Enantioseparation of 5,5?-Dibromo-2,2?-dichloro-3-selanyl-4,4?-bipyridines on Polysaccharide-Based Chiral Stationary Phases: Exploring Chalcogen Bonds in Liquid-Phase Chromatography
Abstract
:1. Introduction
2. Results and Discussion
2.1. Conceptual Bases
2.1.1. Chiral Analytes
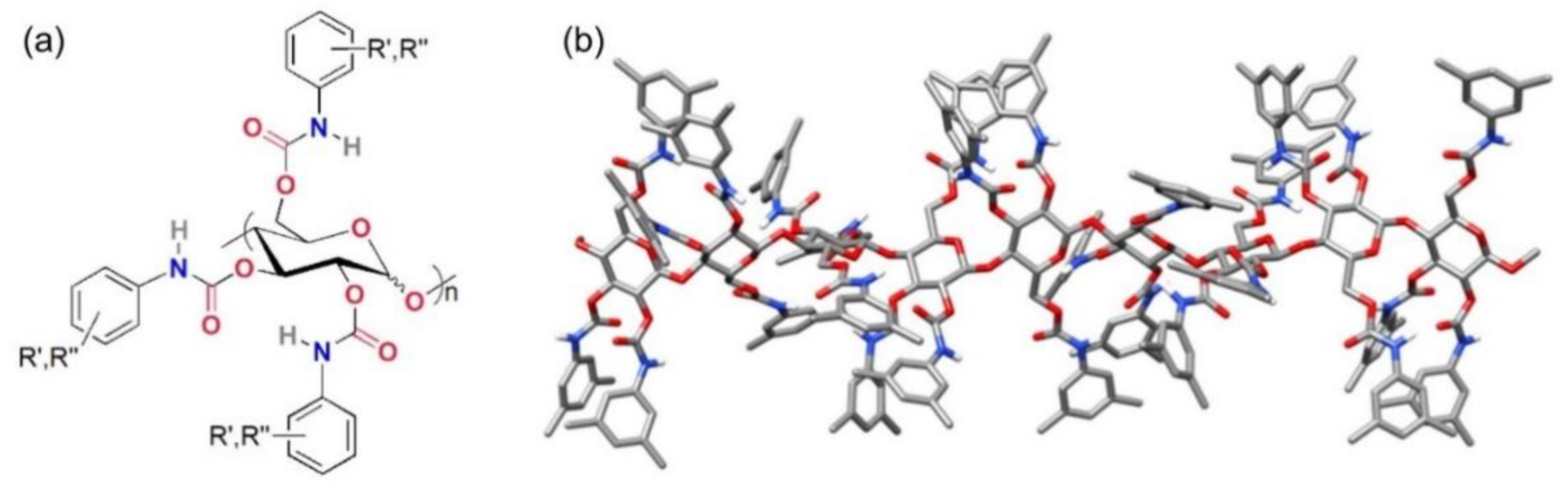
2.1.2. Chiral Selectors
2.1.3. Mobile Phases
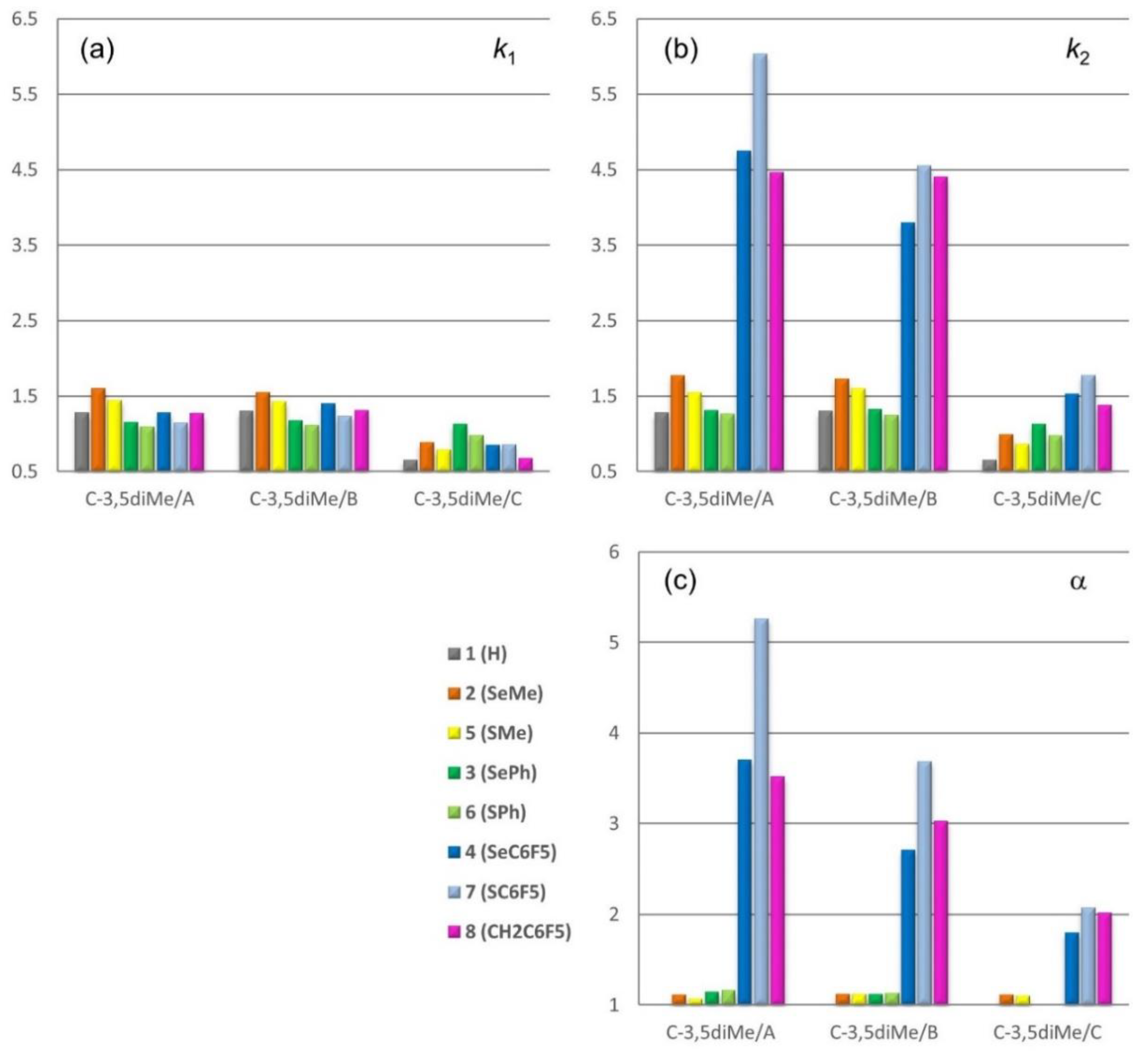
2.2. Chromatographic Screening
2.3. Effect of Methanol on Retention and Selectivity
2.4. Effect of Temperature and Thermodynamic Quantities
- (i)
- enantioseparations were enthalpy-driven in all cases (|ΔΔH°| > |TΔΔS°|);
- (ii)
- change in standard enthalpy and entropy were more negative on C-3,5diMe with mix A and mix B, indicating a stronger adsorption process under these conditions. A different trend was observed for compounds 2 and 5 (R = Me), showing more negative values with the system C-3,5diMe/mix C;
- (iii)
- the ΔΔG° values associated with the enantioseparation of compounds 2 and 5 (R = Me) on the C-3,5diMe CSP showed to be quite different with mix A (ΔΔG° (kJ/mol) = −0.26, −0.19, respectively), whereas they became equal by using the same CSP with mix B (ΔΔG° = −0.27, −0.27), where methanol weakened analyte-CSP electrostatic interactions;
- (iv)
- retention of both first and second eluted enantiomers were enthalpy-driven (|ΔH°| > |TΔS°|) in almost all cases. Entropy-driven retention (|ΔH°| < |TΔS°|) was observed for both enantiomers of compounds 2 and 5 (R = Me), and 6 (Ch = S, R = Ph) with the system C-3,5diMe/mix C. Under the same conditions, positive values of ΔG° were also derived for the first eluted enantiomers of compounds 4, 7, and 8. The first eluted enantiomer of compound 7 gave entropy-driven retention also with the system C-3Cl,4Me/mix A. The adsorption step with positive ΔG° as an independent process is definitely impossible. However, it may be coupled with other endergonic processes facilitating the exergonic adsorption step. The details of this unusual observation are the subject of further studies;
