Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Antibacterial Activity
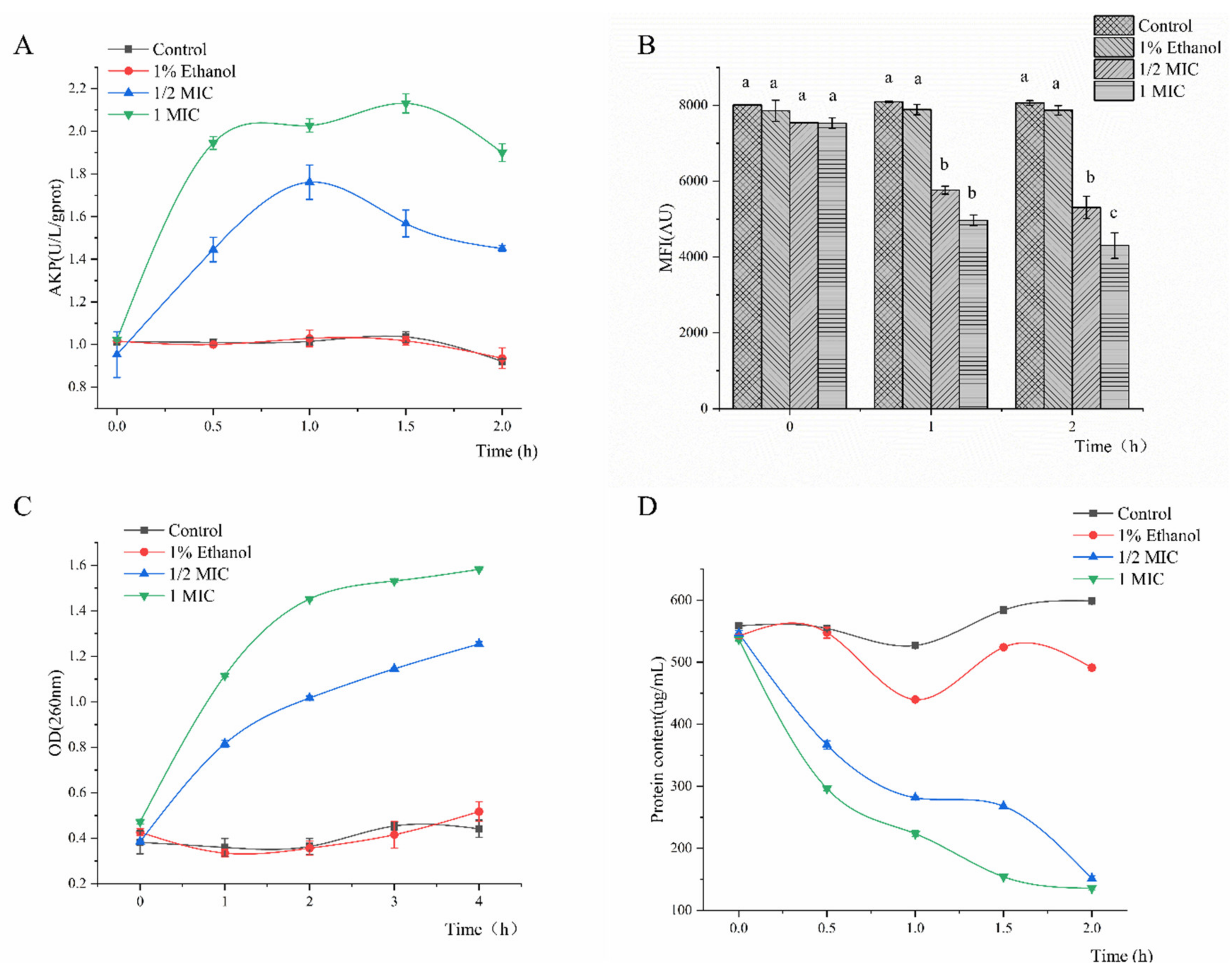
2.2. Cell Wall Permeability
