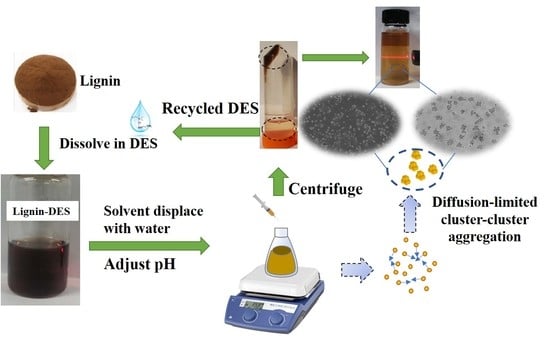
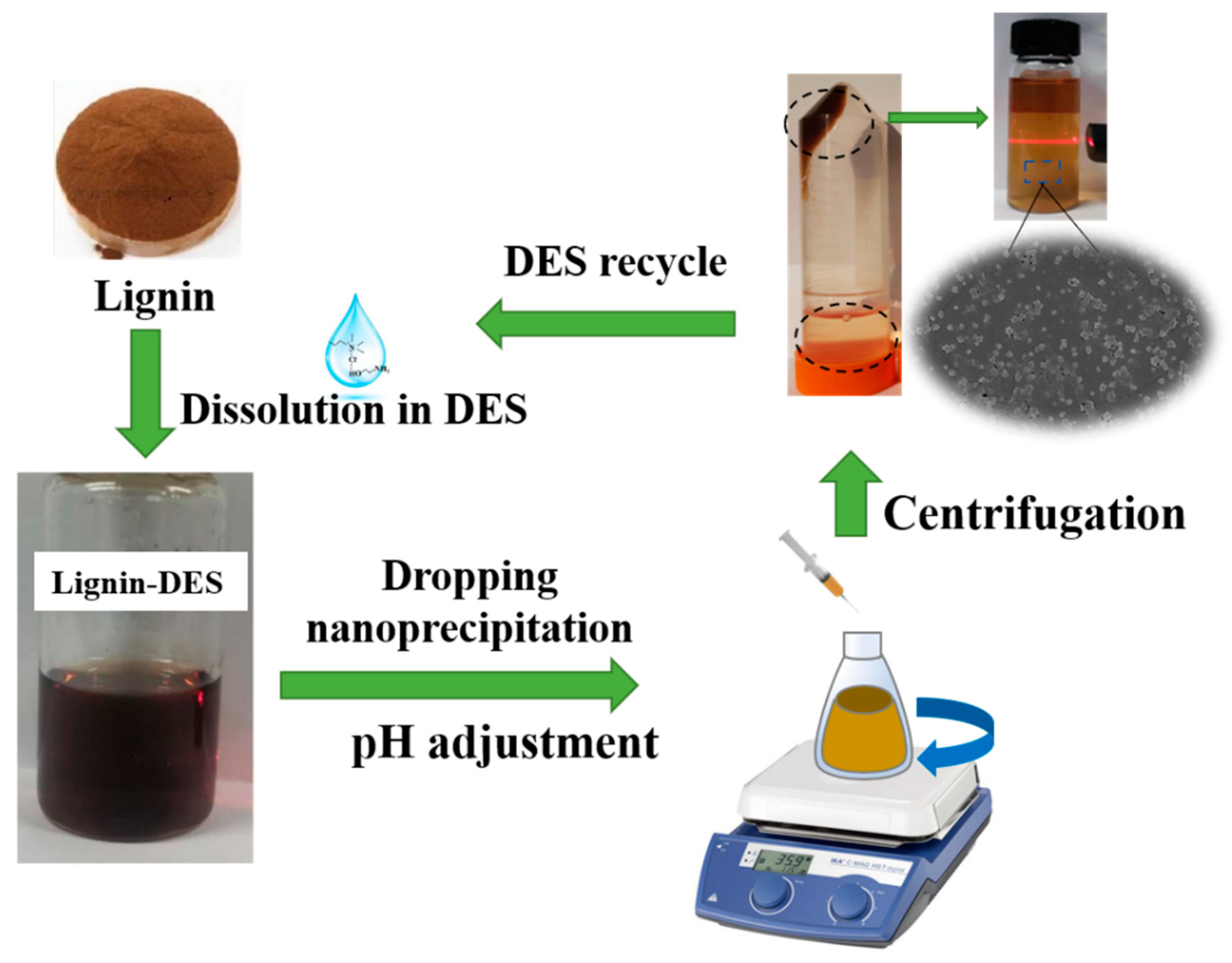
Preparation and Characterization of Size-Controlled Lignin Nanoparticles with Deep Eutectic Solvents by Nanoprecipitation
Abstract
1. Introduction
2. Results and Discussion
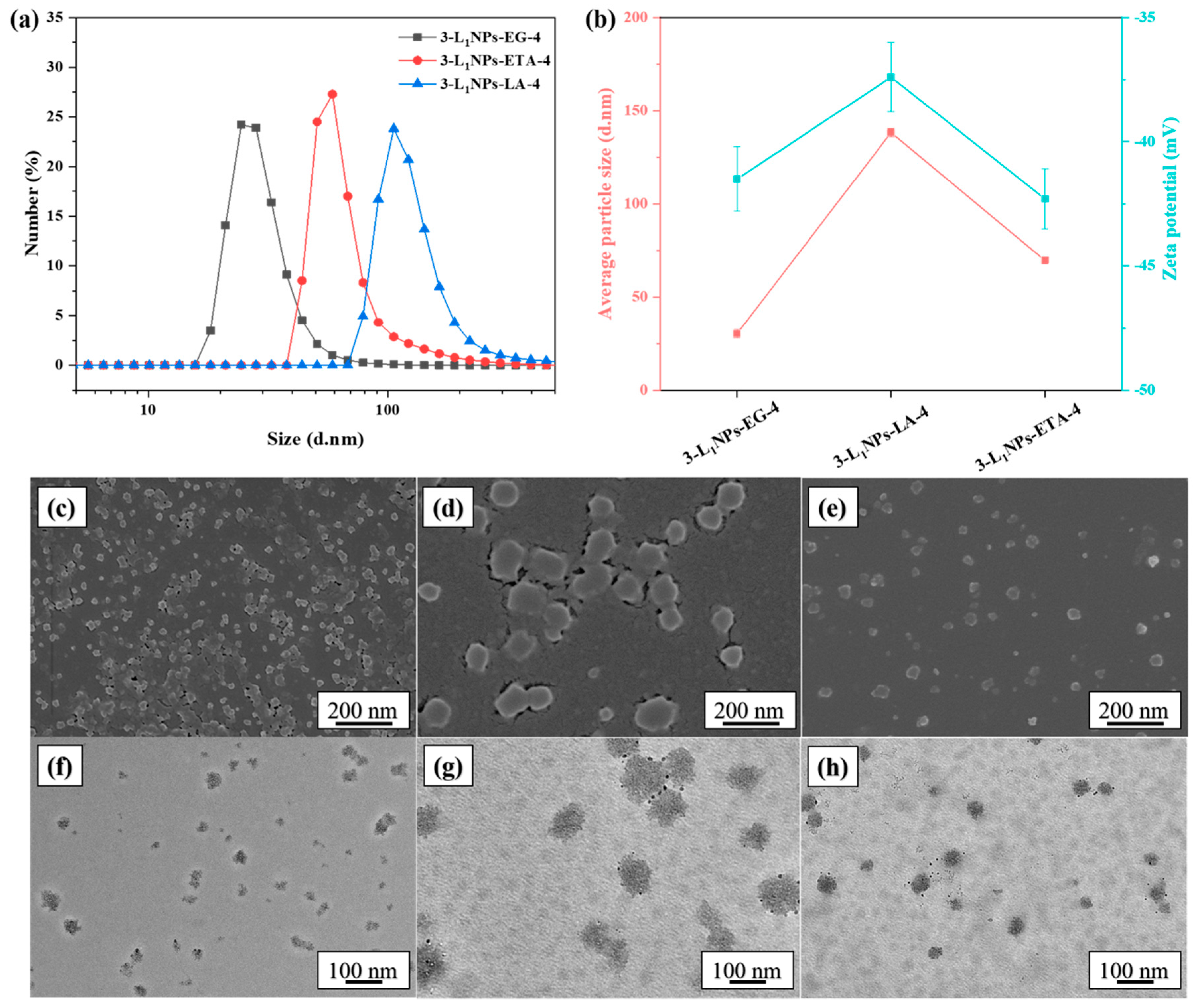
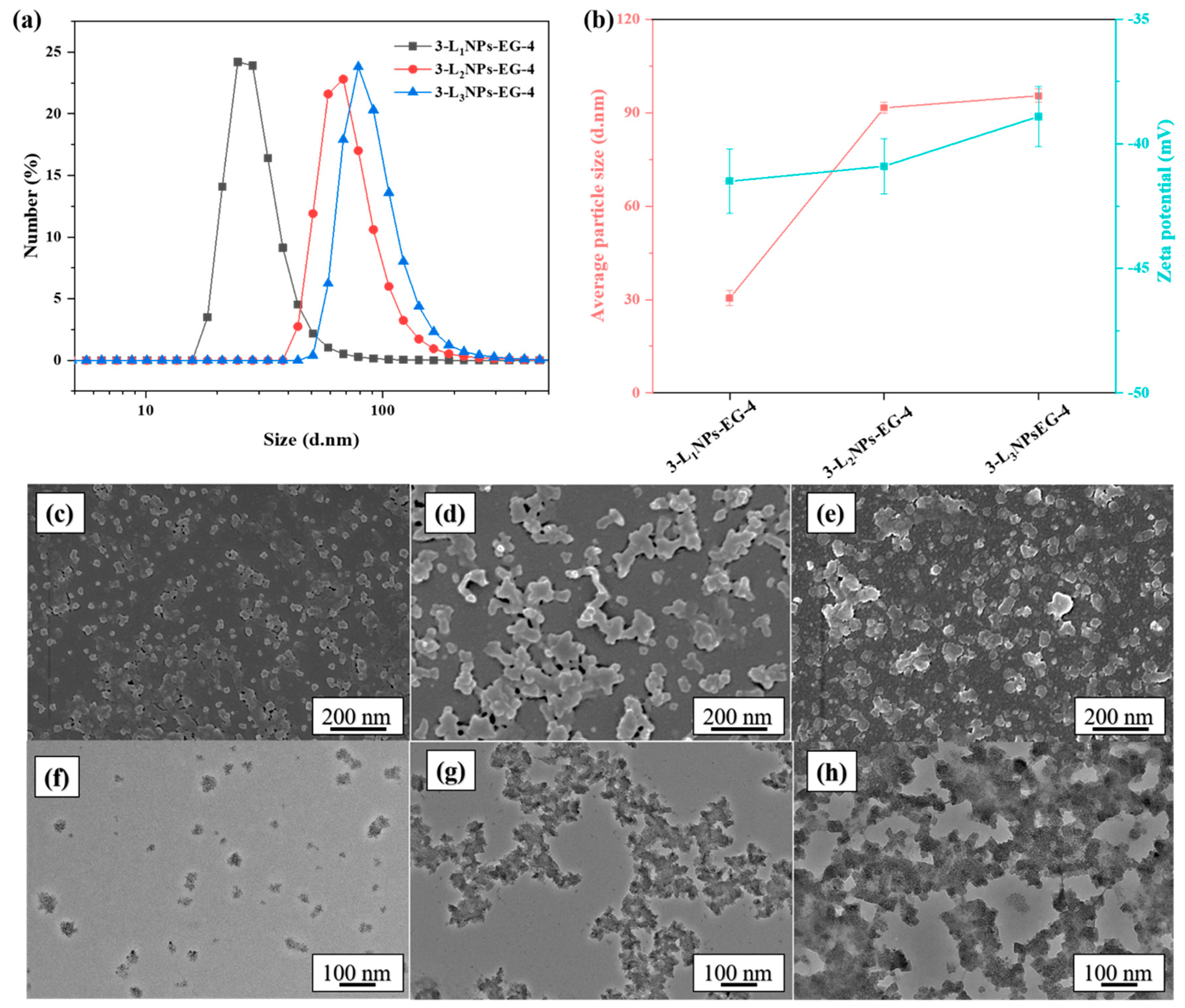
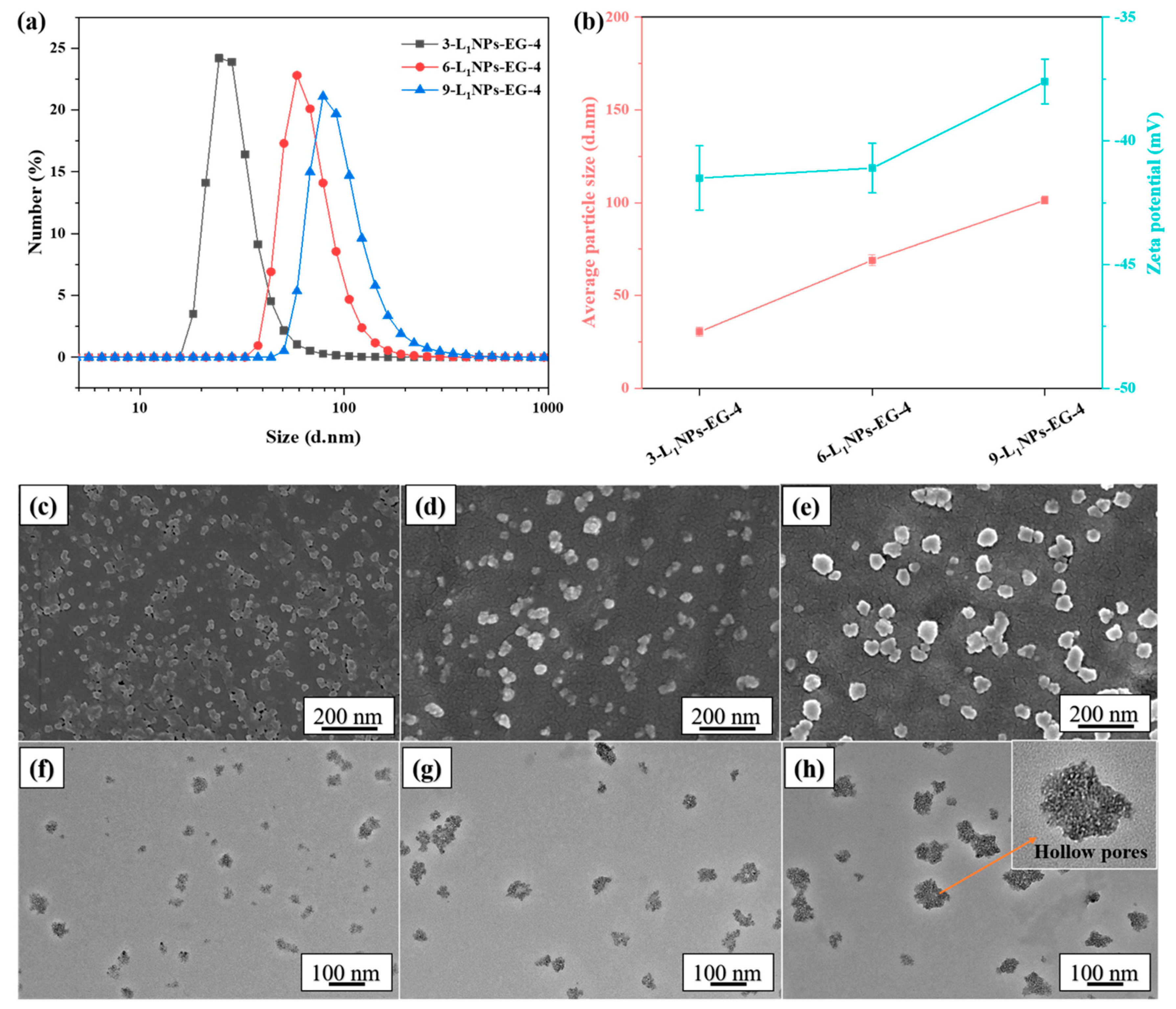
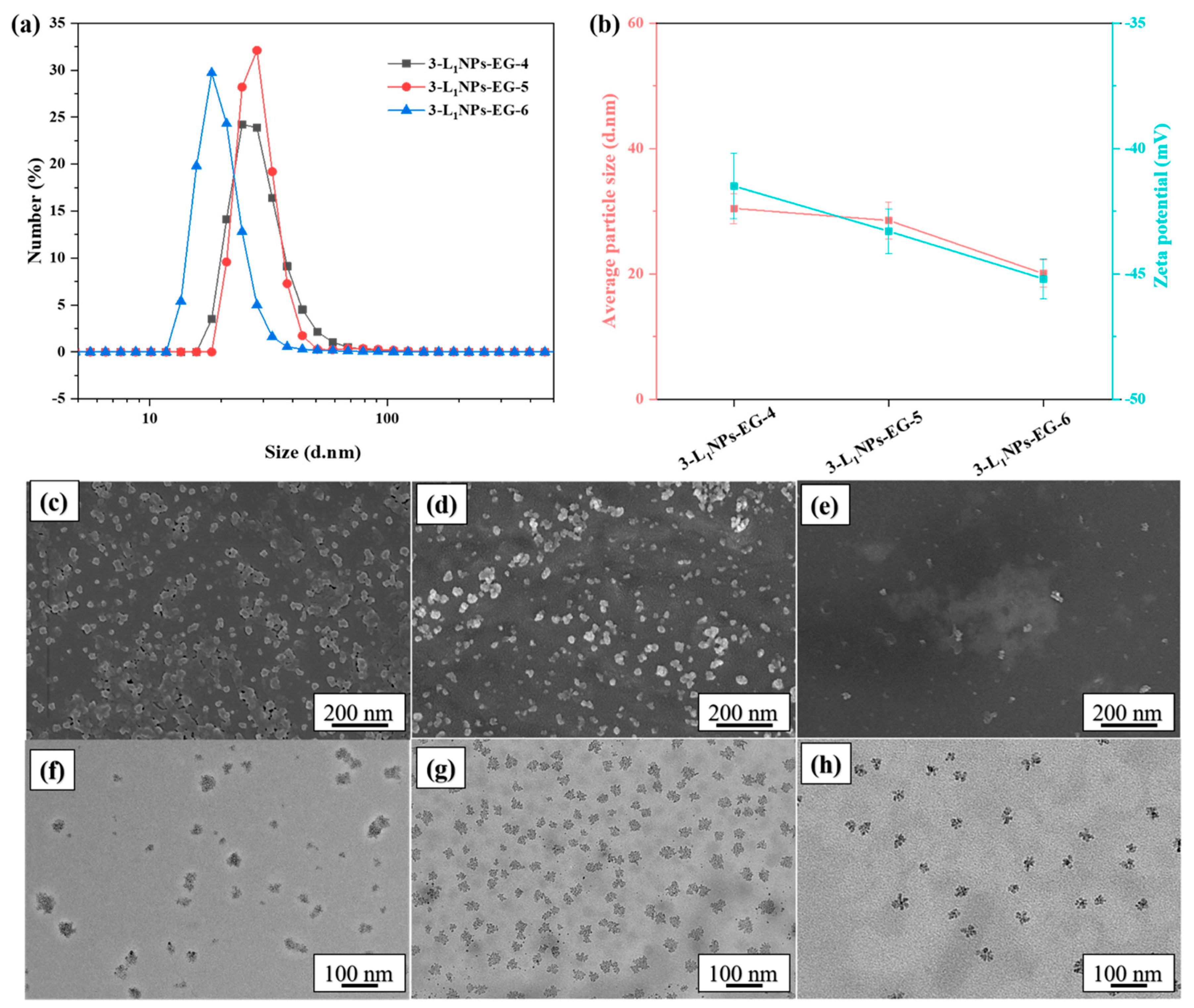
2.1. Effects of Different Conditions on Morphology and Size of LNPs
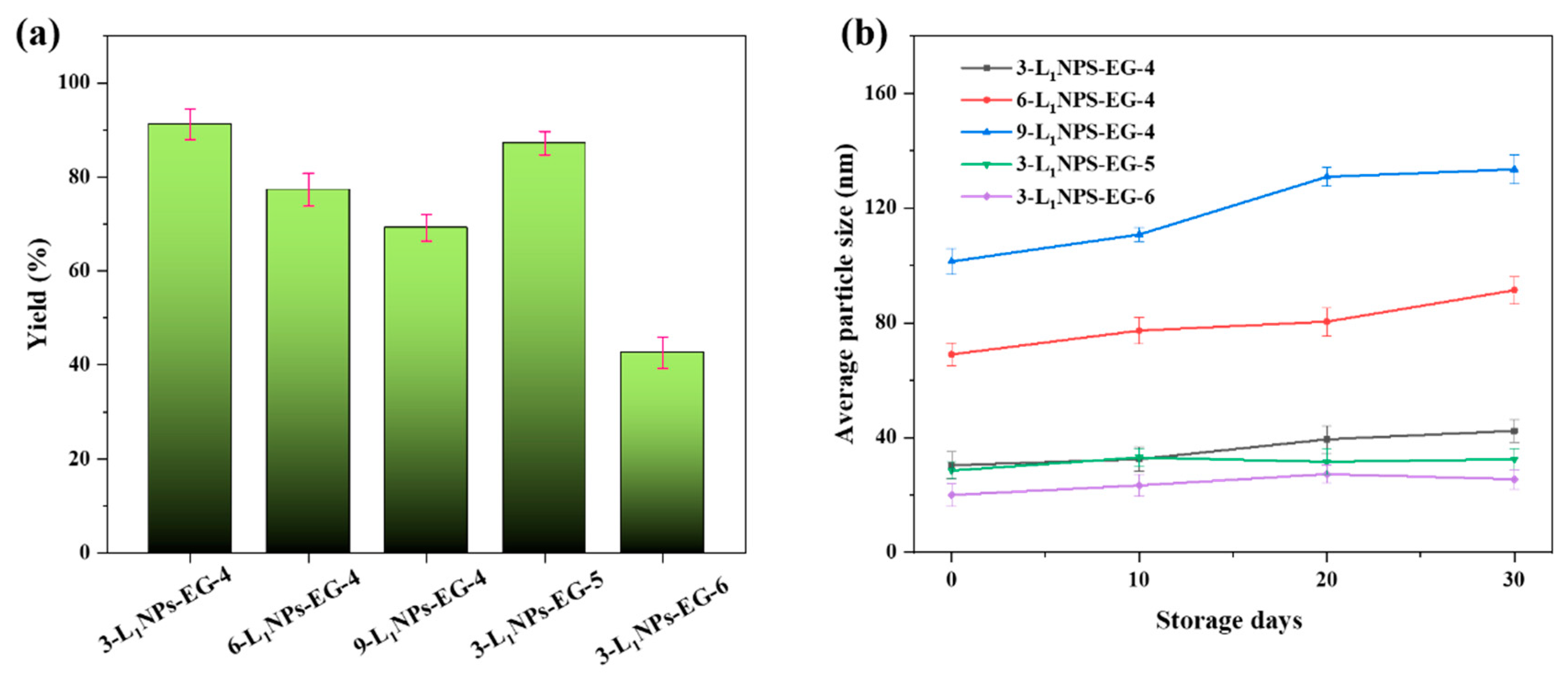
2.2. Yield and Stability of LNPs
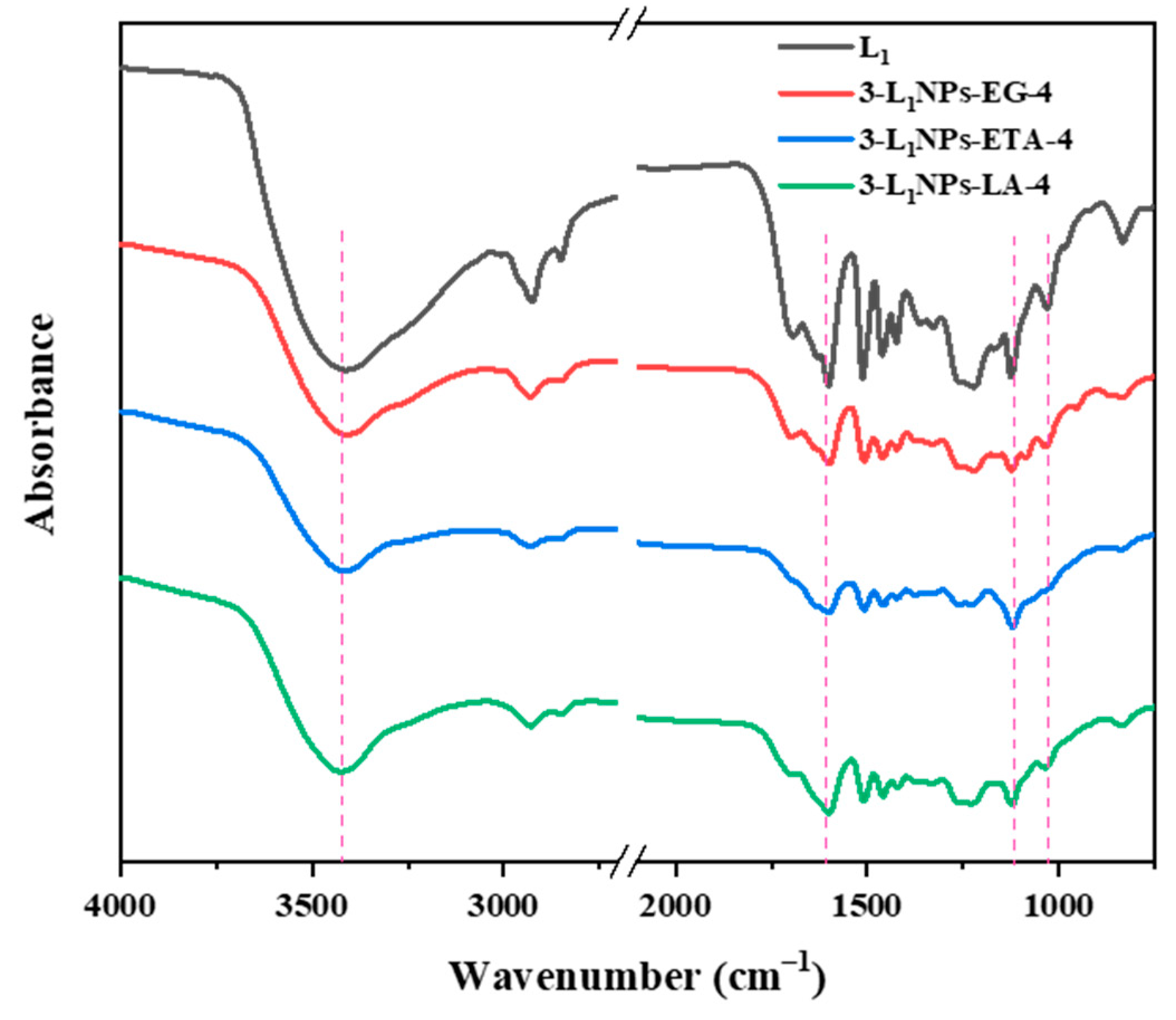
2.3. Physicochemical Properties of Lignin and the LNPs
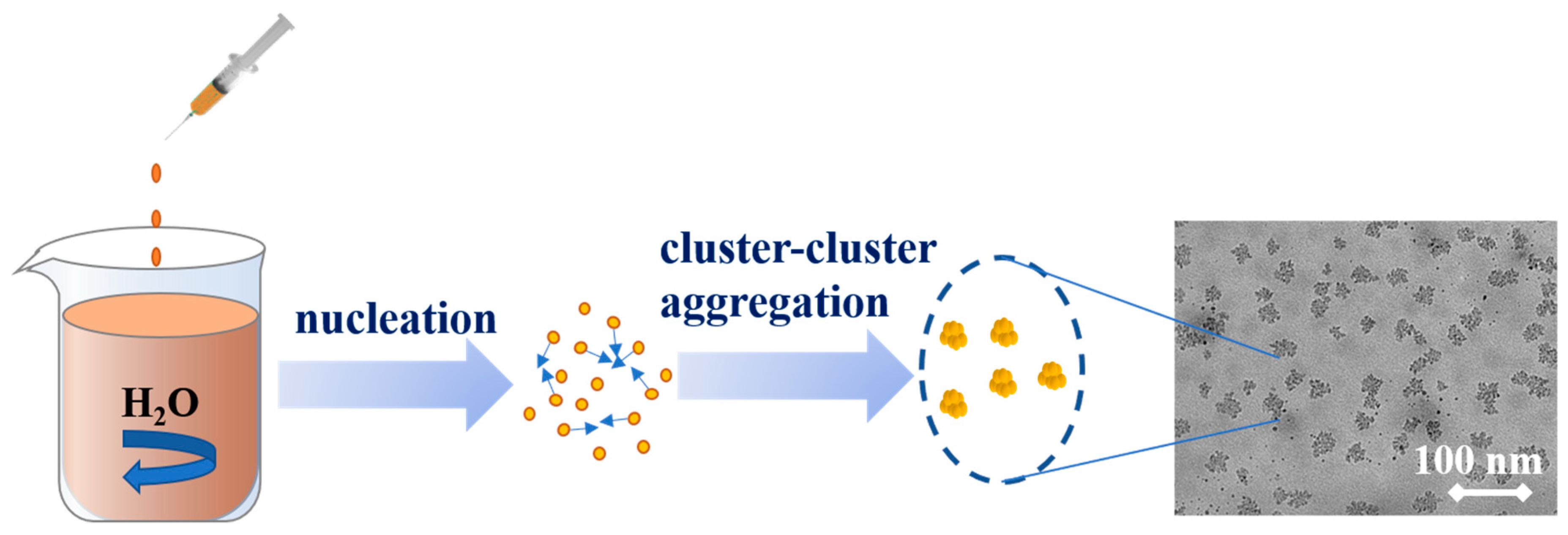
2.4. Formation Mechanism of LNPs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of DESs
3.3. Control of Process Parameters for LNP Synthesis
3.4. Characterizations of LNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buono, P.; Duval, A.; Avérous, L.; Habibi, Y. Clicking Biobased Polyphenols: A Sustainable Platform for Aromatic Polymeric Materials. ChemSusChem 2018, 11, 2472–2491. [Google Scholar] [CrossRef]
