Oligosilanylated Silocanes †
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. NMR Spectroscopy
2.3. Electrochemical Studies
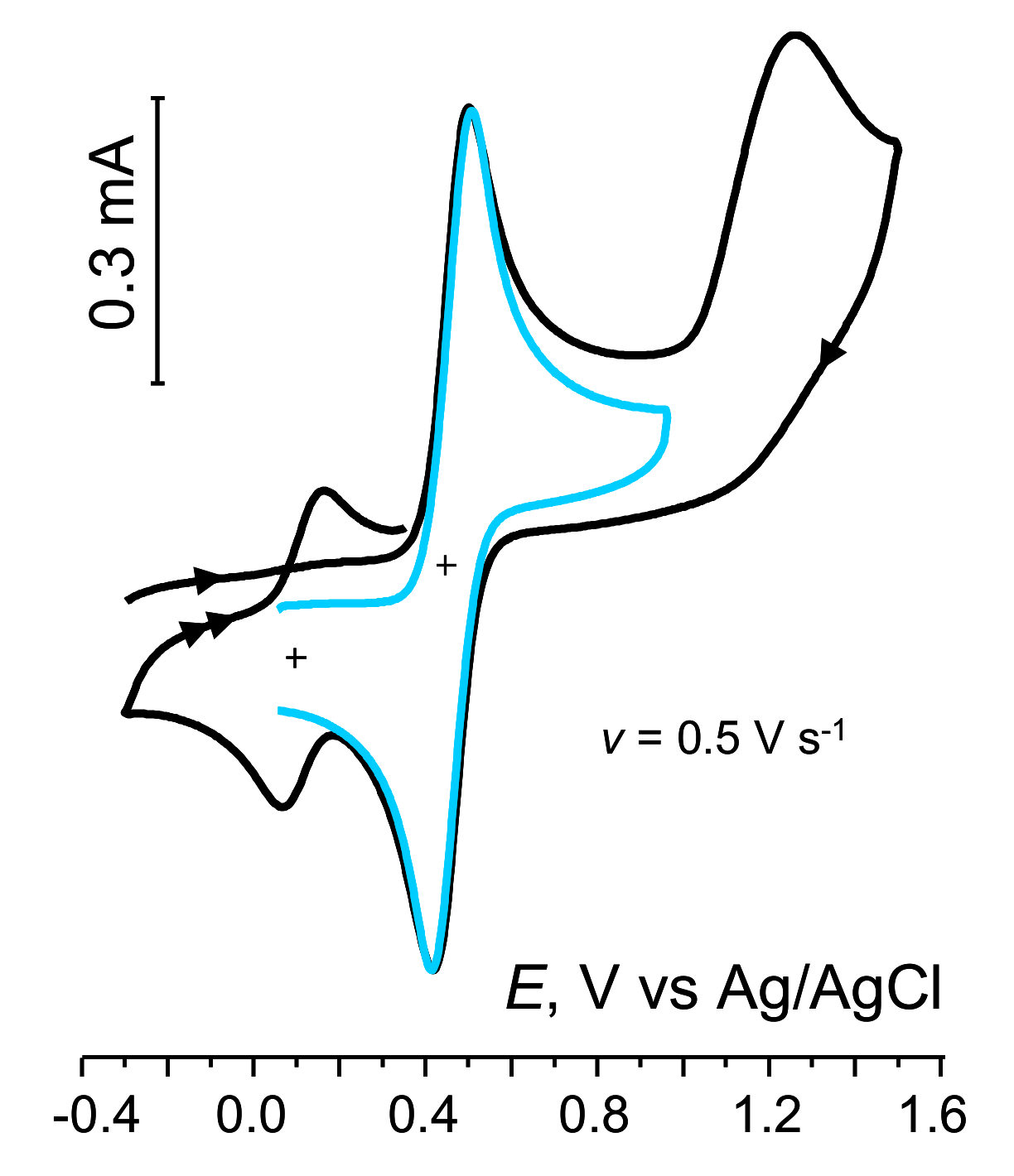
2.3.1. Oxidation
2.3.2. Reduction
2.3.3. DFT Study
3. Conclusions
4. Experimental Section
4.1. Potassiobis(trimethylsilyl)silyl]-2,6-dimethyl-1,3,6,2-dioxazasilocane 18-crown-6 (4)
4.2. 2,2’-(2,2,3,3-Tetramethyl-1,1,4,4-tetrakis(trimethylsilyl)tetrasilane-1,4-diyl)bis(2,6-di methyl-1,3,6,2-dioxazasilocane) (5)
4.3. 2,2-Dimethoxy-6-methyl-1,3,6,2-dioxazasilocane (6)
4.4. 2.2-Dichloro-6-methyl-1,3,6,2-dioxazasilocane (7)
4.5. 2,2-Bis[tris(trimethylsilyl)silyl]-6-methyl-1,3,6,2-dioxazasilocane (8)
4.6. 2,2-Bis[tris(trimethylsilyl)germyl]-6-methyl-1,3,6,2-dioxazasilocane (8a)
4.7. 2,2,3,3,9-Pentamethyl-1,1,4,4-tetrakis(trimethylsilyl)-6,12-dioxa-9-aza-1,2,3,4,5-penta silaspiro[4.7]dodecane (9)
4.8. 2,2,4,4,10-Pentamethyl-1,1,5,5-tetrakis(trimethylsilyl)-3,7,13-trioxa-10-aza-1,2,4,5,6-penta silaspiro[5.7]tridecane (10)
4.9. 2.-[Potassiobis(trimethylsilyl)silyl]-2-tert-butoxy-6-methyl-1,3,6,2-dioxazasilocane (12)
4.10. 2.-[Potassiobis(trimethylsilyl)silyl]-2-[tris(trimethylsilyl)silyl]-6-methyl-1,3,6,2-dioxaza silocane (13)
4.11. 2,2,3,3,9-Pentamethyl-1,4-dipotassio-1,4-bis(trimethylsilyl)-6,12-dioxa-9-aza 1,2,3,4,5-pentasilaspiro[4.7]dodecane (14)
4.12. 2,2,4,4,10-Pentamethyl-1,5-dipotassio-1,5-bis(trimethylsilyl)-3,7,13-trioxa-10-aza 1,2,4,5,6-pentasilaspiro[5.7]tridecane (15)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pestunovich, V.; Kirpichenko, S.; Voronkov, M. Silatranes and Their Tricyclic Analogs. In The Chemistry of Organic Silicon Compounds; Rappoport, Z., Apeloig, Y., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; pp. 1447–1537. ISBN 978-0-470-85725-0. [Google Scholar]
- Kano, N. Penta- and Hexacoordinated Silicon (IV) Compounds. In Organosilicon Compounds; Lee, V.Y., Ed.; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-801981-8. [Google Scholar]
