Medvedev, A.G.; Pedahzur, R.; Lev, O. Zinc Dioxide Nanoparticulates: A Hydrogen Peroxide Source at Moderate pH. Environ. Sci. Technol. 2013, 47, 8769–8774. [Google Scholar] [CrossRef] [PubMed]
- Inorganic Crystal Structure Database. Ver. 4.2.0. 2019. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 1 December 2020).
- Belsky, A.; Hellenbrandt, M.; Karen, V.L.; Luksch, P. New Developments in the Inorganic Crystal Structure Database (ICSD): Accessibility in Support of Materials Research and Design. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 364–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, F.H. The Cambridge Structural Database: A Quarter of a Million Crystal Structures and Rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.F.; Pedersen, B. The Crystal Structure of Sodium Oxalate Perhydrate Na2C2O4·H2O2. Acta Chem. Scand. 1964, 18, 1454–1468. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Jones, W. Potassium Hydrogen Phthalate Hemiperhydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1995, 51, 1128–1130. [Google Scholar] [CrossRef]
- Wallen, C.M.; Bacsa, J.; Scarborough, C.C. Hydrogen Peroxide Complex of Zinc. J. Am. Chem. Soc. 2015, 137, 14606–14609. [Google Scholar] [CrossRef]
- Pedersen, B.F. The Crystal Structure of Ammonium Oxalate Monoperhydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 746–754. [Google Scholar] [CrossRef]
- Pedersen, B.F.; Larsen, T.K.; Soling, H.; Torbjörnsson, L.; Werner, P.-E.; Junggren, U.; Lamm, B.; Samuelsson, B. The Crystal Structure of Lithium Oxalate Monoperhydrate, Li2C2O4·H2O2. Acta Chem. Scand. 1969, 23, 1871–1877. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.F.; Kvick, Å. Neutron Diffraction Study of Potassium Oxalate Monoperhydrate at 123 K. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 21–23. [Google Scholar] [CrossRef]
- Pedersen, B.F.; Seip, H.M.; Santesson, J.; Holmberg, P.; Eriksson, G.; Blinc, R.; Paušak, S.; Ehrenberg, L.; Dumanović, J. The Crystal Structure of Potassium and Rubidium Oxalate Monoperhydrates, K2C2O4·H2O2 and Rb2C2O4·H2O2. Acta Chem. Scand. 1967, 21, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.M.; Pritchard, R.G. The Crystal Structure of Guanidinium Oxalate Dihydrate Monoperhydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1976, 32, 2438–2440. [Google Scholar] [CrossRef] [Green Version]
- Sarin, V.A.; Dudarev, V.Y.; Dobrynina, T.A.; Fykin, L.E.; Zavodnik, V.E. X-ray and Neutron-diffraction Study of KF·2H2O2. Kristallografiya 1976, 21, 929–936. [Google Scholar]
- Sarin, V.A.; Dudarev, V.Y.; Dobrynina, T.A.; Fykin, L.E.; Zavodnik, V.E. X-ray and Neutron-diffraction Studies of RbF·H2O2 Crystals. Kristallografiya 1977, 22, 982–987. [Google Scholar]
- Sarin, V.A.; Dudarev, V.Y.; Dobrynina, T.A.; Zavodnik, V.E. X-ray structural Investigation of NH4F·H2O2 Crystals. Kristallografiya 1979, 24, 824–825. [Google Scholar]
- Pritchard, R.G.; Begum, Z.; Lau, Y.F.; Austin, J. Structures of Na9[SO4]4X·2H2O2, where X = Cl or Br, in which the Halide Anions Orchestrate Extended Orientation Sequences of H2O2 Solvate Molecules. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Churakov, A.V.; Prikhodchenko, P.V.; Howard, J.A.K. The Preparation and Crystal Structures of Novel Perhydrates Ph4X+Hal−·nH2O2: Anionic Hydrogen-bonded Chains Containing Hydrogen Peroxide. CrystEngComm 2005, 7, 664–669. [Google Scholar] [CrossRef]
- Carrondo, M.A.A.F.; De, C.T.; Griffith, W.P.; Jones, D.P.; Skapski, A.C. X-ray Crystal Structure of the Industrial Bleaching Agent ‘Sodium Percarbonate’[Sodium Carbonate–Hydrogen Peroxide (2/3)]. J. Chem. Soc. Dalton Trans. 1977, 2323–2327. [Google Scholar] [CrossRef]
- Pritchard, R.G.; Islam, E. Sodium Percarbonate between 293 and 100 K. Acta Crystallogr. Sect. B Struct. Sci. 2003, 59, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.G.; Mikhaylov, A.A.; Churakov, A.V.; Prikhodchenko, P.V.; Lev, O. Ammonium and Caesium Carbonate Peroxosolvates: Supramolecular Networks Formed by Hydrogen Bonds. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2012, 68, i20–i24. [Google Scholar] [CrossRef] [PubMed]
- Thierbach, D.; Huber, F.; Preut, H. Structure of Triphenylphosphine Oxide Hemiperhydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 974–977. [Google Scholar] [CrossRef]