- Decostanzi, M.; Auvergne, R.; Boutevin, B.; Caillol, S. Biobased phenol and furan derivative coupling for the synthesis of functional monomers. Green Chem. 2019, 21, 724–747. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Osterberg, M. Lignin for Nano- and Microscaled Carrier Systems: Applications, Trends, and Challenges. ChemSusChem 2019, 12, 2039–2054. [Google Scholar] [CrossRef]
- Allegretti, C.; Boumezgane, O.; Rossato, L.; Strini, A.; Troquet, J.; Turri, S.; Griffini, G.; D’Arrigo, P. Tuning Lignin Characteristics by Fractionation: A Versatile Approach Based on Solvent Extraction and Membrane-Assisted Ultrafiltration. Molecules 2020, 25, 2893. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, microand nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Eraghi Kazzaz, A.; AlipoorMazandarani, N.; Hosseinpour Feizi, Z.; Fatehi, P. Production of Flocculants, Adsorbents, and Dispersants from Lignin. Molecules 2018, 23, 868. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Gao, B.; Zhao, Y.; Jiang, Y.; Zha, Z.; Xue, W.; Gong, L. Lignin Nanoparticles: Green Synthesis in a γ-Valerolactone/Water Binary Solvent and Application to Enhance Antimicrobial Activity of Essential Oils. ACS Sustain. Chem. Eng. 2019, 8, 714–722. [Google Scholar] [CrossRef]
- Sun, R. Across the Board: Runcang Sun on Lignin Nanoparticles. ChemSusChem 2020, 13, 1–4. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Posoknistakul, P.; Tangkrakul, C.; Chaosuanphae, P.; Deepentham, S.; Techasawong, W.; Phonphirunrot, N.; Bairak, S.; Sakdaronnarong, C.; Laosiripojana, N. Fabrication and Characterization of Lignin Particles and Their Ultraviolet Protection Ability in PVA Composite Film. ACS Omega 2020, 5, 20976–20982. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, D.; Wang, H.M.; Yuan, T.Q.; Sun, R.C. Green and Facile Preparation of Regular Lignin Nanoparticles with High Yield and Their Natural Broad-Spectrum Sunscreens. ACS Sustain. Chem. Eng. 2018, 7, 2658–2666. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Yang, W.; Rallini, M.; Wang, D.Y.; Gao, D.; Dominici, F.; Torre, L.; Kenny, J.M.; Puglia, D. Role of lignin nanoparticles in UV resistance, thermal and mechanical performance of PMMA nanocomposites prepared by a combined free-radical graft polymerization/masterbatch procedure. Compos. Part A Appl. Sci. Manufac. 2018, 107, 61–69. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 192. [Google Scholar] [CrossRef]
- Osterberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef]
- Rasool, M.A.; Vankelecom, I.F.J. Use of γ-valerolactone and glycerol derivatives as bio-based renewable solvents for membrane preparation. Green Chem. 2019, 21, 1054–1064. [Google Scholar] [CrossRef]