- (v)
- in all cases, thermodynamic quantities associated with retention changed in a narrower range (−1.17 ≤ ΔG° ≤ 0.96 kJ/mol) for the first eluted enantiomers compared to the second eluted ones (−4.45 ≤ ΔG° ≤ 0.36 kJ/mol), this evidence confirming that the adsorption mechanism of the most retained enantiomer is more sensitive to subtle structural variations;
- (vi)
- in the series 4–7–8, compound 7 showed the lowest retention for the first eluted enantiomer (ΔG° = −0.35 kJ/mol), and the highest retention for the second eluted enantiomer (ΔG° = −4.45 kJ/mol) with the system C-3,5diMe/mix A, evidencing the pivotal role of the system Ch = S and R = C6F5 for enantiodiscrimination.
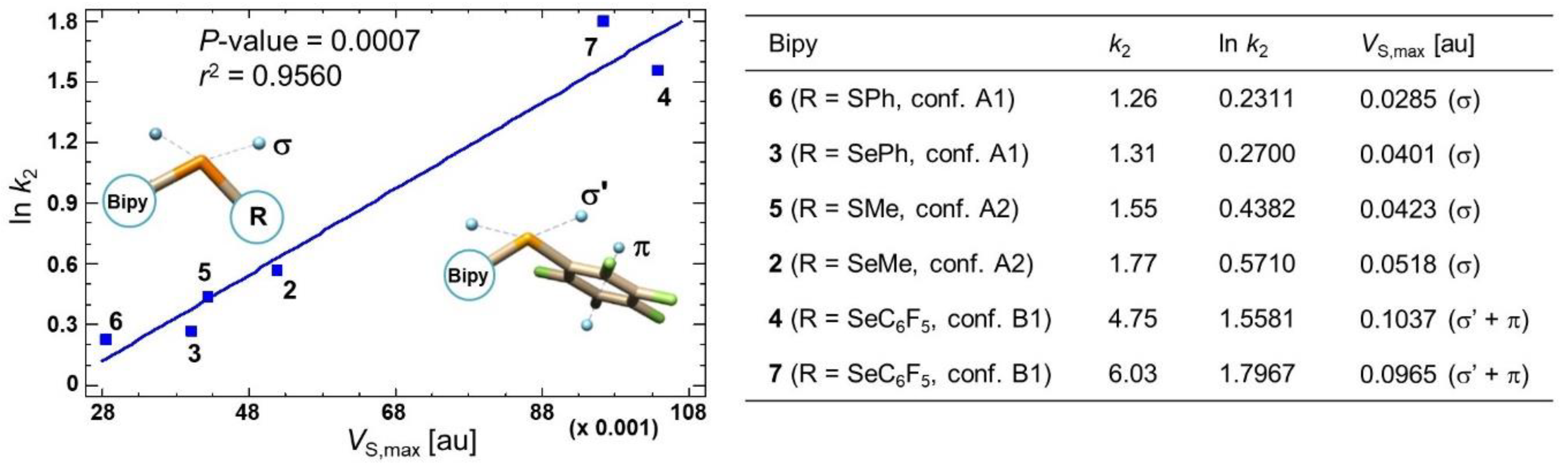
2.5. Electrostatic Potential Analysis to Explore Chiral Recognition Mechanism
2.6. Source Function Reconstruction of the Electrostatic Potential
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.2. Chromatography
4.3. Computationals
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
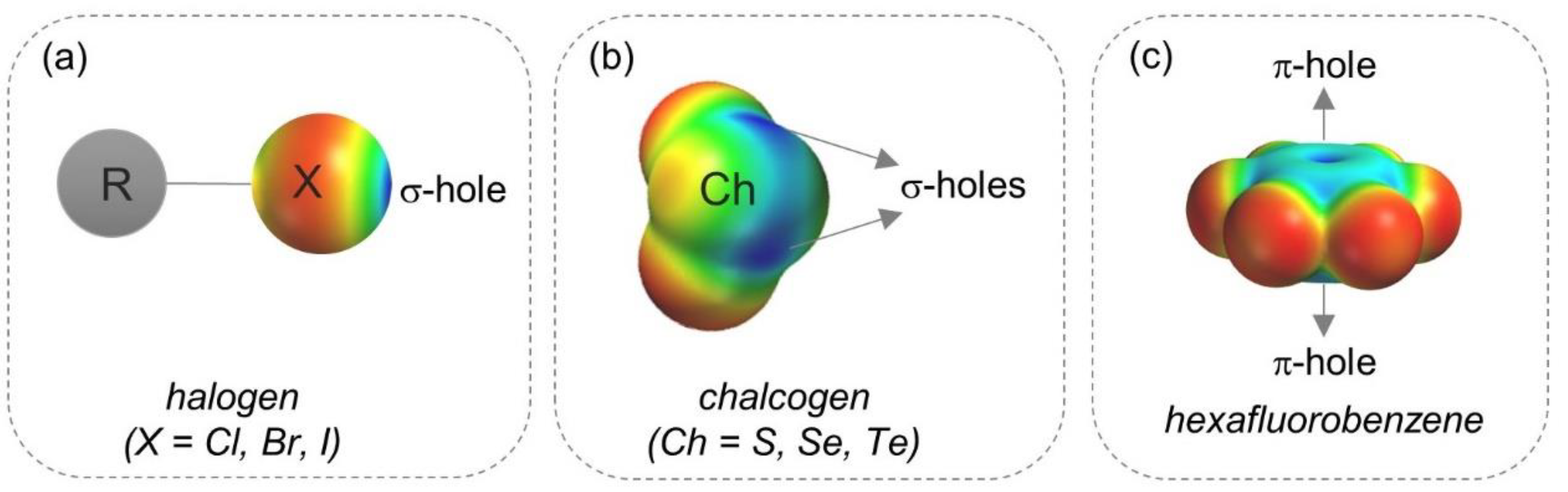
- Politzer, P.; Murray, J.S. σ-Holes and π-holes: Similarities and differences. J. Comput. Chem. 2018, 39, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Kollman, P. A general analysis of noncovalent intermolecular interactions. J. Am. Chem. Soc. 1977, 99, 4875–4894. [Google Scholar] [CrossRef]
- Scrocco, E.; Tomasi, J. The electrostatic molecular as a tool for the interpretation of molecular properties. Top. Curr. Chem. 1973, 42, 95–170. [Google Scholar] [CrossRef]
- Clark, T. Halogen bonds and σ-holes. Faraday Discuss. 2017, 203, 9–27. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Houk, K.N. Through-space effects of substituents dominate molecular electrostatic potentials of substituted arenes. J. Chem. Theory Comput. 2009, 5, 2301–2312. [Google Scholar] [CrossRef]
- Murray, J.S.; Macaveiu, L.; Politzer, P. Factor affecting the strengths of σ-hole electrostatic potentials. J. Comput. Sci. 2014, 5, 590–596. [Google Scholar] [CrossRef]