- Stomberg, R.; Klemets, R.; Lundström, I.; Fontell, K.; Nielsen, C.J.; Urso, F.; Weidlein, J.; Zingaro, R.A. The Crystal Structures of Potassium Bis(oxalato)oxoperoxovanadate(V) Hemihydrate, K3[VO(O2)(C2O4)2]·½H2O, and Potassium Bis(oxalato)dioxovanadate(V) Trihydrate, K3[VO2(C2O4)2]·3H2O. Acta Chem. Scand. 1986, 40a, 168–176. [Google Scholar] [CrossRef]
- Won, T.-J.; Barnes, C.L.; Schlemper, E.O.; Thompson, R.C. Two Crystal Structures Featuring the Tetraperoxovanadate(V) Anion and a Brief Reinvestigation of Peroxovanadate Equilibria in Neutral and Basic Solutions. Inorg. Chem. 1995, 34, 4499–4503. [Google Scholar] [CrossRef]
- Szentivanyi, H.; Stomberg, R.; Hämäläinen, R.; Kohl, F.X.; Seip, R. The Crystal Structure of 2,2′-Bipyridinium(1+) mu-Hydrogen-bis[(2,2′-bipyridine)oxodiperoxovanadate](1-)-x-hydrogen peroxide-(6-x)-water, (Hbipy)[H{VO(O2)2bipy}2]·xH2O2·(6-x)H2O, x ~= 0.5, at −100 degrees C. Acta Chem. Scand. 1984, 38, 101–107. [Google Scholar] [CrossRef]
- Campbell, N.J.; Capparelli, M.V.; Griffith, W.P.; Skapski, A.C. On the Existence of Triperoxo Vanadium Complexes. X-ray Crystal Structures of K3[VO(O2)2(C2O4]·H2O2 and of (NH4)[VO(O2)2(bipy)]·4H2O. Inorg. Chim. Acta 1983, 77, L215–L216. [Google Scholar] [CrossRef]
- Schwendt, P.; Ahmed, M.; Marek, J. Complexation between Vanadium (V) and Phenyllactate: Synthesis, spectral Studies and Crystal Structure of (NEt4)(NH4)3[V2O2(O2)2(R-3-phlact)2][V2O2(O2)2(S-3-phlact)2]·6H2O, [3-phlact=3-phenyllactato(2−)]. Inorg. Chim. Acta 2005, 358, 3572–3580. [Google Scholar] [CrossRef]
- Shao, M.; Dong, X.U.N.; Tang, Y. Crystal Structure Investigation of Vanadyl Complexes of Tridentate Ligand. (II)—Synthese and Crystal Structure of 1-(2-pyridylazo)-2-naphtholato-dioxovanadium(V) Dimer [VO2(C15)H10N3O)]2(H2O2)(CHCl3)2 and Pyridine-(1-(2-pyridylazo)-2-naphtolato)peroxo Oxovanadium(V) VO(O2)(C15H10N3O)(C5H5N). Sci. Sin. Ser. B (Engl. Ed.) 1988, 31, 789–799. [Google Scholar] [CrossRef]
- Šimuneková, M.; Šimunek, J.; Chrappová, J.; Schwendt, P.; Žák, Z.; Pavelčík, F. Dinucleating Role of a Strong Hydrogen Bond in Crystal Structure of [N(C4H9)4]{[VO(HO2)(O2)(phen)][VO(O2)2(phen)]}·3H2O2·H2O. Inorg. Chem. Commun. 2012, 24, 125–128. [Google Scholar] [CrossRef]
- Mathern, G.; Weiss, R. Structure des Complexes Peroxydiques des Métaux de Transition. II. Structure Cristalline du Triperoxo-(o-phénanthroline)niobate de Potassium à Trois Molécules d’Eau et de son Perhydrate KNb(O2)3(C12H8N2)·3H2O et KNb(O2)3(C12H8N2)·3H2O·H2O2. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1971, 27, 1582–1597. [Google Scholar] [CrossRef]
- Bayot, D.; Tinant, B.; Mathieu, B.; Declercq, J.-P.; Devillers, M. Spectroscopic and Structural Characterizations of Novel Water-Soluble Peroxo[polyaminocarboxylato bis(N-oxido)]niobate(V) Complexes. Eur. J. Inorg. Chem. 2003, 2003, 737–743. [Google Scholar] [CrossRef]
- Bayot, D.; Tinant, B.; Devillers, M. Homo- and Heterobimetallic Niobium(V) and Tantalum(V) Peroxo-tartrate Complexes and Their Use as Molecular Precursors for Nb−Ta Mixed Oxides. Inorg. Chem. 2005, 44, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Bayot, D.; Tinant, B.; Devillers, M. Spectroscopic and Structural Characterizations of Novel Water-Soluble Tetraperoxo and Diperoxo[polyaminocarboxylato bis(N-oxido)]tantalate(V) Complexes. Inorg. Chem. 2004, 43, 5999–6005. [Google Scholar] [CrossRef]
- Qiu, J.; Vlaisavljevich, B.; Jouffret, L.; Nguyen, K.; Szymanowski, J.E.S.; Gagliardi, L.; Burns, P.C. Cation Templating and Electronic Structure Effects in Uranyl Cage Clusters Probed by the Isolation of Peroxide-Bridged Uranyl Dimers. Inorg. Chem. 2015, 54, 4445–4455. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Medvedev, A.G.; Churakov, A.V.; Grishanov, D.A.; Prikhodchenko, P.V.; Lev, O. Peroxide Coordination of Tellurium in Aqueous Solutions. Chem. Eur. J. 2016, 22, 2980–2986. [Google Scholar] [CrossRef]
- Mühle, C.; Peters, E.-M.; Jansen, M. New Hydrogen Peroxide Adducts of Alkali Metal Tetracyanoplatinates A2[Pt(CN)4]·H2O2 (A = K, Rb, Cs). Z. Naturforsch. B 2009, 64, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Khodadad, P.; Rodier, N. Trans-Diammine-trans-dichloro-trans-dihydroxoplatine(IV) di(peroxyde d’hydrogène). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 2219–2220. [Google Scholar] [CrossRef]