- Kim, K.H.; Dutta, T.; Sun, J.; Simmons, B.; Singh, S. Biomass pretreatment using deep eutectic solvents from lignin derived phenols. Green Chem. 2018, 20, 809–815. [Google Scholar] [CrossRef]
- Shen, X.J.; Chen, T.; Wang, H.M.; Mei, Q.; Yue, F.; Sun, S.; Wen, J.L.; Yuan, T.Q.; Sun, R.C. Structural and Morphological Transformations of Lignin Macromolecules during Bio-Based Deep Eutectic Solvent (DES) Pretreatment. ACS Sustain. Chem. Eng. 2019, 8, 2130–2137. [Google Scholar] [CrossRef]
- Lou, R.; Ma, R.; Lin, K.T.; Ahamed, A.; Zhang, X. Facile Extraction of Wheat Straw by Deep Eutectic Solvent (DES) to Produce Lignin Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 10248–10256. [Google Scholar] [CrossRef]
- Sosa, F.H.B.; Abranches, D.O.; da Costa Lopes, A.M.; Coutinho, J.A.P.; da Costa, M.C. Kraft Lignin Solubility and Its Chemical Modification in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 18577–18589. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, S.; Wu, C.; Liu, Y.; Yang, G.; Ni, Y. Super-stable, solvent-resistant and uniform lignin nanorods and nanospheres with a high yield in a mild and facile process. Green Chem. 2020, 22, 8734–8744. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Hou, Y. A simple environment-friendly process for preparing high-concentration alkali lignin nanospheres. Eur. Polym. J. 2019, 112, 15–23. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crops Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Shi, Y.; Gao, B.; Wu, J.; Kirk, T.B.; Xu, J.; Xue, W. Green synthesis of lignin nanoparticle in aqueous hydrotropic solution toward broadening the window for its processing and application. Chem. Eng. J. 2018, 346, 217–225. [Google Scholar] [CrossRef]
- Jin, J.; Iyoda, T.; Cao, C.S.; Song, Y.L.; Jiang, L.; Li, T.J.; Zhu, D.B. Self-assembly of uniform spherical aggregates of magnetic nanoparticles through π-π interactions. Angew. Chem. Int. Ed. 2001, 40, 2135–2138. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.Y.; Choi, I.-G.; Choi, J.W. Investigation of Molecular Size Effect on the Formation of Lignin Nanoparticles by Nanoprecipitation. Appl. Sci. 2020, 10, 4910. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.Q.; Zou, Z.-F.; Si, C.L. Lignin Nanoparticle as a Novel Green Carrier for the Efficient Delivery of Resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and Formation Mechanism of Renewable Lignin Hollow Nanospheres with a Single Hole by Self-Assembly. ACS Sustain. Chem. Eng. 2017, 5, 2273–2281. [Google Scholar] [CrossRef]
- Ma, M.; Dai, L.; Si, C.; Hui, L.; Liu, Z.; Ni, Y. A Facile Preparation of Super Long-Term Stable Lignin Nanoparticles from Black Liquor. ChemSusChem 2019, 12, 5239–5245. [Google Scholar] [CrossRef] [PubMed]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.L.; Hult, E.L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Pang, T.; Wang, G.; Sun, H.; Wang, L.; Liu, Q.; Sui, W.; Parvez, A.M.; Si, C. Lignin Fractionation for Reduced Heterogeneity in Self-Assembly Nanosizing: Toward Targeted Preparation of Uniform Lignin Nanoparticles with Small Size. ACS Sustain. Chem. Eng. 2020, 8, 9174–9183. [Google Scholar] [CrossRef]
- Casas, A.; Oliet, M.; Alonso, M.; Rodriguez, F. Dissolution of Pinus radiata and Eucalyptus globulus woods in ionic liquids under microwave radiation: Lignin regeneration and characterization. Sep. Purif. Technol. 2012, 97, 115–122. [Google Scholar] [CrossRef]
- Matsakas, L.; Karnaouri, A.; Cwirzen, A.; Rova, U.; Christakopoulos, P. Formation of Lignin Nanoparticles by Combining Organosolv Pretreatment of Birch Biomass and Homogenization Processes. Molecules 2018, 23, 1822. [Google Scholar] [CrossRef]
- Zhong, L.; Yang, L.; Wang, C.; Ji, X.; Yang, G.; Chen, J.; Lyu, G.; Xu, F.; Yoo, C.G. NaOH-Aided Sulfolane Pretreatment for Effective Fractionation and Utilization of Willow (Salix matsudana cv. Zhuliu). Ind. Eng. Chem. Res. 2020, 59, 17546–17553. [Google Scholar] [CrossRef]