- Aghazadeh Meshgi, M.; Baumgartner, J.; Marschner, C. Oligosilanylsilatranes. Organometallics 2015, 34, 3721–3731. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh Meshgi, M.; Baumgartner, J.; Jouikov, V.V.; Marschner, C. Electron Transfer and Modification of Oligosilanylsilatranes and Related Derivatives. Organometallics 2017, 36, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghazadeh Meshgi, M.; Zitz, R.; Walewska, M.; Baumgartner, J.; Marschner, C. Tuning the Si–N Interaction in Metalated Oligosilanylsilatranes. Organometallics 2017, 36, 1365–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitz, R.; Hlina, J.; Aghazadeh Meshgi, M.; Krenn, H.; Marschner, C.; Szilvási, T.; Baumgartner, J. Using Functionalized Silyl Ligands to Suppress Solvent Coordination to Silyl Lanthanide(II) Complexes. Inorg. Chem. 2017, 56, 5328–5341. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh Meshgi, M.; Zaitsev, K.V.; Vener, M.V.; Churakov, A.V.; Baumgartner, J.; Marschner, C. Hypercoordinated Oligosilanes Based on Aminotrisphenols. ACS Omega 2018, 3, 10317–10330. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Matsubara, H.; Murakami, K.; Yorimitsu, H.; Osuka, A. Activator-Free Palladium-Catalyzed Silylation of Aryl Chlorides with Silylsilatranes. Chem. Asian J. 2015, 10, 219–224. [Google Scholar] [CrossRef]
- Guo, J.-D.; Sasamori, T.; Yamamoto, Y.; Matsubara, H.; Nagase, S.; Yorimitsu, H. Computational Picture of Silyl Transfer from Silylsilatranes to Arylpalladium Chloride. Bull. Chem. Soc. Jpn. 2016, 89, 192–194. [Google Scholar] [CrossRef]
- Song, H.-J.; Jiang, W.-T.; Zhou, Q.-L.; Xu, M.-Y.; Xiao, B. Structure-Modified Germatranes for Pd-Catalyzed Biaryl Synthesis. ACS Catal. 2018, 8, 9287–9291. [Google Scholar] [CrossRef]
- Ralph, G.; Biscoe, M.R. Preparation of Enantioenriched Alkylcarbastannatranes via Nucleophilic Inversion of Alkyl Mesylates for Use in Stereospecific Cross-Coupling Reactions. Organometallics 2019, 38, 3912–3915. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Jiang, W.-T.; Li, Y.; Xu, Q.-H.; Zhou, Q.-L.; Yang, S.; Xiao, B. Alkyl Carbagermatranes Enable Practical Palladium-Catalyzed sp2-sp3 Cross-Coupling. J. Am. Chem. Soc. 2019, 141, 7582–7588. [Google Scholar] [CrossRef]