- Barnard, C.F.J.; Hydes, P.C.; Griffiths, W.P.; Mills, O.S. A Stable Platinum Complex Perhydrate Adduct: Crystal Stucture of cis,trans-[PtCl2(OH)2(2-NH2Pr)2]·0.5H2O2 and water and N,N-dimethylacetamide adducts. J. Chem. Res. Synop. 1983, 302–303. [Google Scholar]
- Vannerberg, N.G. On the System SrO2-H2O-H2O2. I. The Crystal Structure of α -SrO2·2H2O2 and β -SrO2·2H2O2. Ark. Kemi 1958, 13, 29–41. [Google Scholar]
- Vannerberg, N.G. On the System BaO2-H2O-H2O2. I. Investigation of the Existing Phases and their Preparation. Ark. Kemi 1959, 14, 147–149. [Google Scholar]
- Vannerberg, N.G. On the System BaO2-H2O-H2O2. II The Structure of BaO2·H2O2. Ark. Kemi 1959, 14, 149–159. [Google Scholar]
- Vannerberg, N.G. On the System BaO2-H2O-H2O2. III. The Crystal Structure of α-BaO2, β-BaO2, and γ-BaO2·2H2O2 and BaO2·H2O2·2H2O. Ark. Kemi 1959, 14, 125–145. [Google Scholar]
- Arp, F.F.; Ahn, S.H.; Bhuvanesh, N.; Blümel, J. Selective Synthesis and Stabilization of Peroxides via Phosphine Oxides. New J. Chem. 2019, 43, 17174–17181. [Google Scholar] [CrossRef]
- Arp, F.F.; Bhuvanesh, N.; Blümel, J. Hydrogen Peroxide Adducts of Triarylphosphine Oxides. Dalton Trans. 2019, 48, 14312–14325. [Google Scholar] [CrossRef]
- Ahn, S.H.; Cluff, K.J.; Bhuvanesh, N.; Blümel, J. Hydrogen Peroxide and Di(hydroperoxy)propane Adducts of Phosphine Oxides as Stoichiometric and Soluble Oxidizing Agents. Angew. Chem. Int. Ed. 2015, 54, 13341–13345. [Google Scholar] [CrossRef]
- Hilliard, C.R.; Bhuvanesh, N.; Gladysz, J.A.; Blümel, J. Synthesis, Purification, and Characterization of Phosphine Oxides and their Hydrogen Peroxide Adducts. Dalton Trans. 2012, 41, 1742–1754. [Google Scholar] [CrossRef]
- Čermák, J.; Kvíčalová, M.; Šabata, S.; Blechta, V.; Vojtíšek, P.; Podlaha, J.; Shaw, B.L. Diphosphinoazines (Z,Z)-R2PCH2C(But)=NN=C(But)CH2PR2 with R Groups of Various Sizes and Complexes {[(Z,Z)-R2PCH2C(But)=NN=C(But)CH2PR2]-[η3-CH2C(CH3)=CH2PdCl]2}. Inorg. Chim. Acta 2001, 313, 77–86. [Google Scholar] [CrossRef]
- Neda, I.; Kaukorat, T.; Fischer, A.; Jones, P.G.; Schmutzler, R. Oxidationsreaktionen an 2-[2-(N,N-Dimethylamino)ethyl-methylamino]-1,3,5-trimethyl-1,3,5-triaza-2λ3-phosphorinan-4,6-dion; Hydrolyse und Thermolyse eines Perfluorpinakolylsubstituierten Spirophosphorans. J. Fluor. Chem. 1994, 69, 35–40. [Google Scholar] [CrossRef]
- Sevcik, R.; Necas, M.; Novosad, J. The Synthesis and Characterization of Three Oxidized Derivatives of bis(diphenylphosphino)pyridine and their Sn(IV) Complexes. Polyhedron 2003, 22, 1585–1593. [Google Scholar] [CrossRef]
- Wiscons, R.A.; Bellas, M.K.; Bennion, J.C.; Matzger, A.J. Detonation Performance of Ten Forms of 5,5′-Dinitro-2H,2H′-3,3′-bi-1,2,4-triazole (DNBT). Cryst. Growth Des. 2018, 18, 7701–7707. [Google Scholar] [CrossRef]
- Laus, G.; Schwärzler, A.; Bentivoglio, G.; Hummel, M.; Kahlenberg, V.; Wurst, K.; Kristeva, E.; Schütz, J.; Kopacka, H.; Kreutz, C.; et al. Synthesis and Crystal Structures of 1-Alkoxy-3-alkylimidazolium Salts Including Ionic Liquids, 1-Alkylimidazole 3-oxides and 1-Alkylimidazole Perhydrates. Z. Naturforsch. B 2008, 63, 447–464. [Google Scholar] [CrossRef]
- Jakob, F.; Herdtweck, E.; Bach, T. Synthesis and Properties of Chiral Pyrazolidines Derived from (+)-Pulegone. Chem. Eur. J. 2010, 16, 7537–7546. [Google Scholar] [CrossRef] [PubMed]
- Navasardyan, M.A.; Bezzubov, S.I.; Kuz’mina, L.G.; Prikhodchenko, P.V.; Churakov, A.V. Crystal Structure of 2,3,5,6-tetrakis(pyridin-2-yl)pyrazine Hydrogen Peroxide 4.75-solvate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1793–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churakov, A.V.; Chetina, O.V.; Howard, J.A.K. Dicyclohexylamine Hydrogen Peroxide Hemisolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o3503–o3505. [Google Scholar] [CrossRef]
- Serra, M.A.; Dorner, B.K.; Silver, M.E. Structure of an Adenine-hydrogen Peroxide Adduct. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1992, 48, 1957–1960. [Google Scholar] [CrossRef]
- Kersten, K.M.; Breen, M.E.; Mapp, A.K.; Matzger, A.J. Pharmaceutical Solvate Formation for the Incorporation of the Antimicrobial Agent Hydrogen Peroxide. Chem. Commun. 2018, 54, 9286–9289. [Google Scholar] [CrossRef]
- Bennion, J.C.; Chowdhury, N.; Kampf, J.W.; Matzger, A.J. Hydrogen Peroxide Solvates of 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane. Angew. Chem. Int. Ed. 2016, 55, 13118–13121. [Google Scholar] [CrossRef]