- Gatti, C.; Dessì, A.; Dallocchio, R.; Mamane, V.; Cossu, S.; Weiss, R.; Pale, P.; Aubert, E.; Peluso, P. Factors impacting σ- and π-hole regions as revealed by the electrostatic potential and its source function reconstruction: The case of 4,4′-bipyridine derivatives. Molecules 2020, 25, 4409. [Google Scholar] [CrossRef]
- Wonner, P.; Steinke, T.; Huber, S.M. Activation of quinolines by cationic chalcogen bond donors. Synlett 2019, 30, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.; Aubert, E.; Peluso, P.; Cossu, S.; Pale, P.; Mamane, V. Chiral chalcogen bond donors based on the 4,4′-bipyridine scaffold. Molecules 2019, 24, 4484. [Google Scholar] [CrossRef] [Green Version]
- Forni, A.; Franchini, D.; Dapiaggi, F.; Pieraccini, S.; Sironi, M.; Scilabra, P.; Pilati, T.; Petko, K.I.; Resnati, G.; Yagupolkii, Y.L. Featuring I···N halogen bond and weaker interactions in iodoperfluoroalkylimidazoles: An experimental and theoretical charge density study. Cryst. Growth Des. 2019, 19, 1621–1631. [Google Scholar] [CrossRef] [Green Version]
- Politzer, P.; Murray, J.S. An overview of strengths and directionalities of noncovalent interactions: σ-holes and π-holes. Crystals 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The bright future of unconventional σ/π-hole interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Shing Ho, P.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013. Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendations 2019. Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Chalcogen bonding in synthesis, catalysis and design of materials. Dalton Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef] [Green Version]
- Bauzá, A.; Frontera, A.; Mooibroek, T.J. π-Hole interactions involving nitro aromatic ligands in protein structures. Chem. Eur. J. 2019, 25, 13436–13443. [Google Scholar] [CrossRef] [Green Version]
- Mamane, V.; Peluso, P.; Aubert, E.; Weiss, R.; Wenger, E.; Cossu, S.; Pale, P. Disubstituted ferrocenyl iodo- and chalcogenoalkynes as chiral halogen and chalcogen bond donors. Organometallics 2020, 39, 3936–3960. [Google Scholar] [CrossRef]
