Kinetic Study on Alpha-Form Crystallization of Mixed-Acid Triacylglycerols POP, PPO, and Their Mixture
Abstract
:1. Introduction
2. Results and Discussion
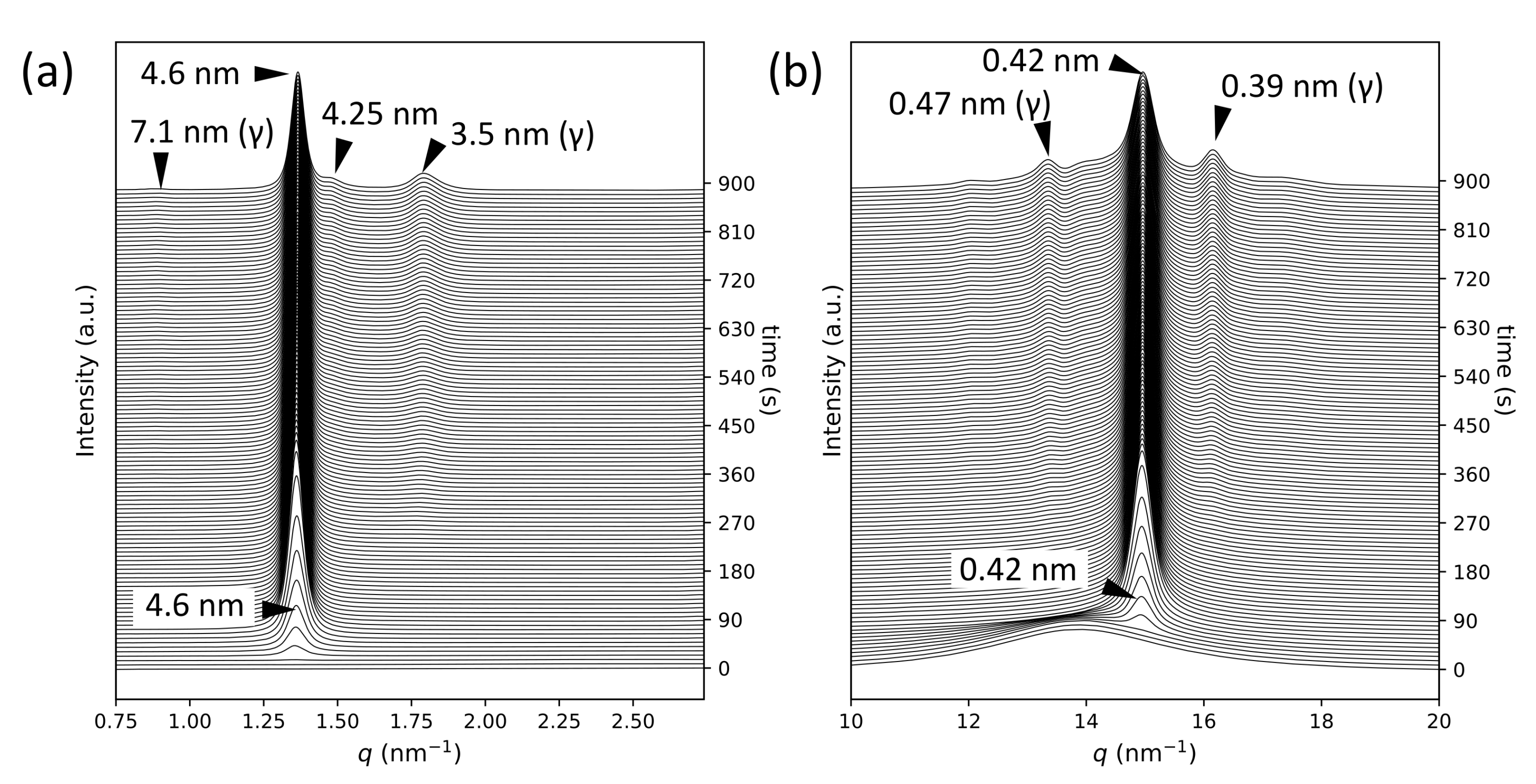
2.1. SR-TRXRD Measurements
2.2. POM Observations
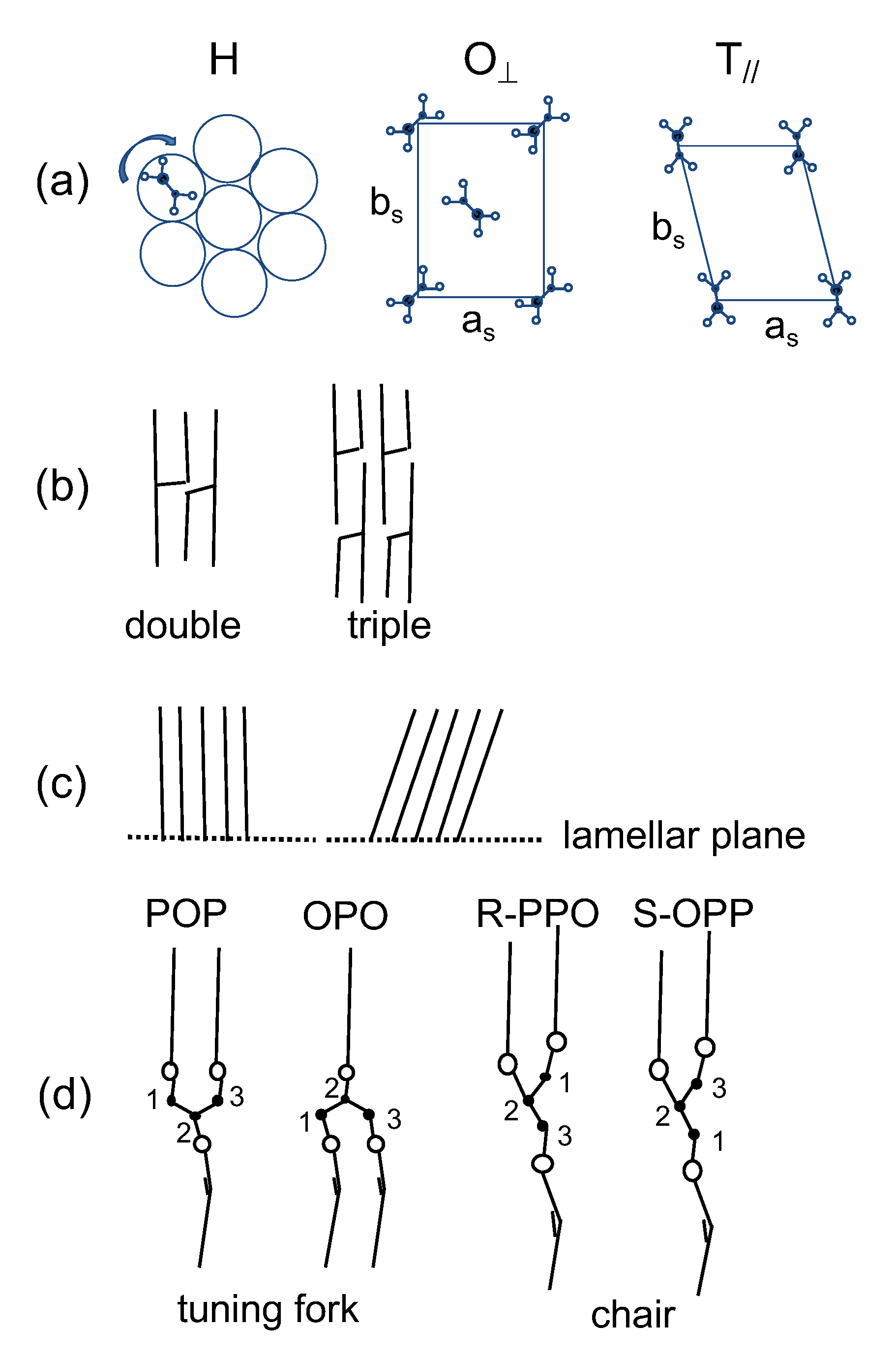
2.3. Complex Crystallization Behavior of Forms
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MC | molecular compound |
| POP | 1,3-dipalmitoyl-2-oleoyl-sn-glycerol |
| rac-PPO | 1,2-dipalmitoyl-3-rac-oleoyl-glycerol |
| OPO | 1,3-dioleoyl-2-palmitoyl-sn-glycerol |
| LS | long spacing |
| SS | short spacing |
| SR-TRXRD | synchrotron radiation time-resolved X-ray diffraction |
| POM | polarized optical microscopy |
| WAXS | wide angle X-ray scattering |
| SAXS | small-angle X-ray scattering |
References
- Sato, K. (Ed.) Polymorphism of lipid crystals. In Crystallization of Lipids. Fundamentals and Applications in Food, Cosmetics, and Pharmaceuticals; Wiley-Blackwell: Hoboken, NJ, USA, 2018; Chapter 2; pp. 17–60. [Google Scholar]
- Sato, K.; Bayés-García, L.; Calvet, T.; Cuevas-Diarte, M.; Satoru, U. External factors affecting polymorphic crystallization of lipids. Eur. J. Lipid Sci. Technol. 2013, 115, 1224–1238. [Google Scholar] [CrossRef]
- Acevedo, N.; Marangoni, A. Nanostructure and microstructure of fats. Annu. Rev. Food Sci. Technol. 2015, 6, 71–96. [Google Scholar] [CrossRef]
- Bayés-García, L.; Patel, A.; Dewettinck, K.; Rousseau, D.; Sato, K.; Ueno, S. Lipid crystallization kinetics-roles of external factors influencing functionality of end products. Curr. Opin. Food Sci. 2015, 32–38. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles. Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Cholakova, D.; Glushkova, D.; Tcholakova, S.; Denkov, N. Nanopore and Nanoparticle Formation with Lipids Undergoing Polymorphic Phase Transitions. Cryst. Growth Des. 2020, 14, 8594–8604. [Google Scholar] [CrossRef] [PubMed]
- Awad, T.S.; Helgason, T.; Kristbergsson, K.; Decker, E.A.; Weiss, J.; McClements, D.J. Effect of cooling and heating rates on polymorphic transformations and gelation of tripalmitin solid lipid nanoparticle (SLN) suspensions. Food Biophys. 2008, 3, 155–162. [Google Scholar] [CrossRef]