- Erickson, K.A.; Cibuzar, M.P.; Mucha, N.T.; Waterman, R. Catalytic N–Si Coupling as a Vehicle for Silane Dehydrocoupling via α-Silylene Elimination. Dalton Trans. 2018, 47, 2138–2142. [Google Scholar] [CrossRef] [PubMed]
- Hegyes, P.; Földeák, S.; Hencsei, P.; Zsombok, G.; Nagy, J. Synthesis and Structural Study of 1,3-Dioxa-6-aza-2-silacyclooctanes. J. Organomet. Chem. 1983, 251, 289–294. [Google Scholar] [CrossRef]
- Zyablikova, T.A.; Ishmaeva, E.A.; Kataev, V.E.; Vereshchagina, Y.A.; Bazhanova, Z.G.; Il’yasov, A.V.; Terent’eva, S.A.; Pudovik, M.A. Conformational Analysis of 1,3,6,2-Dioxazaphosphocanes and 1,3,6,2-Dioxazasilocanes. Russ. J. Gen. Chem. 2004, 74, 1171–1176. [Google Scholar] [CrossRef]
- Kemme, A.; Bleidelis, J.; Urtane, I.; Zelchan, G.; Lukevics, E. X-Ray Analysis of 1,3-Dioxa-6-Aza-2-Silacyclooctane Derivatives. J. Organomet. Chem. 1980, 202, 115–121. [Google Scholar] [CrossRef]
- Lutter, M.; Iovkova-Berends, L.; Dietz, C.; Jouikov, V.; Jurkschat, K. N-Aryl-Substituted 5-Aza-2,8-dioxasilabicyclo[3.3.01.5]octanes: Syntheses, Molecular Structures, DFT Calculations and Cyclovoltammetric Studies. Main Group Met. Chem. 2012, 35, 41–52. [Google Scholar] [CrossRef]
- Shekar, S.; Brown, S.N. Mechanism and Selectivity of Methyl and Phenyl Migrations in Hypervalent Silylated Iminoquinones. J. Org. Chem. 2014, 79, 12047–12055. [Google Scholar] [CrossRef]
- Salazar-Hernandez, M.M.; Layva-Ramirez, M.A.; Guiterrez, A. Neutral Alkoxysilanes from Silica Gel and N-Phenyldiethanolamine. Polyhedron 2009, 28, 4044–4050. [Google Scholar] [CrossRef]
- Shekar, S.; Brown, S.N. Migrations of Alkyl and Aryl Groups from Silicon to Nitrogen in Silylated Aryloxyiminoquinones. Organometallics 2013, 32, 556–564. [Google Scholar] [CrossRef]
- Kemme, A.A.; Bleidelis, Y.Y.; Urtane, I.P.; Zelchan, G.I.; Dukevits, E.I. Zh. Strukt. Khim. 1984, 25, 165–171.