- Grishanov, D.A.; Navasardyan, M.A.; Medvedev, A.G.; Lev, O.; Prikhodchenko, P.V.; Churakov, A.V. Hydrogen Peroxide Insular Dodecameric and Pentameric Clusters in Peroxosolvate Structures. Angew. Chem. Int. Ed. 2017, 56, 15241–15245. [Google Scholar] [CrossRef]
- Ravikumar, K.; Sridhar, B.; Manjunatha, S.G.; Thomas, S. Risperidone N-oxide Hydrogen Peroxide Methanol Solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, o2515–o2517. [Google Scholar] [CrossRef]
- Kay Hon, P.; Mak, T.C.W. Isolation and Crystal Structures of 1,3 Molecular Complexes of TriethylenediamineN,N’-dioxide with Hydrogen Peroxide and Water. J. Crystallogr. Spectrosc. Res. 1987, 17, 419–429. [Google Scholar] [CrossRef]
- Churakov, A.V.; Prikhodchenko, P.V.; Medvedev, A.G.; Mikhaylov, A.A. Crystal Structure of (Z)-N-benzylidene-1-phenylmethanamine Oxide Hydrogen Peroxide Monosolvate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1666–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, W.; Padgett, C.W. 2,2′-Disulfanediylbis(pyridine N-oxide)–hydrogen Peroxide (1/1). IUCrData 2018, 3, x180320. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Timosheva, N.V.; Day, R.O.; Holmes, R.R. Pseudoheptacoordination and Pseudohexacoordination in Tris(2-N,N-dimethylbenzylamino)phosphane. Inorg. Chem. 2002, 41, 5235–5240. [Google Scholar] [CrossRef]
- Mak, T.C.W.; Lam, Y.-S. Hexamethylenetetramine Oxide–hydrogen Peroxide–water (1:1:1). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 1732–1735. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Huang, C.; Qi, X.; Wang, K.; Liu, Y.; Zhang, Q. Hunting for Advanced High-energy-density Materials with Well-balanced Energy and Safety through an Energetic Host–guest Inclusion Strategy. J. Mater. Chem. A 2019, 7, 19248–19257. [Google Scholar] [CrossRef]
- Laus, G.; Kahlenberg, V.; Wurst, K.; Lörting, T.; Schottenberger, H. Hydrogen Bonding in the Perhydrate and Hydrates of 1,4-diazabicyclo[2.2.2]octane (DABCO). CrystEngComm 2008, 10, 1638–1644. [Google Scholar] [CrossRef]
- Churakov, A.V.; Prikhodchenko, P.V.; Howard, J.A.K.; Lev, O. Glycine and L-serine Crystalline Perhydrates. Chem. Commun. 2009, 4224–4226. [Google Scholar] [CrossRef]
- Prikhodchenko, P.V.; Medvedev, A.G.; Tripol’skaya, T.A.; Churakov, A.V.; Wolanov, Y.; Howard, J.A.K.; Lev, O. Crystal Structures of Natural Amino Acid Perhydrates. CrystEngComm 2011, 13, 2399–2407. [Google Scholar] [CrossRef]
- Navasardyan, M.A.; Grishanov, D.A.; Tripol’skaya, T.A.; Kuz’mina, L.G.; Prikhodchenko, P.V.; Churakov, A.V. Crystal Structures of Non-proteinogenic Amino Acid Peroxosolvates: Rare Example of H-bonded Hydrogen Peroxide Chains. CrystEngComm 2018, 20, 7413–7416. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Mikhailov, A.A.; Prikhodchenko, P.V.; Tripol’skaya, T.A.; Lev, O.; Churakov, A.V. Crystal Structures of Pyridinemonocarboxylic Acid Peroxosolvates. Russ. Chem. Bull. 2013, 62, 1871–1876. [Google Scholar] [CrossRef]
- Tegenfeldt, J.; Olovsson, I. Hydrogen Bond Studies. X. The Crystal Structure of Ammonium Hydrogenperoxide. Acta Crystallogr. 1966, 21, 934–942. [Google Scholar] [CrossRef]
- Adams, J.M.; Ramdas, V. The Crystal Structure of Guanidinium Pyromellitate Triperhydrate. Inorg. Chim. Acta 1979, 34, L225–L227. [Google Scholar] [CrossRef]
- Adams, J.M.; Ramdas, V. The Crystal Structure of Guanidinium Pyromellitate Trihydrate Monoperhydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 2781–2785. [Google Scholar] [CrossRef]
- Adams, J.M.; Ramdas, V. The Crystal Structure of Guanidinium Pyrophosphate Monoperhydrate Sesquihydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 2150–2156. [Google Scholar] [CrossRef]
- Churakov, A.V.; Legurova, E.A.; Dutov, A.A.; Prikhodchenko, P.V.; Tripol’skaya, T.A. Peroxide Derivatives of Heteropoly Compounds with Keggin Anions [PW12O40]3− and [SiW12O40]4−: Synthesis and Structure. Russ. J. Inorg. Chem. 2008, 53, 1187–1192. [Google Scholar] [CrossRef]
- Farkens, M.; Meyer, T.G.; Neda, I.; Sonnenburg, R.; Müller, C.; Fischer, A.K.; Jones, P.G.; Schmutzler, R. Zur Chemie der l,3,5-Triaza-2-phosphinan-4,6-dione. Teil VI. Darstellung von 1,3,5-Triaza-2 λ3-, 1,3,5-Triaza-2 λ4- und 1,3,5-Triaza-2 λ5-phosphinan-4,6-dionen / Chemistry of the 1,3,5-Triaza-2-phosphinane-4,6-diones. Part VI. Synthesis of 1,3,5-Triaza-2 λ3-, 1,3,5-Triaza-2 λ4- and 1,3,5-Triaza-2 λ5-phosphinan-4,6-diones. Z. Naturforsch. B 1994, 49, 145–164. [Google Scholar] [CrossRef]