- da Silva Santos, V.; Ribeiro, A.P.B.; Santana, M.L.A. Solid lipid nanoparticles as carriers for lipophilic compounds for applications in foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef]
- Schoenitz, M.; Joseph, S.; Bunjes, H.; Scholl, S. Application of ultrasound in a micro heat exchanger for crystallization of solid lipid nanoparticles. Chem. Eng. Technol. 2013, 36, 1075–1079. [Google Scholar] [CrossRef]
- Joseph, S.; Rappolt, M.; Schoenitz, M.; Huzhalska, V.; Augustin, W.; Scholl, S.; Bunjes, H. Stability of the metastable α-polymorph in solid triglyceride drug-carrier nanoparticles. Langmuir 2015, 31, 6663–6674. [Google Scholar] [CrossRef]
- Riewe, J.; Erfle, P.; Melzig, S.; Kwade, A.; Dietzel, A.; Bunjes, H. Antisolvent precipitation of lipid nanoparticles in microfluidic systems—A comparative study. Int. J. Pharm. 2020, 579, 119167–119178. [Google Scholar] [CrossRef]
- Rønholt, S.; Kirkensgaard, J.; Pedersen, T.; Mortensen, K.; Knudsen, J. Polymorphism, microstructure and rheology of butter. Effects of cream heat treatment. Food Chem. 2012, 135, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Rønholt, S.; Mortensen, K.; Knudsen, J.C. The Effective Factors on the Structure of Butter and Other Milk Fat-Based Products. Compr. Rev. Food Sci. Food Saf. 2013, 12, 468–482. [Google Scholar] [CrossRef]
- Pande, P.; Akoh, C.C.; Shewfelt, R.L. Utilization of enzymatically interesterified cottonseed oil and palm stearin-based structured lipid in the production of trans-free margarine. Biocatal. Agric. Biotechnol. 2013, 2, 76–84. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Xie, H.; Liang, Z.; Su, L.; Liu, G.; Li, B. Effect of Temperature on the crystalline form and fat crystal network of two model palm oil-based shortenings during storage. Food Bioprocess Technol. 2014, 7, 887–900. [Google Scholar] [CrossRef]
- Sato, K.; Arishima, T.; Wang, Z.; Okima, K.; Sagi, N.; Mori, H. Polymorphism of POP and SOS. I. Occurrence and polymorphic transformation. J. Am. Oil Chem. Soc. 1989, 66, 664–674. [Google Scholar] [CrossRef]