- Voronkov, M.G.; Grebneva, E.A.; Albanov, A.I.; Zel’bst, E.A.; Trofimova, O.M.; Vasil’ev, A.D.; Chernov, N.F.; Timofeeva, E.N. Neutral Pentacoordinate Silicon Complexes with SiO2FC Skeleton: Synthesis, Structural Characterization and Stereodynamical Behavior. J. Organomet. Chem. 2014, 768, 10–14. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Korlyukov, A.A.; Zelbst, É.A.; Grebneva, E.A.; Trofimova, O.M.; Antipin, M.Y. Molecular Structure of 1-Phenyl-1-Fluoro-5-Methylquasisilatrane (2-Phenyl-2-Fluoro-1,3-Dioxa-6-Aza-6-Methyl-2-Silacyclooctane). J. Struct. Chem. 2008, 49, 378–381. [Google Scholar] [CrossRef]
- Ignatyev, I.S.; Kochina, T.A.; Avrorin, V.V.; Gurzhiy, V.V.; Fundamensky, V.S. Molecular and Crystal Structures of 2-Phenyl-2-Hydro-6-Methyl-1,3-Dioxa-6-Aza-2-Silacyclooctane. J. Mol. Struct. 2015, 1094, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Ignatyev, I.S.; Montejo, M.; Rodriguez Ortega, P.G.; Kochina, T.A.; López González, J.J. DFT Study of the Hydrolysis Reaction in Atranes and Ocanes: The Influence of Transannular Bonding. J. Mol. Model. 2015, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.M.; Brun, M.-C.; Cervantes-Lee, F.; Pannell, K.H. Synthesis, Structure, and Reactivity of the Permethylated Decasilane (Me3Si)3SiSiMe2SiMe2SiMe3)3. J. Organomet. Chem. 1995, 499, 247–252. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Day, R.O.; Holmes, R.R. A New Class of Silatranes: Structure and Dynamic NMR Behavior. J. Am. Chem. Soc. 2000, 122, 1066–1072. [Google Scholar] [CrossRef]
- Szpakolski, K.; Latham, K.; Rix, C.; Rani, R.A.; Kalantar-zadeh, K. Silane: A New Linker for Chromophores in Dye-Sensitised Solar Cells. Polyhedron 2013, 52, 719–732. [Google Scholar] [CrossRef]
- Baumgartner, J.; Frank, D.; Kayser, C.; Marschner, C. Comparative Study of Structural Aspects of Branched Oligosilanes. Organometallics 2005, 24, 750–761. [Google Scholar] [CrossRef]
- Fischer, J.; Baumgartner, J.; Marschner, C. Silylgermylpotassium Compounds. Organometallics 2005, 24, 1263–1268. [Google Scholar] [CrossRef]
- Fischer, R.; Frank, D.; Gaderbauer, W.; Kayser, C.; Mechtler, C.; Baumgartner, J.; Marschner, C. α,ω-Oligosilyl Dianions and Their Application in the Synthesis of Homo- and Heterocyclosilanes. Organometallics 2003, 22, 3723–3731. [Google Scholar] [CrossRef]
- Klare, H.F.T.; Bergander, K.; Oestreich, M. Taming the Silylium Ion for Low-Temperature Diels-Alder Reactions. Angew. Chem. Int. Ed. 2009, 48, 9077–9079. [Google Scholar] [CrossRef]
- Selina, A.; Karlov, S.; Zaitseva, G. Metallocanes of Group 14 Elements. 1. Derivatives of Silicon and Germanium. (Review). Chem. Heterocycl. Compd. 2006, 42, 1518–1556. [Google Scholar] [CrossRef]