- Dhaka, A.; Jeannin, O.; Jeon, I.-R.; Aubert, E.; Espinosa, E.; Fourmigué, M. Activating chalcogen bonding (ChB) in alkylseleno/alkyltelluroacetylenes toward ChB directionality control. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Peluso, P.; Mamane, V.; Dessì, A.; Dallocchio, R.; Aubert, E.; Gatti, C.; Mangelings, D.; Cossu, S. Halogen bond in separation science: A critical analysis across experimental and theoretical results. J. Chromatogr. A 2020, 1616, 460788. [Google Scholar] [CrossRef]
- Peluso, P.; Mamane, V.; Aubert, E.; Cossu, S. Insights into the impact of shape and electronic properties on the enantioseparation of polyhalogenated 4,4′-bipyridines on polysaccharide-type selectors. Evidence of stereoselective halogen bonding interactions. J. Chromatogr. A 2014, 1345, 182–192. [Google Scholar] [CrossRef]
- Peluso, P.; Mamane, V.; Aubert, E.; Dessì, A.; Dallocchio, R.; Dore, A.; Pale, P.; Cossu, S. Insights into halogen bond-driven enantioseparations. J. Chromatogr. A 2016, 1467, 228–238. [Google Scholar] [CrossRef]
- Dallocchio, R.; Dessì, A.; Solinas, M.; Arras, A.; Cossu, S.; Aubert, E.; Mamane, V.; Peluso, P. Halogen bond in high-performance liquid chromatography enantioseparations: Description, features and modelling. J. Chromatogr. A 2018, 1563, 71–81. [Google Scholar] [CrossRef]
- Peluso, P.; Mamane, V.; Dallocchio, R.; Dessì, A.; Villano, R.; Sanna, D.; Aubert, E.; Pale, P.; Cossu, S. Polysaccharide-based chiral stationary phases as halogen bond acceptors: A novel strategy for detection of stereoselective σ-hole bonds in solution. J. Sep. Sci. 2018, 41, 1247–1256. [Google Scholar] [CrossRef]
- Peluso, P.; Gatti, C.; Dessì, A.; Dallocchio, R.; Weiss, R.; Aubert, E.; Pale, P.; Cossu, S.; Mamane, V. Enantioseparation of fluorinated 3-arylthio-4,4’-bipyridines: Insights into chalcogen and π-hole bonds in high-performance liquid chromatography. J. Chromatogr. A 2018, 1567, 119–129. [Google Scholar] [CrossRef]