- Le Révérend, B.; Fryer, P.J.; Coles, S.; Bakalis, S. A method to qualify and quantify the crystalline state of cocoa butter in industrial chocolate. J. Am. Oil Chem. Soc. 2010, 87, 239–246. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. New insights into the β polymorphism of 1,3-palmitoyl-stearoyl-2-oleoyl glycerol. Cryst. Growth Des. 2018, 18, 4811–4814. [Google Scholar] [CrossRef]
- Ghazani, S.; Marangoni, A. The triclinic polymorphism of cocoa butter is dictated by its major molecular species, 1-palmitoyl, 2-oleoyl, 3-stearoyl glycerol (POS). Cryst. Growth Des. 2019, 19, 90–97. [Google Scholar] [CrossRef]
- Ghazani, S.; Marangoni, A. The ternary solid state phase behavior of triclinic POP, POS and SOS and its relationship to CB and CBE properties. Cryst. Growth Des. 2019, 19, 704–713. [Google Scholar] [CrossRef]
- Higaki, K.; Sasakura, Y.; Koyano, T.; Hachiya, I.; Sato, K. Physical analyses of gel-like behavior of binary mixtures of high- and low-melting fats. J. Am. Oil Chem. Soc. 2003, 80, 263–270. [Google Scholar] [CrossRef]
- Higaki, K.; Koyano, T.; Hachiya, I.; Sato, K.; Suzuki, K. Rheological properties of β-fat gel made of binary mixtures of high-melting and low-melting fats. Food Res. Int. 2004, 37, 799–804. [Google Scholar] [CrossRef]
- Chrysan, M.M. Margarines and Spreads. In Bailey’s Industrial Oil and Fat Products; American Cancer Society: Atlanta, GA, USA, 2005; pp. 33–82. [Google Scholar] [CrossRef]
- Rajah, K.K. Fats in Food Technology; Wiley Blackwell: Chichester, UK, 2014; Chapter 6; pp. 213–252. [Google Scholar]
- Macridachis-González, J.; Bayés-García, L.; Calvet, T. An insight into the solid-state miscibility of triacylglycerol crystals. Molecules 2020, 25, 4562. [Google Scholar] [CrossRef] [PubMed]
- Minato, A.; Ueno, S.; Yano, J.; Smith, K.; Seto, H.; Amemiya, Y.; Sato, K. Thermal and structural properties of sn-1,3-dipalmitoyl-oleoylglycerol and sn-1,3-dioleoyl-2-palmitoylglycerol binary mixtures examined with synchrotron radiation X-ray diffraction. J. Am. Oil Chem. Soc. 1997, 74, 1213–1220. [Google Scholar] [CrossRef]