- Hammerich, O. Methods for Studies of Electrochemical Reactions. In Organic Electrochemistry, 4th ed.; Hammerich, O., Lund, H., Eds.; CRC Press: Boca Raton, FL, USA, 2000; p. 102. ISBN 978-0-8247-0430-8. [Google Scholar]
- Hub, W.; Schneider, S.; Doerr, F.; Oxman, J.D.; Lewis, F.D. Trans-Stilbene-Amine Exciplexes. Stereoelectronic Control of Amine Dimer Cation Radical Formation. J. Am. Chem. Soc. 1984, 106, 701–708. [Google Scholar] [CrossRef]
- Broka, K.; Stradiņš, J.; Glezer, V.; Zelčāns, G.; Lukevics, E. Electrochemical Oxidation of Silatranes. J. Electroanal. Chem. 1993, 351, 199–206. [Google Scholar] [CrossRef]
- Sidorkin, V.F.; Belogolova, E.F.; Wang, Y.; Jouikov, V.; Doronina, E.P. Electrochemical Oxidation and Radical Cations of Structurally Non-rigid Hypervalent Silatranes: Theoretical and Experimental Studies. Chem. Eur. J. 2017, 23, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Romanovs, V.; Sidorkin, V.; Belogolova, E.; Jouikov, V. Radical Cations of Phenyl Silatrane. Dalton Trans. 2017, 46, 8849–8854. [Google Scholar] [CrossRef]
- Jouikov, V. Electrochemical Oxidation and Cation Radicals of All-Five and All-Six 1-Substituted Metallatranes (M = Si, Ge): Spectroelectrochemical Study. ECS Trans. 2010, 28, 5–16. [Google Scholar] [CrossRef]
- Mann, C.K. Cyclic Stationary Electrode Voltammetry of Some Aliphatic Amines. Anal. Chem. 1964, 36, 2424–2426. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Adenier, A.; Chehimi, M.M.; Gallardo, I.; Pinson, J.; Vilà, N. Electrochemical Oxidation of Aliphatic Amines and Their Attachment to Carbon and Metal Surfaces. Langmuir 2004, 20, 8243–8253. [Google Scholar] [CrossRef]
- Diaz, A.; Miller, R.D. Electro-Oxidation of Substituted Silane High Polymers. J. Electrochem. Soc. 1985, 132, 834–837. [Google Scholar] [CrossRef]
- Mochida, K.; Itani, A.; Yokoyama, M.; Tsuchiya, T.; Worley, S.D.; Kochi, J.K. A Correlation of Electrochemical Oxidation and Ionization Potentials of Group 4B Dimetals. Bull. Chem. Soc. Jpn. 1985, 58, 2149–2150. [Google Scholar] [CrossRef]
- Boberski, W.G.; Allred, A.L. Properties of Long-Chain Permethylpolysilanes. J. Organomet. Chem. 1975, 88, 65–72. [Google Scholar] [CrossRef]
- Diaz, A.F.; Baier, M.; Wallraff, G.M.; Miller, R.D.; Nelson, J.; Pietro, W. Electro-Oxidation of Some Soluble Alkyl and Aryl Substituted Polysilane Homopolymers. J. Electrochem. Soc. 1991, 138, 742. [Google Scholar] [CrossRef]
- Diaz, A.; Seymour, M.; Pannell, K.H.; Rozell, J.M. Electrochemistry of Polysilanes with Bound Ferrocene. J. Electrochem. Soc. 1990, 137, 503–506. [Google Scholar] [CrossRef]