- Lazard, M.; Dauplais, M.; Blanquet, S.; Plateau, P. Recent advances in the mechanism of selenoamino acids toxicity in eukaryotic cells. BioMol. Concepts 2017, 8, 93–104. [Google Scholar] [CrossRef]
- Clark, L.C.; Combs, G.; Turnball, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, D.K.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. J. Am. Med. Ass. 1996, 276, 1957–1963. [Google Scholar] [CrossRef]
- Thompson, H.J.; Meeker, L.D.; Kokoska, S. Effect of an inorganic and organic form of dietary selenium on the promotional stage of mammary carcinogenesis in the rat. Cancer Res. 1984, 44, 2803–2806. [Google Scholar]
- Weiss, J.; Möckel, H.J.; Müller, A.; Diemann, E.; Walberg, H.-J. Retention of thio- and selenometalates in mobile-phase ion chromatography. J. Chromatogr. 1988, 439, 93–108. [Google Scholar] [CrossRef]
- Vespalec, R.; Corstjens, H.; Billiet, H.A.H.; Frank, J.; Luyben, K.C.A.M. Enantiomeric separation of sulfur- and selenium-containing amino acids by capillary electrophoresis using vancomycin as a chiral selector. Anal. Chem. 1995, 67, 3223–3228. [Google Scholar] [CrossRef]
- Hou, J.-G.; Han, X.-Q.; Liu, H.-T.; Wang, Y.-L.; Gao, J.-Z. Chiral separation of glycidyl selenide and glycidyl sulfide racemates on cellulose-based chiral stationary phases. J. Chromatogr. Sci. 2001, 39, 388–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peluso, P.; Mamane, V.; Aubert, E.; Cossu, S. High-performance liquid chromatography enantioseparation of atropisomeric 4,4′-bipyridines on polysaccharide-type chiral stationary phases: Impact of substituents and electronic properties. J. Chromatogr. A 2012, 1251, 91–100. [Google Scholar] [CrossRef]
- Peluso, P.; Mamane, V.; Aubert, E.; Cossu, S. High-performance liquid chromatography enantioseparation of polyhalogentaed 4,4′-bipyridines on polysaccharide-based chiral stationary phases under multimodal elution. J. Sep. Sci. 2014, 37, 2481–2489. [Google Scholar] [CrossRef]
- Abboud, M.; Mamane, V.; Aubert, E.; Lecomte, C.; Fort, Y. Synthesis of polyhalogenated 4,4′-bipyridines via a simple dimerization procedure. J. Org. Chem. 2010, 75, 3224–3231. [Google Scholar] [CrossRef]