- Lepeltier, E.; Bourgaux, C.; Couvreur, P. Nanoprecipitation and the “Ouzo effect”: Application. Adv. Drug Deliv. Rev. 2014, 71, 86–97. [Google Scholar] [CrossRef]
- Zhong, L.; Xu, M.; Wang, C.; Shao, L.; Mao, J.; Jiang, W.; Ji, X.; Yang, G.; Chen, J.; Lyu, G.; et al. Pretreatment of willow using the alkaline-catalyzed sulfolane/water solution for high-purity and antioxidative lignin production. Int. J. Biol. Macromol. 2020, 159, 287–294. [Google Scholar] [CrossRef]
| Sample | Material | DES System | Pre-Dropping Concentration | pH Value |
|---|---|---|---|---|
| 3-L1NPs-EG-4 | L1 | ChCl and EG | 3 wt % | 4 |
| 6-L1NPs-EG-4 | L1 | ChCl and EG | 6 wt % | 4 |
| 9-L1NPs-EG-4 | L1 | ChCl and EG | 9 wt % | 4 |
| 3-L1NPs-EG-5 | L1 | ChCl and EG | 3 wt % | 5 |
| 3-L1NPs-EG-6 | L1 | ChCl and EG | 3 wt % | 6 |
| 3-L1NPs-ETA-4 | L1 | ChCl and ETA | 3 wt % | 4 |
| 3-L1NPs-LA-4 | L1 | ChCl and LA | 3 wt % | 4 |
| 3-L2NPs-EG-4 | L2 | ChCl and EG | 3 wt % | 4 |
| 3-L3NPs-EG-4 | L3 | ChCl and EG | 3 wt % | 4 |
| Sample | Mn | Mw | PDI |
|---|---|---|---|
| L1 | 1400 | 3400 | 2.43 |
| L2 | 1800 | 3900 | 2.17 |
| L3 | 1100 | 2700 | 2.45 |
| 3-L1NPs-EG-4 | 1300 | 3000 | 2.31 |
| 6-L1NPs-EG-4 | 1600 | 3400 | 2.13 |
| 9-L1NPs-EG-4 | 1500 | 3100 | 2.07 |
| 3-L1NPs-EG-5 | 1600 | 3500 | 2.19 |
| 3-L1NPs-EG-6 | 1300 | 4200 | 3.23 |