- Biran, C.; Bordeau, M.; Pons, P.; Léger, M.-P.; Dunoguès, J. L’électrosynthèse, une voie simple d’accès aux di-et polysilanes. J. Organomet. Chem. 1990, 382, C17–C20. [Google Scholar] [CrossRef]
- Peureux, C.; Jouikov, V. Covalent Grafting of Silatranes to Carbon Interfaces. Chem. Eur. J. 2014, 20, 9290–9294. [Google Scholar] [CrossRef]
- Zhuikov, V. Electrochemical Oxidation of Hexaalkyldisilanes. Russ. J. Gen. Chem. 1999, 69, 1906–1911. [Google Scholar]
- Cleij, T.J.; King, J.K.; Jenneskens, L.W. Occurrence of Radical Cation Localization in Chemically Modified Poly(methylphenylsilane): Poly(methylphenyl-co-4-dimethylaminophenylmethylsilane)s and Poly(methylphenyl-co-4-bromophenylmethylsilane)s. Chem. Mater. 2000, 12, 84–89. [Google Scholar] [CrossRef]
- Imae, I.; Minami, T.; Kawakami, Y. Electrochemical Properties and Estimation of HOMO and LUMO Levels of Permethylated Oligosilanes with Well-Defined Structures. Des. Monomers Polym. 2004, 7, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Cerveau, G.; Chuit, C.; Colomer, E.; Corriu, R.J.P.; Reye, C. Ferrocenyl Compounds Containing Two Hypervalent Silicon Species. Electrochemical Studies. Organometallics 1990, 9, 2415–2417. [Google Scholar] [CrossRef]
- Pangborn, A.B.; Giardello, M.A.; Grubbs, R.H.; Rosen, R.K.; Timmers, F.J. Safe and Convenient Procedure for Solvent Purification. Organometallics 1996, 15, 1518–1520. [Google Scholar] [CrossRef]
- Lukens, W.W.; Matsunaga, P.T.; Andersen, R.A. Synthesis and Structure of Cp*2TiH, Cp*2TiH2Li(tmed), and [Cp*2TiOLi(THF)]2. Organometallics 1998, 17, 5240–5247. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kumada, M.; Sakurai, H. Preparation of Some Polysilicon Halides by Aluminum Halide Catalyzed Interchange of Methyl and Halogen on Silicon. J. Organomet. Chem. 1970, 23, 63–69. [Google Scholar] [CrossRef]
- Marschner, C.; Baumgartner, J. 4.4.5 Product Subclass 5: Disilanes and Oligosilanes. In Science of Synthesis: Houben-Weyl Methods of Molecular Transformations; Oestreich, M., Ed.; Thieme: Stuttgart, Germany, 2013. [Google Scholar] [CrossRef]
- Brook, A.G.; Abdesaken, F.; Söllradl, H. Synthesis of Some Tris(trimethylsilyl)germyl Compounds. J. Organomet. Chem. 1986, 299, 9–13. [Google Scholar] [CrossRef]
- Morris, G.A.; Freeman, R. Enhancement of Nuclear Magnetic Resonance Signals by Polarization Transfer. J. Am. Chem. Soc. 1979, 101, 760–762. [Google Scholar] [CrossRef]
- Blinka, T.A.; Helmer, B.J.; West, R. Polarization Transfer NMR Spectroscopy for Silicon-29: The INEPT and DEPT Techniques. Adv. Organomet. Chem. 1984, 23, 193–218. [Google Scholar] [CrossRef]
- SAINTPLUS: Software Reference Manual, Version 6.45; Bruker-AXS: Madison, WI, USA, 1997–2003.
