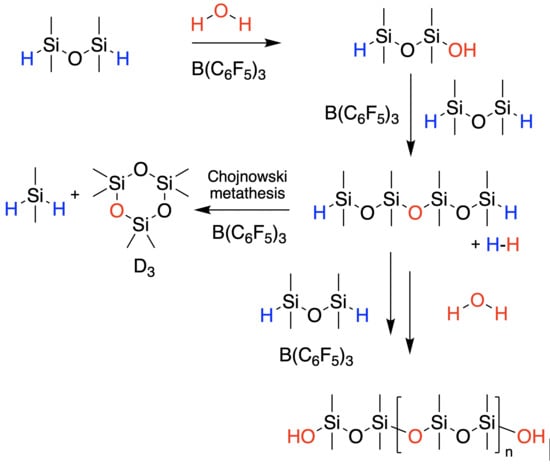
When Attempting Chain Extension, Even Without Solvent, It Is Not Possible to Avoid Chojnowski Metathesis Giving D3
Abstract
:1. Introduction
2. Results
2.1. High-Molecular-Weight PDMS Preparation Using Hydrolysis
2.2. Managing Cyclics
3. Experimental Section
3.1. Materials
3.2. Methods
3.3. Experimental Procedures for High-Molecular-Weight PDMS Preparation Using Hydrolysis
3.3.1. One-Shot Addition
3.3.2. Portion by Portion Addition
3.3.3. Capturing the Volatiles, Entries 7, 8
3.4. Experimental Procedure for Controlled Growth of Linear PDMS Using MHMH and Water
3.4.1. In Neat Water, or in Water/Toluene Mixtures
3.4.2. Chain Extension
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Dedication
Sample Availability
References
- Chojnowski, J.; Cypryk, M. Synthesis of Linear Polysiloxanes. In Silicon-Containing Polymers: The Science and Technology of Their Synthesis; Jones, R.G., Ando, W., Chojnowski, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–42. [Google Scholar]
- Kantor, S.W.; Grubb, W.T.; Osthoff, R.C. The Mechanism of the Acid- and Base-catalyzed Equilibration of Siloxanes. J. Am. Chem. Soc. 1954, 76, 5190–5197. [Google Scholar] [CrossRef]
- Chojnowski, J. Polymerization. In Siloxane Polymers; Clarson, S.J., Semlyen, J.A., Eds.; Prentice Hall: Englewood Cliffs, NJ, USA, 1993; pp. 1–71. [Google Scholar]
- Wright, P.V.; Semlyen, J.A. Equilibrium ring concentrations and the statistical conformations of polymer chains: Part 3. Substituent effects in polysiloxane systems. Polymer 1970, 11, 462–471. [Google Scholar] [CrossRef]
- Franzen, A.; Greene, T.; Van Landingham, C.; Gentry, R. Toxicology of octamethylcyclotetrasiloxane (D4). Toxic. Lett. 2017, 279, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Steer, H.; Tait, T.; Williams, Z.; Pacepavicius, G.; Young, T.; Ng, T.; Smyth, S.A.; Kinsman, L.; Alaee, M. Concentrations of cyclic volatile methylsiloxanes in biosolid amended soil, influent, effluent, receiving water, and sediment of wastewater treatment plants in Canada. Chemosphere 2013, 93, 766–773. [Google Scholar] [CrossRef]
- European Chemical Agency. Restriction proposal for siloxanes (D4, D5, D6) in personal care products. ECHA 2020. Available online: http://echa.europa.eu/-/echa-s-committees-conclude-on-five-restrictions (accessed on 3 January 2021).
- Environment Canada Regulation on D4. Available online: www.canada.ca/en/environment-climate-change/services/management-toxic-substances/list-canadian-environmental-protection-act/slioxane-d4.html (accessed on 3 January 2021).