- Schölkopf, T.; Van, N.-D.; Schleid, T. Rb2[B12(OH)12]·2H2O and Rb2[B12(OH)12]·2H2O2: Hydrate and perhydrolate of rubidium dodecahydroxo-closo-dodecaborate. Inorg. Chim. Acta 2011, 374, 181–186. [Google Scholar] [CrossRef]
- Fidalgo, E.G.; Neels, A.; Stoeckli-Evans, H.; Süss-Fink, G. New Iso and Heteropolyoxomolybdates: Synthesis and Molecular Structure of the Anions [Mo(VI)8O26(OH)]5−, [Has(III)As(V)Mo(V)Mo(VI)8O34]6− and [HAs(III)As(V)Mo(V)Mo(VI)8O34{Co(C5H5N)2(H2O)3}]4−. Polyhedron 2002, 21, 1921–1928. [Google Scholar] [CrossRef]
- Sousa, D.P.; Bigelow, J.O.; Sundberg, J.; Que, L.; McKenzie, C.J. Caught! Crystal Trapping of a Side-on Peroxo Bound to Cr(IV). Chem. Commun. 2015, 51, 2802–2805. [Google Scholar] [CrossRef] [Green Version]
- Stomberg, R.; Szentivanyi, H.; Hämäläinen, R.; Kohl, F.X.; Seip, R. The Crystal Structure of 2,2′-Bipyridinium(1+) (2,2′-Bipyridine)oxodiperoxovanadate(1-)-(3+x)-hydrogen peroxide-(2-x)-water, (C10H9N2)[VO(O2)2(C10H8N2)]·(3+x)H2O2·(2-x)H2O, x = 0.4, at -100 degrees C. Acta Chem. Scand. 1984, 38a, 121–128. [Google Scholar] [CrossRef]
- Chohan, S.; Pritchard, R.G. Tripotassium tris(oxalato-κ 2 O,O′)aluminate bis(hydrogen peroxide) Hydrate, the First Example of a Cyclic Hydrogen-bonded H2O2 Dimer. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2003, 59, m187–m189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infantes, L.; Motherwell, S. Water Clusters in Organic Molecular Crystals. CrystEngComm 2002, 4, 454–461. [Google Scholar] [CrossRef]
- Hinrichs, F.; Adam, A. Ein neues Salz der Monoperoxokohlensäure: K2(O2)CO2·3.5H2O2. Z. Anorg. Allg. Chem. 2011, 637, 426–429. [Google Scholar] [CrossRef]
- Churakov, A.V.; Howard, J.A.K. Thymine Hydrogen Peroxide 0.55-solvate 0.45-hydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, o4483. [Google Scholar] [CrossRef]
- Navasardyan, M.A.; Grishanov, D.A.; Prikhodchenko, P.V.; Churakov, A.V. DL-Piperidinium-2-carboxylate bis(hydrogen peroxide): Unusual Hydrogen-bonded Peroxide Chains. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1331–1335. [Google Scholar] [CrossRef]
- Zubatyuk, R.I.; Sinelshchikova, A.A.; Enakieva, Y.Y.; Gorbunova, Y.G.; Tsivadze, A.Y.; Nefedov, S.E.; Bessmertnykh-Lemeune, A.; Guilard, R.; Shishkin, O.V. Insights into the Crystal Packing of Phosphorylporphyrins based on the Topology of their Intermolecular Interaction Energies. CrystEngComm 2014, 16, 10428–10438. [Google Scholar] [CrossRef]
- Colombo, V.; Presti, L.L.; Gavezzotti, A. Two-component Organic Crystals without Hydrogen Bonding: Structure and Intermolecular Interactions in Bimolecular Stacking. CrystEngComm 2017, 19, 2413–2423. [Google Scholar] [CrossRef] [Green Version]
- Masunov, A.E.; Torres, K.; Dyakov, A.A.; Yushina, I.D.; Bartashevich, E.V. First-Principles Crystal Engineering of Nonlinear Optical Materials. II. Effect of Halogen Bonds on the Structure and Properties of Triiodobenzenes. J. Phys. Chem. C 2018, 122, 22622–22631. [Google Scholar] [CrossRef]
- Evarestov, R.A. Quantum Chemistry of Solids; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–734. [Google Scholar]
- Deringer, V.L.; George, J.; Dronskowski, R.; Englert, U. Plane-Wave Density Functional Theory Meets Molecular Crystals: Thermal Ellipsoids and Intermolecular Interactions. Acc. Chem. Res. 2017, 50, 1231–1239. [Google Scholar] [CrossRef]
- Červinka, C.; Fulem, M.; Růžička, K. CCSD(T)/CBS Fragment-based Calculations of Lattice Energy of Molecular Crystals. J. Chem. Phys. 2016, 144, 064505. [Google Scholar] [CrossRef] [PubMed]
- Basilevsky, M.V.; Odinokov, A.V.; Komarova, K.G. Charge-Transfer Mobility Parameters in Photoelectronic Devices: The Advanced Miller–Abrahams Computation. J. Phys. Chem. B 2015, 119, 7430–7438. [Google Scholar] [CrossRef] [PubMed]
- Sosorev, A.Y. Role of Intermolecular Charge Delocalization and its Dimensionality in Efficient band-like Electron Transport in Crystalline 2,5-difluoro-7,7,8,8-tetracyanoquinodimethane (F2-TCNQ). Phys. Chem. Chem. Phys. 2017, 19, 25478–25486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernyshov, I.Y.; Vener, M.V.; Shenderovich, I.G. Local-structure Effects on 31P NMR Chemical Shift Tensors in Solid State. J. Chem. Phys. 2019, 150, 144706. [Google Scholar] [CrossRef]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models, 2nd ed.; Wiley: Chichester, UK, 2004; pp. 1–562. [Google Scholar]