- Ikeda, E.; Ueno, S.; Miyamoto, R.; Sato, K. Phase behavior of a binary mixture of 1,3-dipalmitoyl-2-oleoylsn-glycerol and 1,3-dioleoyl-2-palmitoyl-sn-glycerol in n-dodecane solution. J. Phys. Chem. B 2010, 114, 10961–10969. [Google Scholar] [CrossRef]
- Sibbald, A.N.; Carney, J.R.; Marangoni, A.G. Enhanced structuring of fat with reduced saturates using mixed molecular compounds. J. Am. Oil Chem. Soc. 2016, 93, 1441–1452. [Google Scholar] [CrossRef]
- Nakanishi, K.; Mikiya, Y.; Ishiguro, T.; Ueno, S. Crystallization Behavior of Molecular Compound in Binary Mixture System of 1,3-Dioleoyl-2-Palmitoyl-sn-Glycerol and 1,3-Dipalmitoyl-2-Oleoyl-sn-Glycero. J. Am. Oil Chem. Soc. 2018, 95, 51–59. [Google Scholar] [CrossRef]
- Bunjes, H.; Steiniger, F.; Richter, W. Visualizing the structure of triglyceride nanoparticles in different crystal modifications. Langmuir 2007, 23, 4005–4011. [Google Scholar] [CrossRef]
- Minato, A.; Ueno, S.; Smith, K.; Amemiya, Y.; Sato, K. Thermodynamic and Kinetic Study on Phase Behavior of Binary Mixtures of POP and PPO Forming Molecular Compound Systems. J. Phys. Chem. B 1997, 101, 3498–3505. [Google Scholar] [CrossRef]
- Hondoh, H.; Ueno, S.; Sato, K. Crystallization of lipids. Fundamentals and applications. In Food, Cosmetics, and Pharmaceuticals; Wiley-Blackwell: Hoboken, NJ, USA, 2018; Chapter 4; pp. 105–141. [Google Scholar]
- Oswell, N.J.; Gunstone, F.D.; Pegg, R.B. Vegetable Oils. In Bailey’s Industrial Oil and Fat Products; American Cancer Society: Atlanta, GA, USA, 2020; pp. 1–30. [Google Scholar] [CrossRef]
- Kodali, D.R. 1-Trans Fats: Health, Chemistry, Functionality, and Potential Replacement Solutions. In Trans Fats Replacement Solutions; Kodali, D.R., Ed.; AOCS Press: Champaign, IL, USA, 2014; pp. 1–39. [Google Scholar] [CrossRef]
- Gregersen, S.B.; Miller, R.L.; Andersen, M.D.; Hammershøj, M.; Wiking, L. Inhomogeneous consistency of crystallized fat. Eur. J. Lipid Sci. Technol. 2015, 117, 1782–1791. [Google Scholar] [CrossRef]
- Watanabe, S.; Yoshikawa, S.; Sato, K. Formation and properties of dark chocolate prepared using fat mixtures of cocoa butter and symmetric/asymmetric stearic-oleic mixed-acid triacylglycerols: Impact of molecular compound crystals. Food Chem. 2021, 339, 127808. [Google Scholar] [CrossRef] [PubMed]