- Peters, M.A.; Belu, A.M.; Linton, R.W.; Dupray, L.; Meyer, T.J.; DeSimone, J.M. Termination of Living Anionic Polymerizations Using Chlorosilane Derivatives: A General Synthetic Methodology for the Synthesis of End-Functionalized Polymers. J. Am. Chem. Soc. 1995, 117, 3380–3388. [Google Scholar] [CrossRef]
- Frye, C.L.; Salinger, R.M.; Fearon, F.W.G.; Klosowski, J.M.; DeYoung, T. Reactions of organolithium reagents with siloxane substrates. J. Org. Chem. 1970, 35, 1308–1314. [Google Scholar] [CrossRef]
- Maschke, U.; Wagner, T.; Coqueret, X. Synthesis of high-molecular-weight poly(dimethylsiloxane) of uniform size by anionic polymerization, 1. Initiation by a monofunctional lithium siloxanolate. Makromol. Chem. 1992, 193, 2453–2466. [Google Scholar] [CrossRef]
- Fuchise, K.; Igarashi, M.; Sato, K.; Shimada, S. Organocatalytic controlled/living ring-opening polymerization of cyclotrisiloxanes initiated by water with strong organic base catalysts. Chem. Sci. 2018, 9, 2879–2891. [Google Scholar] [CrossRef] [Green Version]
- Brook, M.A. Silicon in Organic, Organometallic, and Polymer Chemistry; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Pesti, J.; Larson, G.L. Tetramethyldisiloxane: A Practical Organosilane Reducing Agent. Org. Proc. Res. Devel. 2016, 20, 1164–1181. [Google Scholar] [CrossRef] [Green Version]
- Larson, G.L. Silicon-Based Reducing Agents. Gelest. 2004. Available online: https://www.gelest.com/wp-content/uploads/Silicon-Based_Reducing_Agents.pdf (accessed on 3 January 2021).
- Schneider, A.F.; Chen, Y.; Brook, M.A. Trace water affects tris(pentafluorophenyl)borane catalytic activity in the Piers–Rubinsztajn reaction. Dalton Trans. 2019, 48, 13599–13606. [Google Scholar] [CrossRef]
- Piers, W.E. The Chemistry of Perfluoroaryl Boranes. In Advances in Organometallic Chemistry; Academic Press: Cambridge, MA, USA, 2004; Volume 52, pp. 1–76. [Google Scholar]
- Jacobsen, H.; Berke, H.; Doring, S.; Kehr, G.; Erker, G.; Frohlich, R.; Meyer, O. Lewis acid properties of tris(pentafluorophenyl)borane. Structure and bonding in L-B(C6F5)3 complexes. Organometallics 1999, 18, 1724–1735. [Google Scholar] [CrossRef]
- Zheng, S.; Liao, M.; Chen, Y.; Brook, M.A. Dissolving used rubber tires. Green Chem. 2020, 22, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Rubinsztajn, S.; Cella, J.A. A New Polycondensation Process for the Preparation of Polysiloxane Copolymers. Macromolecules 2005, 38, 1061–1063. [Google Scholar] [CrossRef]
- Chojnowski, J.; Rubinsztajn, S.; Cella, J.A.; Fortuniak, W.; Cypryk, M.; Kurjata, J.; Kaźmierski, K. Mechanism of the B(C6F5)3-Catalyzed Reaction of Silyl Hydrides with Alkoxysilanes. Kinetic and Spectroscopic Studies. Organometallics 2005, 24, 6077–6084. [Google Scholar] [CrossRef]
- Chojnowski, J.; Fortuniak, W.; Kurjata, J.; Rubinsztajn, S.; Cella, J.A. Oligomerization of Hydrosiloxanes in the Presence of Tris(pentafluorophenyl)borane. Macromolecules 2006, 39, 3802–3807. [Google Scholar] [CrossRef]