- Chernyshov, I.Y.; Vener, M.V.; Feldman, E.V.; Paraschuk, D.Y.; Sosorev, A.Y. Inhibiting Low-Frequency Vibrations Explains Exceptionally High Electron Mobility in 2,5-Difluoro-7,7,8,8-tetracyanoquinodimethane (F2-TCNQ) Single Crystals. J. Phys. Chem. Lett. 2017, 8, 2875–2880. [Google Scholar] [CrossRef]
- Voronin, A.P.; Surov, A.O.; Churakov, A.V.; Parashchuk, O.D.; Rykounov, A.A.; Vener, M.V. Combined X-ray Crystallographic, IR/Raman Spectroscopic, and Periodic DFT Investigations of New Multicomponent Crystalline Forms of Anthelmintic Drugs: A Case Study of Carbendazim Maleate. Molecules 2020, 25, 2386. [Google Scholar] [CrossRef]
- Korlyukov, A.A.; Antipin, M.Y. Structural Studies of Crystals of Organic and Organoelement Compounds Using Modern Quantum Chemical Calculations within the Framework of the Density Functional Theory. Russ. Chem. Rev. 2012, 81, 105–129. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M. Periodic DFT Calculations—Review of Applications in the Pharmaceutical Sciences. Pharmaceutics 2020, 12, 415. [Google Scholar] [CrossRef]
- Tosoni, S.; Tuma, C.; Sauer, J.; Civalleri, B.; Ugliengo, P. A Comparison Between Plane Wave and Gaussian-type Orbital Basis Sets for Hydrogen Bonded Systems: Formic Acid as a Test Case. J. Chem. Phys. 2007, 127, 154102. [Google Scholar] [CrossRef]
- Melikova, S.M.; Voronin, A.P.; Panek, J.; Frolov, N.E.; Shishkina, A.V.; Rykounov, A.A.; Tretyakov, P.Y.; Vener, M.V. Interplay of π-stacking and Inter-stacking Interactions in Two-component Crystals of Neutral Closed-shell Aromatic Compounds: Periodic DFT Study. RSC Adv. 2020, 10, 27899–27910. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships Between Interaction Energy, Intermolecular Distance and Electron Density Properties in Hydrogen Bonded Complexes under External Electric Fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]
- Kuznetsov, M.L. Can Halogen Bond Energy be Reliably Estimated from Electron Density Properties at Bond Critical Point? The Case of the (A)nZ—Y⋯X − (X, Y = F, Cl, Br) interactions. Int. J. Quantum Chem. 2019, 119, e25869. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef] [PubMed]
- Korlyukov, A.A.; Nelyubina, Y.V. Quantum Chemical Methods in Charge Density Studies from X-ray Diffraction Data. Russ. Chem. Rev. 2019, 88, 677–716. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Tsirelson, V.G. Hydrogen Bonds and O⋯O Interactions in Proton-ordered Ices. DFT Computations with Periodic Boundary Conditions. Chem. Phys. Lett. 2010, 500, 272–276. [Google Scholar] [CrossRef]
- Borissova, A.O.; Korlyukov, A.A.; Antipin, M.Y.; Lyssenko, K.A. Estimation of Dissociation Energy in Donor−Acceptor Complex AuCl·PPh3 via Topological Analysis of the Experimental Electron Density Distribution Function. J. Phys. Chem. A 2008, 112, 11519–11522. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.V.; Yushina, I.D.; Stash, A.I.; Tsirelson, V.G. Halogen Bonding and Other Iodine Interactions in Crystals of Dihydrothiazolo(oxazino)quinolinium Oligoiodides from the Electron-Density Viewpoint. Cryst. Growth Des. 2014, 14, 5674–5684. [Google Scholar] [CrossRef]
- Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Structure-directing sulfur⋯metal Noncovalent Semicoordination Bonding. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 436–449. [Google Scholar] [CrossRef]
- Dem’yanov, P.; Polestshuk, P. A Bond Path and an Attractive Ehrenfest Force Do Not Necessarily Indicate Bonding Interactions: Case Study on M2X2 (M=Li, Na, K; X=H, OH, F, Cl). Chem. Eur. J. 2012, 18, 4982–4993. [Google Scholar] [CrossRef]
- Shahbazian, S. Why Bond Critical Points Are Not “Bond” Critical Points. Chem. Eur. J. 2018, 24, 5401–5405. [Google Scholar] [CrossRef]
- Iogansen, A.V. Direct Proportionality of the Hydrogen Bonding Energy and the Intensification of the Stretching ν(XH) Vibration in Infrared Spectra. Spectrochim. Acta Part A 1999, 55, 1585–1612. [Google Scholar] [CrossRef]
- Rozenberg, M.; Loewenschuss, A.; Marcus, Y. An Empirical Correlation Between Stretching Vibration Redshift and Hydrogen Bond Length. Phys. Chem. Chem. Phys. 2000, 2, 2699–2702. [Google Scholar] [CrossRef]