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta Cryst. A 1995, 51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS. Version 2.10; Bruker AXS Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Princeton Applied Research. PowerSuite 2.58, I/O Library 2.43.0. Princeton Applied Research; Advanced Measurement Technology, Inc.: Oak Ridge, TN, USA, 2003. [Google Scholar]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; International Series of Monographs on Chemistry; Oxford University Press: Oxford, NY, USA, 1994; ISBN 978-0-19-855865-1. [Google Scholar]
- Keith, T.A.; Gristmill, T.K. AIMAll; TK Gristmill Software: Overland Park, KS, USA, 2019. [Google Scholar]







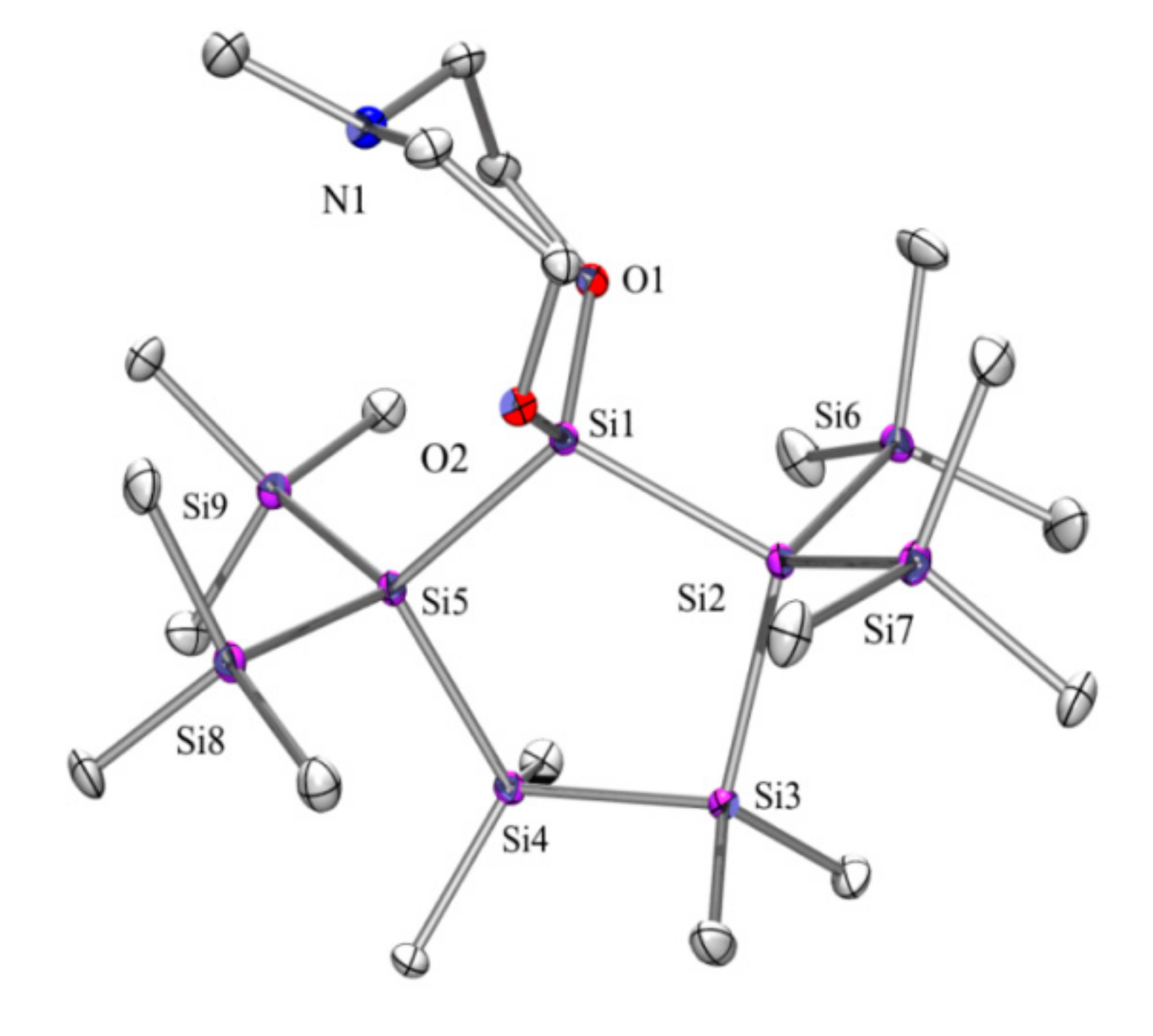










| Compd | C1 (N-Me) | C2 (CH2-N) | C3 (CH2-O) | C4 (Si-Me) | Si1 (SiO2) | Si2 (Siq) | Si3 SiMe3 | Si4 |
|---|---|---|---|---|---|---|---|---|
| 1 | 43.6 | 54.6 | 59.7 | 3.9 | −56.3 | n.a. | n.a. | n.a. |
| 3 a, d | 44.3 | 58.3 | 62.3 | 3.7 | 3.5 | −134.8 | −10.1 | n.a. |
| 4 b | 46.2 | 60.7 | 63.0 | 6.4 | 35.9 | −210.2 | −5.4 | n.a. |
| 5 a | 43.9 | 58.7 | 62.3 | 3.6 | 2.5 | −129.6 | −9.6 | −31.0 |
| 6 b | 43.1 | 55.3 | 60.2 | n.a. | −90.7 | n.a. | n.a. | n.a. |
| 7 a | 44.9 | 53.8 | 58.9 | n.a. | −89.4 | n.a. | n.a. | n.a. |
| 8 a | 47.3 | 59.3 | 66.4 | n.a. | 19.6 | −121.0 | −9.6 | n.a. |