- Zhou, D.; Kawakami, Y. Tris(pentafluorophenyl)borane as a Superior Catalyst in the Synthesis of Optically Active SiO-Containing Polymers. Macromolecules 2005, 38, 6902–6908. [Google Scholar] [CrossRef]
- Brook, M.A.; Grande, J.B.; Ganachaud, F. New Synthetic Strategies for Structured Silicones Using B(C6F5)3. In Silicon Polymers; Muzafarov, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 161–183. [Google Scholar] [CrossRef]
- Brook, M.A. New Control Over Silicone Synthesis using SiH Chemistry: The Piers–Rubinsztajn Reaction. Chem. Eur. J. 2018, 24, 8458–8469. [Google Scholar] [CrossRef]
- Liao, M.; Schneider, A.F.; Laengert, S.E.; Gale, C.B.; Chen, Y.; Brook, M.A. Living synthesis of silicone polymers controlled by humidity. Eur. Polym. J. 2018, 107, 287–293. [Google Scholar] [CrossRef]
- Schneider, A.F.; Brook, M.A. High-Throughput Synthesis and Characterization of Aryl Silicones Using the Piers-Rubinsztajn Reaction. Chem. Eur. J. 2019, 25, 15367–15374. [Google Scholar] [CrossRef]
- Schneider, A.F.; Lu, E.K.; Lu, G.; Brook, M.A. Facile synthesis of phenyl-rich functional siloxanes from simple silanes. J. Polym. Sci. 2020, 58, 3095–3106. [Google Scholar] [CrossRef]
- Morgan, J.; Chen, T.; Hayes, R.; Dickie, T.; Urlich, T.; Brook, M.A. Facile synthesis of dendron-branched silicone polymers. Polym. Chem. 2017, 8, 2743–2746. [Google Scholar] [CrossRef]
- Thompson, D.B.; Brook, M.A. Rapid Assembly of Complex 3D Siloxane Architectures. J. Am. Chem. Soc. 2008, 130, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Liang, S.; Chen, Y.; Brook, M.A. Hyperbranched Silicone MDTQ Tack Promoters. Molecules 2019, 24, 4133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longuet, C.; Joly-Duhamel, C.; Ganachaud, F. Copolycondensation of regular functional silane and siloxane in aqueous emulsion using B(C6F5)3 as a catalyst. Macromol. Chem. Phys. 2007, 208, 1883–1892. [Google Scholar] [CrossRef]
- Chojnowski, J.; Rubinsztajn, S.; Fortuniak, W.; Kurjata, J. Oligomer and Polymer Formation in Hexamethylcyclotrisiloxane (D3) -Hydrosilane Systems Under Catalysis by tris(pentafluorophenyl)borane. J. Inorg. Organomet. Polym. Mat. 2007, 17, 173–187. [Google Scholar] [CrossRef]
- Babonneau, F.; Gualandris, V.; Maquet, J.; Massiot, D.; Janicke, M.T.; Chmelka, B.F. Newly Applied Two-Dimensional Solid-State NMR Correlation Techniques for the Characterization of Organically Modified Silicates. J. Sol-Gel Sci. Technol. 2000, 19, 113–117. [Google Scholar] [CrossRef]
- Taylor, R.B.; Parhboo, B.; Fillmore, D.M.; Fillmore, D.M. Nuclear Magnetic Resonance Spectroscopy. In Analysis of Silicones; Smith, A.L., Ed.; Wiley: Hoboken, NJ, USA, 1991; Chapter 12; pp. 347–420. [Google Scholar]
- Mrozek, R.A.; Cole, P.J.; Otim, K.J.; Shull, K.R.; Lenhart, J.L. Influence of solvent size on the mechanical properties and rheology of polydimethylsiloxane-based polymeric gels. Polymer 2011, 52, 3422–3430. [Google Scholar] [CrossRef]