- Garrett, G.E.; Carrera, E.I.; Seferos, D.S.; Taylor, M.S. Anion recognition by a bidentate chalcogen bond donor. Chem. Commun. 2016, 52, 9881–9884. [Google Scholar] [CrossRef] [Green Version]
- Esrafili, M.D.; Asadollahi, S.; Shahamat, Y.D. Competition between chalcogen bond and halogen bond interactions in YOX4:NH3 (Y = S, Se; X = F, Cl, Br) complexes: An ab initio investigation. Struct. Chem. 2016, 27, 1439–1447. [Google Scholar] [CrossRef]
- Shukla, R.; Khan, I.; Ibrar, A.; Simpson, J.; Chopra, D. Complex electronic interplay of σ-hole and π-hole interactions in crystals of halogen substituted 1,3,4-oxadiazol-2(3H)-thiones. CrystEngComm 2017, 19, 3485–3498. [Google Scholar] [CrossRef]
- Chankvetadze, B. Recent trends in preparation, investigation and application of polysaccharide-based chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Yamamoto, C.; Okamoto, Y. Enantioseparation of selected chiral sulfoxides using polysaccharide-type chiral stationary phases and polar organic, polar aqueous–organic and normal-phase eluents. J. Chromatogr. A 2001, 922, 127–137. [Google Scholar] [CrossRef]
- Dossou, K.S.S.; Chiap, P.; Chankvetadze, B.; Servais, A.-C.; Fillet, M.; Crommen, J. Enantioresolution of basic pharmaceuticals using cellulose tris(4-chloro-3-methylphenylcarbamate) as chiral stationary phase and polar organic mobile phases. J. Chromatogr. A 2009, 1216, 7450–7455. [Google Scholar] [CrossRef] [PubMed]
- Peluso, P.; Mamane, V.; Dallocchio, R.; Dessì, A.; Cossu, S. Noncovalent interactions in high-performance liquid chromatography enantioseparations of polysaccharide-based chiral selectors. J. Chromatogr. A 2020, 1623, 461202. [Google Scholar] [CrossRef]
- Gotmar, G.; Fornstedt, T.; Guiochon, G. Apparent and true enantioselectivity in enantioseparations. Chirality 2000, 12, 558–564. [Google Scholar] [CrossRef]
- Fornstedt, T.; Sajonz, P.; Guiochon, G. Thermodynamic study of an unusual chiral separation. Propranolol enantiomers on an immobilized cellulose. J. Am. Chem. Soc. 1997, 119, 1254–1264. [Google Scholar] [CrossRef]