- Yukhnevich, G.V. Relationship Between the Lengths of Covalent and Intermolecular Bonds in X-H⋯Y bridges. Crystallogr. Rep. 2010, 55, 377–380. [Google Scholar] [CrossRef]
- Busing, W.R.; Levy, H.A. Crystal and Molecular Structure of Hydrogen Peroxide: A Neutron-Diffraction Study. J. Chem. Phys. 1965, 42, 3054–3059. [Google Scholar] [CrossRef]
- Vener, M.V.; Levina, E.O.; Astakhov, A.A.; Tsirelson, V.G. Specific Features of the Extra Strong Intermolecular Hydrogen Bonds in Crystals: Insights from the Theoretical Charge Density Analysis. Chem. Phys. Lett. 2015, 638, 233–236. [Google Scholar] [CrossRef]
- Medvedev, A.G.G.; Shishkina, A.V.V.; Prikhodchenko, P.V.V.; Lev, O.; Vener, M.V.V. The Applicability of the Dimeric Heterosynthon Concept to Molecules with Equivalent Binding Sites. A DFT Study of Crystalline Urea–H2O2. RSC Adv. 2015, 5, 29601–29608. [Google Scholar] [CrossRef] [Green Version]
- Katsyuba, S.A.; Vener, M.V.; Zvereva, E.E.; Fei, Z.; Scopelliti, R.; Brandenburg, J.G.; Siankevich, S.; Dyson, P.J. Quantification of Conventional and Nonconventional Charge-Assisted Hydrogen Bonds in the Condensed and Gas Phases. J. Phys. Chem. Lett. 2015, 6, 4431–4436. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Mikhaylov, A.A.; Chernyshov, I.Y.; Vener, M.V.; Lev, O.; Prikhodchenko, P.V. Effect of Aluminum Vacancies on the H2O2 or H2O Interaction with a gamma-AlOOH Surface. A Solid-state DFT Study. Int. J. Quantum Chem. 2019, 119, e25920. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Churakov, A.V.; Tsirelson, V.G. Intermolecular Hydrogen Bond Energies in Crystals Evaluated Using Electron Density Properties: DFT Computations with Periodic Boundary Conditions. J. Comput. Chem. 2012, 33, 2303–2309. [Google Scholar] [CrossRef]
- Vener, M.V.; Manaev, A.V.; Egorova, A.N.; Tsirelson, V.G. QTAIM Study of Strong H-Bonds with the O−H···A Fragment (A = O, N) in Three-Dimensional Periodical Crystals. J. Phys. Chem. A 2007, 111, 1155–1162. [Google Scholar] [CrossRef]
- Grabowski, S.J. What Is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- McKillop, A.; Sanderson, W.R. Sodium perborate and sodium percarbonate: Cheap, Safe and Versatile Oxidising Agents for Organic Synthesis. Tetrahedron 1995, 51, 6145–6166. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Kapustin, E.A.; Minkov, V.S.; Boldyreva, E.V. Oxidative Stress of H2O2 on N,N-dimethylglycine: Formation of Perhydrate Crystals and More. CrystEngComm 2014, 16, 10165–10168. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press: Oxford, UK, 2009; pp. 1–318. [Google Scholar]
- Nelyubina, Y.V.; Antipin, M.Y.; Lyssenko, K.A. Hydrogen Bonds between Zwitterions: Intermediate between Classical and Charge-Assisted Ones. A Case Study. J. Phys. Chem. A 2009, 113, 3615–3620. [Google Scholar] [CrossRef]
- Olovsson, I.; Templeton, D.H.; Rundqvist, S.; Varde, E.; Westin, G. The Crystal Structure of Hydrogen Peroxide Dihydrate. Acta Chem. Scand. 1960, 14, 1325–1332. [Google Scholar] [CrossRef] [Green Version]
- Biliškov, N.; Kojić-Prodić, B.; Mali, G.; Molćanov, K.; Stare, J. A Partial Proton Transfer in Hydrogen Bond O−H···O in Crystals of Anhydrous Potassium and Rubidium Complex Chloranilates. J. Phys. Chem. A 2011, 115, 3154–3166. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Vener, M.V.; Medvedev, A.G.; Churakov, A.V.; Prikhodchenko, P.V.; Tripol’skaya, T.A.; Lev, O. H-Bond Network in Amino Acid Cocrystals with H2O or H2O2. The DFT Study of Serine–H2O and Serine–H2O2. J. Phys. Chem. A 2011, 115, 13657–13663. [Google Scholar] [CrossRef]
- Redington, R.L.; Olson, W.B.; Cross, P.C. Studies of Hydrogen Peroxide: The Infrared Spectrum and the Internal Rotation Problem. J. Chem. Phys. 1962, 36, 1311–1326. [Google Scholar] [CrossRef]
- Oelfke, W.C.; Gordy, W. Millimeter-Wave Spectrum of Hydrogen Peroxide. J. Chem. Phys. 1969, 51, 5336–5343. [Google Scholar] [CrossRef]
- Savariault, J.M.; Lehmann, M.S. Experimental Determination of the Deformation Electron Density in Hydrogen Peroxide by Combination of X-ray and Neutron Diffraction Measurements. J. Am. Chem. Soc. 1980, 102, 1298–1303. [Google Scholar] [CrossRef]