| 3-L1NPs-ETA-4 | 1500 | 3000 | 2.00 |
| 3-L1NPs-LA-4 | 1400 | 3000 | 2.14 |
| 3-L2NPs-EG-4 | 1700 | 3700 | 2.18 |
| 3-L3NPs-EG-4 | 1000 | 2500 | 2.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Wang, C.; Ji, X.; Yang, G.; Chen, J.; Janaswamy, S.; Lyu, G. Preparation and Characterization of Size-Controlled Lignin Nanoparticles with Deep Eutectic Solvents by Nanoprecipitation. Molecules 2021, 26, 218. https://doi.org/10.3390/molecules26010218
Luo T, Wang C, Ji X, Yang G, Chen J, Janaswamy S, Lyu G. Preparation and Characterization of Size-Controlled Lignin Nanoparticles with Deep Eutectic Solvents by Nanoprecipitation. Molecules. 2021; 26(1):218. https://doi.org/10.3390/molecules26010218
Chicago/Turabian StyleLuo, Tong, Chao Wang, Xingxiang Ji, Guihua Yang, Jiachuan Chen, Srinivas Janaswamy, and Gaojin Lyu. 2021. "Preparation and Characterization of Size-Controlled Lignin Nanoparticles with Deep Eutectic Solvents by Nanoprecipitation" Molecules 26, no. 1: 218. https://doi.org/10.3390/molecules26010218
APA StyleLuo, T., Wang, C., Ji, X., Yang, G., Chen, J., Janaswamy, S., & Lyu, G. (2021). Preparation and Characterization of Size-Controlled Lignin Nanoparticles with Deep Eutectic Solvents by Nanoprecipitation. Molecules, 26(1), 218. https://doi.org/10.3390/molecules26010218