- Tanács, D.; Orosz, T.; Szakonyi, Z.; Minh Le, T.; Fülöp, F.; Lindner, W.; Ilisz, I.; Péter, A. High-performance liquid chromatographic enantioseparation of isopulegol-based β-amino lactone and β-amino amide analogs on polysaccharide-based chiral stationary phases focusing on the change of the enantiomer elution order. J. Chromatogr. A 2020, 1621, 461054. [Google Scholar] [CrossRef]
- Peluso, P.; Sechi, B.; Lai, G.; Dessì, A.; Dallocchio, R.; Cossu, S.; Aubert, E.; Weiss, R.; Pale, P.; Mamane, V.; et al. Comparative enantioseparation of chiral 4,4′-bipyridine derivatives on coated and immobilized amylose-based chiral stationary phases. J. Chromatogr. A 2020, 1625, 461303. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. The molecular bases of chiral recognition in 2-(benzylsulfinyl)benzamide enantioseparation. Anal. Chim. Acta 2021, 1141, 194–205. [Google Scholar] [CrossRef]
- Gatti, C.; Cargnoni, F.; Bertini, L. Chemical information from the source function. J. Comput. Chem. 2003, 24, 422–436. [Google Scholar] [CrossRef]
- Gatti, C. The source function descriptor as a tool to extract chemical information from theoretical and experimental electron densities. Struct. Bond. 2012, 147, 193–286. [Google Scholar] [CrossRef]
- Koller, H.; Rimböck, K.-E.; Mannschreck, A. High-pressure liquid chromatography on triacetylcellulose: Characterization of a sorbent for the separation of enantiomers. J. Chromatogr. A 1983, 282, 89–94. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B. 01; Gaussian. Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyser. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved marching tetrahedra algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
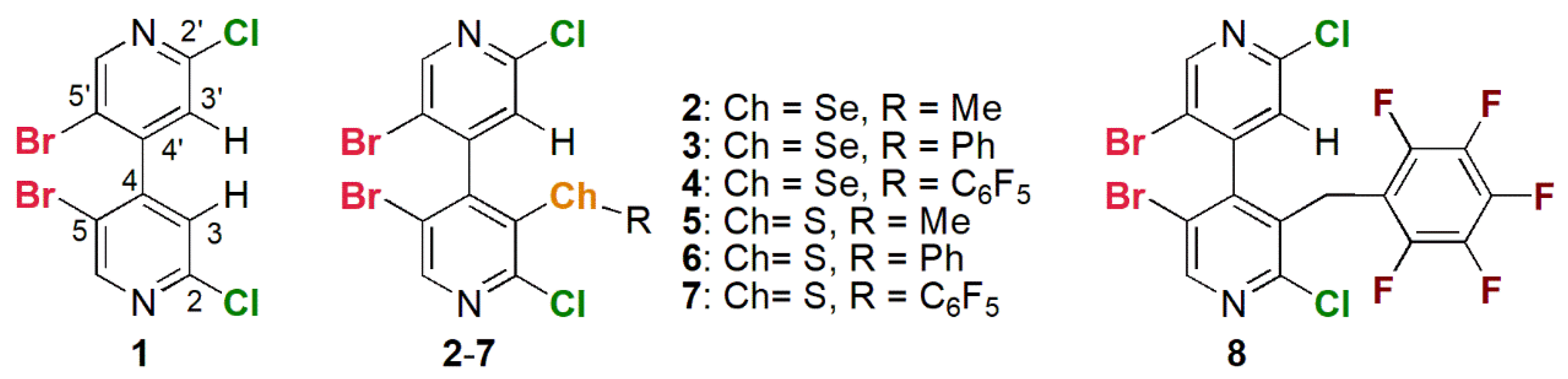



| Column 1 | Backbone | Ar (R’,R’’-C6H4) | Abbreviation | VS,min C=O (au) 2 | VS,max N-H (au) 2 |
|---|---|---|---|---|---|
| Lux Cellulose-1 | Cellulose | 3,5-dimethyl | C-3,5diMe | −0.0660 | 0.0788 |