- Lutton, E. The Polymorphism of the Disaturated Triglycerides—OSS, OPP, POS, OPS and OSP. J. Am. Chem. Soc. 1951, 73, 1567–1572. [Google Scholar] [CrossRef]
- Sato, K.; Ueno, S.; Yano, J. Molecular interactions and kinetic properties of fats. Prog. Lipid Res. 1999, 38, 91–116. [Google Scholar] [CrossRef]
- Mizobe, H.; Tanaka, T.; Hatakeyama, N.; Nagai, T.; Ichioka, K.; Hondoh, H.; Ueno, S.; Sato, K. Structures and Binary Mixing Characteristics of Enantiomers of 1-Oleoyl-2,3-dipalmitoyl-sn-glycerol (S-OPP) and 1,2-Dipalmitoyl-3-oleoyl-sn-glycerol (R-PPO). J. Am. Oil Chem. Soc. 2013, 90, 1809–1817. [Google Scholar] [CrossRef]
- Yagi, N.; Inoue, K. CMOS flatpanel detectors for SAXS/WAXS experiments. J. Appl. Crystallogr. 2007, 40, s439–s441. [Google Scholar] [CrossRef] [Green Version]
- Nozue, Y.; Shinohara, Y.; Ogawa, Y.; Sakurai, T.; Hori, H.; Kasahara, T.; Yamaguchi, N.; Yagi, N.; Amemiya, Y. Deformation Behavior of Isotactic Polypropylene Spherulite during Hot Drawing Investigated by Simultaneous Microbeam SAXS-WAXS and POM Measurement. Macromolecules 2007, 40, 2036–2045. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taguchi, K.; Toda, A.; Hondoh, H.; Ueno, S.; Sato, K. Kinetic Study on Alpha-Form Crystallization of Mixed-Acid Triacylglycerols POP, PPO, and Their Mixture. Molecules 2021, 26, 220. https://doi.org/10.3390/molecules26010220
Taguchi K, Toda A, Hondoh H, Ueno S, Sato K. Kinetic Study on Alpha-Form Crystallization of Mixed-Acid Triacylglycerols POP, PPO, and Their Mixture. Molecules. 2021; 26(1):220. https://doi.org/10.3390/molecules26010220
Chicago/Turabian StyleTaguchi, Ken, Akihiko Toda, Hironori Hondoh, Satoru Ueno, and Kiyotaka Sato. 2021. "Kinetic Study on Alpha-Form Crystallization of Mixed-Acid Triacylglycerols POP, PPO, and Their Mixture" Molecules 26, no. 1: 220. https://doi.org/10.3390/molecules26010220
APA StyleTaguchi, K., Toda, A., Hondoh, H., Ueno, S., & Sato, K. (2021). Kinetic Study on Alpha-Form Crystallization of Mixed-Acid Triacylglycerols POP, PPO, and Their Mixture. Molecules, 26(1), 220. https://doi.org/10.3390/molecules26010220