- Minkov, V.S.; Kapustin, E.A.; Boldyreva, E.V. Betaine 0.77-perhydrate 0.23-hydrate and Common Structural Motifs in Crystals of Amino Acid Perhydrates. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2013, 69, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Rozas, I.; Alkorta, I.; Elguero, J. Bifurcated Hydrogen Bonds: Three-Centered Interactions. J. Phys. Chem. A 1998, 102, 9925–9932. [Google Scholar] [CrossRef]
- Tupikina, E.Y.; Bodensteiner, M.; Tolstoy, P.M.; Denisov, G.S.; Shenderovich, I.G. P═O Moiety as an Ambidextrous Hydrogen Bond Acceptor. J. Phys. Chem. C 2018, 122, 1711–1720. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical Cocrystals: Walking the Talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Serushkina, O.V.; Muravyev, N.V.; Meerov, D.B.; Miroshnichenko, E.A.; Kon’kova, T.S.; Suponitsky, K.Y.; Vener, M.V.; Sheremetev, A.B. Azasydnone—Novel “Green” Building Block for Designing High Energetic Compounds. J. Mater. Chem. A 2018, 6, 18669–18676. [Google Scholar] [CrossRef]
- Lehn, J.M. Cryptates: Inclusion Complexes of Macropolycyclic Receptor Molecules. Pure Appl. Chem. 1978, 50, 871–892. [Google Scholar] [CrossRef]
- Liu, Z.; Nalluri, S.K.M.; Stoddart, J.F. Surveying Macrocyclic Chemistry: From Flexible Crown Ethers to Rigid Cyclophanes. Chem. Soc. Rev. 2017, 46, 2459–2478. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapötke, T.M. Nitrogen-Rich Bis-1,2,4-triazoles-A Comparative Study of Structural and Energetic Properties. Chem. Eur. J. 2012, 18, 16742–16753. [Google Scholar] [CrossRef]
- Luo, J.; Xia, H.; Zhang, W.; Song, S.; Zhang, Q. A Promising Hydrogen Peroxide Adduct of Ammonium Cyclopentazolate as a Green Propellant Component. J. Mater. Chem. A 2020, 8, 12334–12338. [Google Scholar] [CrossRef]
- Manin, A.N.; Voronin, A.P.; Shishkina, A.V.; Vener, M.V.; Churakov, A.V.; Perlovich, G.L. Influence of Secondary Interactions on the Structure, Sublimation Thermodynamics, and Solubility of Salicylate: 4-Hydroxybenzamide Cocrystals. Combined Experimental and Theoretical Study. J. Phys. Chem. B 2015, 119, 10466–10477. [Google Scholar] [CrossRef] [PubMed]


















| Crystal (Coformer) | The Number of H-Bonds | d(O⋯O−)/Å | |
|---|---|---|---|
| 1 | 2 | ||
| l-Phenylalanine | 2 | 2.634(2) | 2.631(2) |
| l-Isoleucine | 2 | 2.652(2) | 2.634(2) |
| l-Isoleucine | 2 | 2.707(2) | 2.678(2) |
| 2-Aminobutiric acid | 2 | 2.697(1) | 2.776(1) |
| 2 | 2.607(1) | 2.664(1) | |
| 2 | 2.682(1) | 2.717(1) | |
| l-Serine | 1 | 2.706(2) | - |
| Glycine | 2 | 2.648(1) | 2.671(1) |
| 2 | 2.671(1) | 2.636(1) | |
| 2 | 2.645(1) | 2.635(1) | |
| l-Tyrosine | 1 | 2.604(4) | 2.760(3) |
| l-Threonine | 1 | 2.637(2) | - |
| β-Alanine | 2 | 2.666(1) | 2.686(1) |
| 2 | 2.725(1) | 2.753(1) | |
| 26 H-bonds | 2.604–2.776 | ||
| Mean value | 2.67 | ||
| Crystal (Coformer) | The Number of H-Bonds | The Proton Donor Group |
|---|---|---|
| l-Phenylalanine | 2 | –NH3+, –NH3+ |
| l-Isoleucine | 2 | –NH3+, –NH3+ |
| l-Isoleucine | 1 | –NH3+ |
| 2-Aminobutiric acid | 2 | –NH3+, –NH3+ |
| 1 | –NH3+ | |
| 1 | –NH3+ | |
| l-Serine | 2 | –NH3+, –NH3+ |
| Glycine | 2 | –NH3+, –NH3+ |
| 2 | –NH3+, –NH3+ | |
| 1 | –NH3+ | |
| l-Tyrosine | 2 | –NH3+, H2O2 |
| 1 | –NH3+ | |
| l-Threonine | 3 | –NH3+, –NH3+, –NH3+ |
| β-Alanine | 2 | –NH3+, –NH3+ |
| 0 | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medvedev, A.G.; Churakov, A.V.; Prikhodchenko, P.V.; Lev, O.; Vener, M.V. Crystalline Peroxosolvates: Nature of the Coformer, Hydrogen-Bonded Networks and Clusters, Intermolecular Interactions. Molecules 2021, 26, 26. https://doi.org/10.3390/molecules26010026
Medvedev AG, Churakov AV, Prikhodchenko PV, Lev O, Vener MV. Crystalline Peroxosolvates: Nature of the Coformer, Hydrogen-Bonded Networks and Clusters, Intermolecular Interactions. Molecules. 2021; 26(1):26. https://doi.org/10.3390/molecules26010026
Chicago/Turabian StyleMedvedev, Alexander G., Andrei V. Churakov, Petr V. Prikhodchenko, Ovadia Lev, and Mikhail V. Vener. 2021. "Crystalline Peroxosolvates: Nature of the Coformer, Hydrogen-Bonded Networks and Clusters, Intermolecular Interactions" Molecules 26, no. 1: 26. https://doi.org/10.3390/molecules26010026
APA StyleMedvedev, A. G., Churakov, A. V., Prikhodchenko, P. V., Lev, O., & Vener, M. V. (2021). Crystalline Peroxosolvates: Nature of the Coformer, Hydrogen-Bonded Networks and Clusters, Intermolecular Interactions. Molecules, 26(1), 26. https://doi.org/10.3390/molecules26010026