| Lux Cellulose-2 | Cellulose | 3-chloro-4-methyl | C-3Cl,4Me | −0.0606 | 0.0868 |
| Lux Amylose-1 | Amylose | 3,5-dimethyl | A-3,5diMe | −0.0660 | 0.0788 |
| Lux i-Amylose-1 | Amylose | 3,5-dimethyl | iA-3,5diMe | −0.0660 | 0.0788 |
| Lux Amylose-2 | Amylose | 5-chloro-2-methyl | A-5Cl,2Me | −0.0618 | 0.0798 |
| Lux i-Amylose-3 | Amylose | 3-chloro-5-methyl | iA-3Cl,5Me | −0.0594 | 0.0871 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peluso, P.; Dessì, A.; Dallocchio, R.; Sechi, B.; Gatti, C.; Chankvetadze, B.; Mamane, V.; Weiss, R.; Pale, P.; Aubert, E.; et al. Enantioseparation of 5,5?-Dibromo-2,2?-dichloro-3-selanyl-4,4?-bipyridines on Polysaccharide-Based Chiral Stationary Phases: Exploring Chalcogen Bonds in Liquid-Phase Chromatography. Molecules 2021, 26, 221. https://doi.org/10.3390/molecules26010221
Peluso P, Dessì A, Dallocchio R, Sechi B, Gatti C, Chankvetadze B, Mamane V, Weiss R, Pale P, Aubert E, et al. Enantioseparation of 5,5?-Dibromo-2,2?-dichloro-3-selanyl-4,4?-bipyridines on Polysaccharide-Based Chiral Stationary Phases: Exploring Chalcogen Bonds in Liquid-Phase Chromatography. Molecules. 2021; 26(1):221. https://doi.org/10.3390/molecules26010221
Chicago/Turabian StylePeluso, Paola, Alessandro Dessì, Roberto Dallocchio, Barbara Sechi, Carlo Gatti, Bezhan Chankvetadze, Victor Mamane, Robin Weiss, Patrick Pale, Emmanuel Aubert, and et al. 2021. "Enantioseparation of 5,5?-Dibromo-2,2?-dichloro-3-selanyl-4,4?-bipyridines on Polysaccharide-Based Chiral Stationary Phases: Exploring Chalcogen Bonds in Liquid-Phase Chromatography" Molecules 26, no. 1: 221. https://doi.org/10.3390/molecules26010221
APA StylePeluso, P., Dessì, A., Dallocchio, R., Sechi, B., Gatti, C., Chankvetadze, B., Mamane, V., Weiss, R., Pale, P., Aubert, E., & Cossu, S. (2021). Enantioseparation of 5,5?-Dibromo-2,2?-dichloro-3-selanyl-4,4?-bipyridines on Polysaccharide-Based Chiral Stationary Phases: Exploring Chalcogen Bonds in Liquid-Phase Chromatography. Molecules, 26(1), 221. https://doi.org/10.3390/molecules26010221