| 8a a | 47.4 | 59.3 | 66.1 | n.a. | 17.7 | n.a. | −4.6 | n.a. |
| 9 a | 45.9 | 58.2 | 65.1 | n.a. | 33.1 | −133.2. | −7.7 | −30.4 |
| 10 b | 47.1 | 58.6 | 66.0 | n.a. | 19.2 | −131.8 | −9.5 | 12.5 |
| 12 c | 48.1 | 61.2 | 62.8 | n.a. | −13.0 | −208.7 | −5.4 | n.a. |
| 14 c | 47.3 | 60.6 | 64.8 | n.a. | 91.5 | −182.9 | −9.0 | −17.7 |
| 15 c | 47.9 | 61.7 | 64.5 | n.a. | 74.0 | −180.9 | −10.2 | 22.5 |
| Compd | lN…Si, Å | Ep, V | Ep-p/2, mV | Epa−Epc, mV | n | ks, cm s−1 |
|---|---|---|---|---|---|---|
| 5 | 2.934 | 1.173 | 75 | 85 | 1.0 | 0.03 |
| 7 | 2.171 a | 1.325 | 104 | 250 | 1.0 | 1.3 × 10−3 |
| 8 | 3.624 | 1.345 | 90 | 1.0 | 0.7 × 10−2 | |
| 9 | 3.425 | 1.365 | 81 | 210 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshgi, M.A.; Pöcheim, A.; Baumgartner, J.; Jouikov, V.V.; Marschner, C. Oligosilanylated Silocanes. Molecules 2021, 26, 244. https://doi.org/10.3390/molecules26010244
Meshgi MA, Pöcheim A, Baumgartner J, Jouikov VV, Marschner C. Oligosilanylated Silocanes. Molecules. 2021; 26(1):244. https://doi.org/10.3390/molecules26010244
Chicago/Turabian StyleMeshgi, Mohammad Aghazadeh, Alexander Pöcheim, Judith Baumgartner, Viatcheslav V. Jouikov, and Christoph Marschner. 2021. "Oligosilanylated Silocanes" Molecules 26, no. 1: 244. https://doi.org/10.3390/molecules26010244