- Williams, E.A. NMR Spectroscopy of Organosilicon Compounds. In The Chemistry of Organic Silicon Compounds; Patai, S., Rappoport, Z., Eds.; John Wiley & Sons: Chichester, UK, 1989; Volume 1, p. 511. [Google Scholar]
- Rabanzo-Castillo, K.M.; Kumar, V.B.; Söhnel, T.; Leitao, E.M. Catalytic Synthesis of Oligosiloxanes Mediated by an Air Stable Catalyst, (C6F5)3B(OH2). Front. Chem. 2020, 8, 477. [Google Scholar] [CrossRef]





| Entry a | [BCF]/ [SiH] mol% | [OH]/ [SiH] a | Addition b | MHMH/DCM c (g·mL−1) | Cyclics % d | PDMS % d | Mn (g·mol−1) | Mw (g·mol−1) | ĐM | Mass Bal e |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 1 | O | - | 2 | 98 | 43,700 | 129,200 | 2.97 | 70.1 |
| 2 | 0.02 | 1 | O | - | 26 | 74 | 52,700 | 105,400 | 2.00 | 71.3 |
| 3 | 0.02 | 3.2 | O | - | 5.9 | 94 | 41,600 | 101,500 | 2.44 | 70.6 |
| 4 | 0.008 | 1 | P | - | 5.9 | 92 | 20,700 | 32,500 | 1.57 | 79.3 |
| 5 | 0.006 | 0.73 | P | 1.3 | 12.9 | 87 | 111,400 | 221,500 | 1.99 | 95.6 |
| 6 | 0.004 | 0.75 | P | 1 | 5.7 | 94 | 152,500 | 314,000 | 2.06 | 82.9 |
| MHMH/PhCH3 c (g·mL−1) | ||||||||||
| 7 | 0.02 | 1 | O | - | 18.9 | 81.2 | 20,500 | 37,400 | 1.82 | 73.8 f |
| 8 | 0.02 | 1 | O | 1.53 | 47.9 | 52.1 | 40,000 | 63,100 | 1.58 | 89.1 g |

| Neat | H2O/Toluene a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Time(min) | Mn | MW | ĐM | MW b | Mn | MW | ĐM | MW b |
| 1 | 0 | - | - | - | 134 | - | - | - | 134 |
| 2 | 2 | - | - | - | 240 | - | - | - | 250 |
| 3 | 4 | - | - | - | 300 | - | - | - | 310 |
| 4 | 6 | - | - | - | 520 c | - | - | - | 330 |
| 5 | 8 | - | - | - | 310 | - | - | - | 440 |
| 6 | 10 | - | - | - | 330 | - | - | - | 1030 |
| 7 | 30 | 21,600 | 54,000 | 2.50 | - | 39,000 | 69,700 | 1.79 | - |
| 8 | 60 | 21,800 | 63,000 | 2.90 | - a | 45,300 | 97,100 | 2.14 | - |
| 9 | 180 | 31,200 | 82,900 | 2.66 | - a | 45,500 | 79,900 | 1.76 | - |
| Chain | Extension d | ||||||||
| Starting | Polymer | Product | Polymer | ||||||
| 10 | 21,300 | 33,200 | 1.56 | → | 41,900 | 137,000 | 3.27 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, M.; Chen, Y.; Brook, M.A. When Attempting Chain Extension, Even Without Solvent, It Is Not Possible to Avoid Chojnowski Metathesis Giving D3. Molecules 2021, 26, 231. https://doi.org/10.3390/molecules26010231
Liao M, Chen Y, Brook MA. When Attempting Chain Extension, Even Without Solvent, It Is Not Possible to Avoid Chojnowski Metathesis Giving D3. Molecules. 2021; 26(1):231. https://doi.org/10.3390/molecules26010231
Chicago/Turabian StyleLiao, Mengchen, Yang Chen, and Michael A. Brook. 2021. "When Attempting Chain Extension, Even Without Solvent, It Is Not Possible to Avoid Chojnowski Metathesis Giving D3" Molecules 26, no. 1: 231. https://doi.org/10.3390/molecules26010231
APA StyleLiao, M., Chen, Y., & Brook, M. A. (2021). When Attempting Chain Extension, Even Without Solvent, It Is Not Possible to Avoid Chojnowski Metathesis Giving D3. Molecules, 26(1), 231. https://doi.org/10.3390/molecules26010231