2.3. Membrane Potential (MP)
2.4. Effect of Linalool on Macromolecules
2.5. Effect of Linalool on Proteins
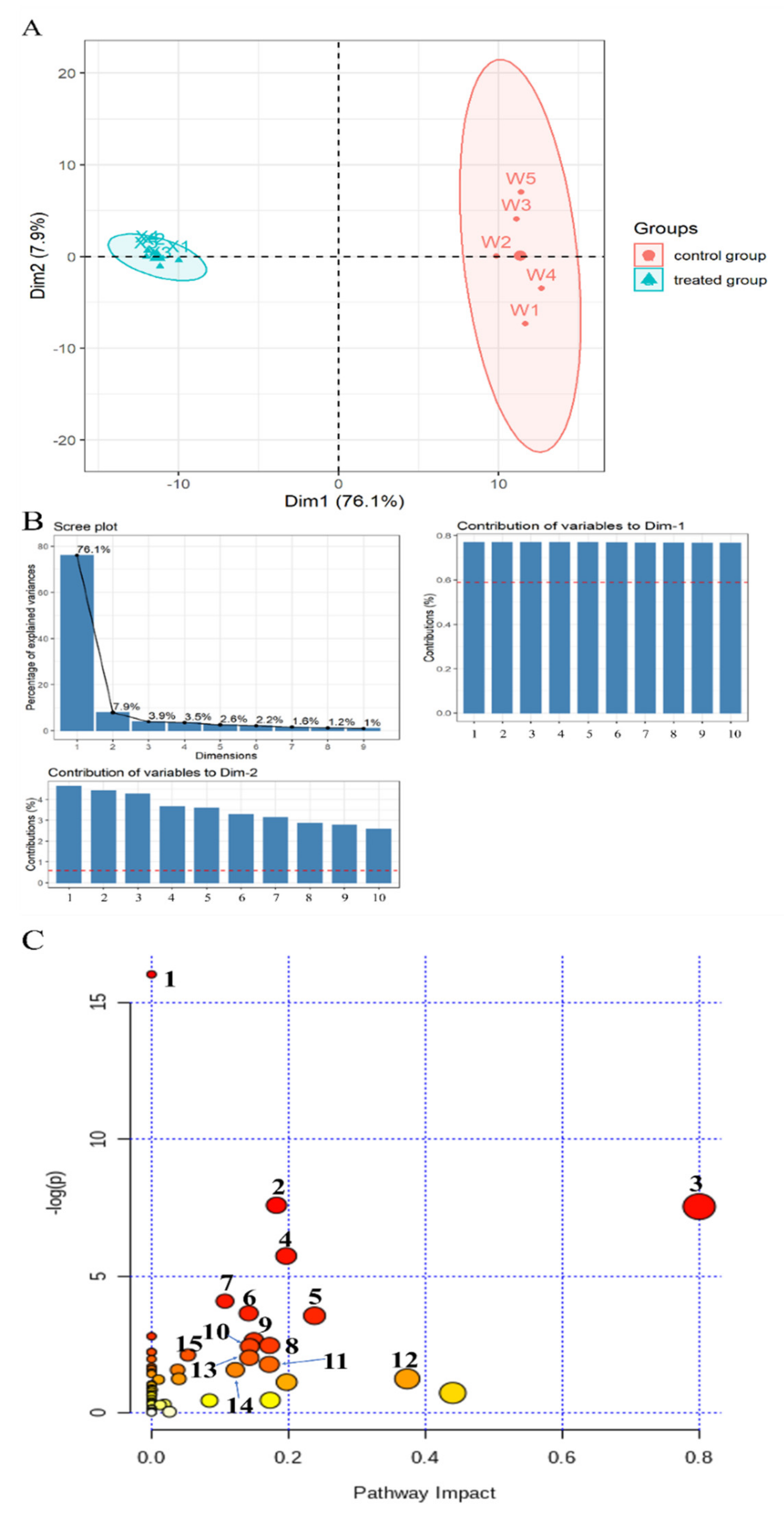
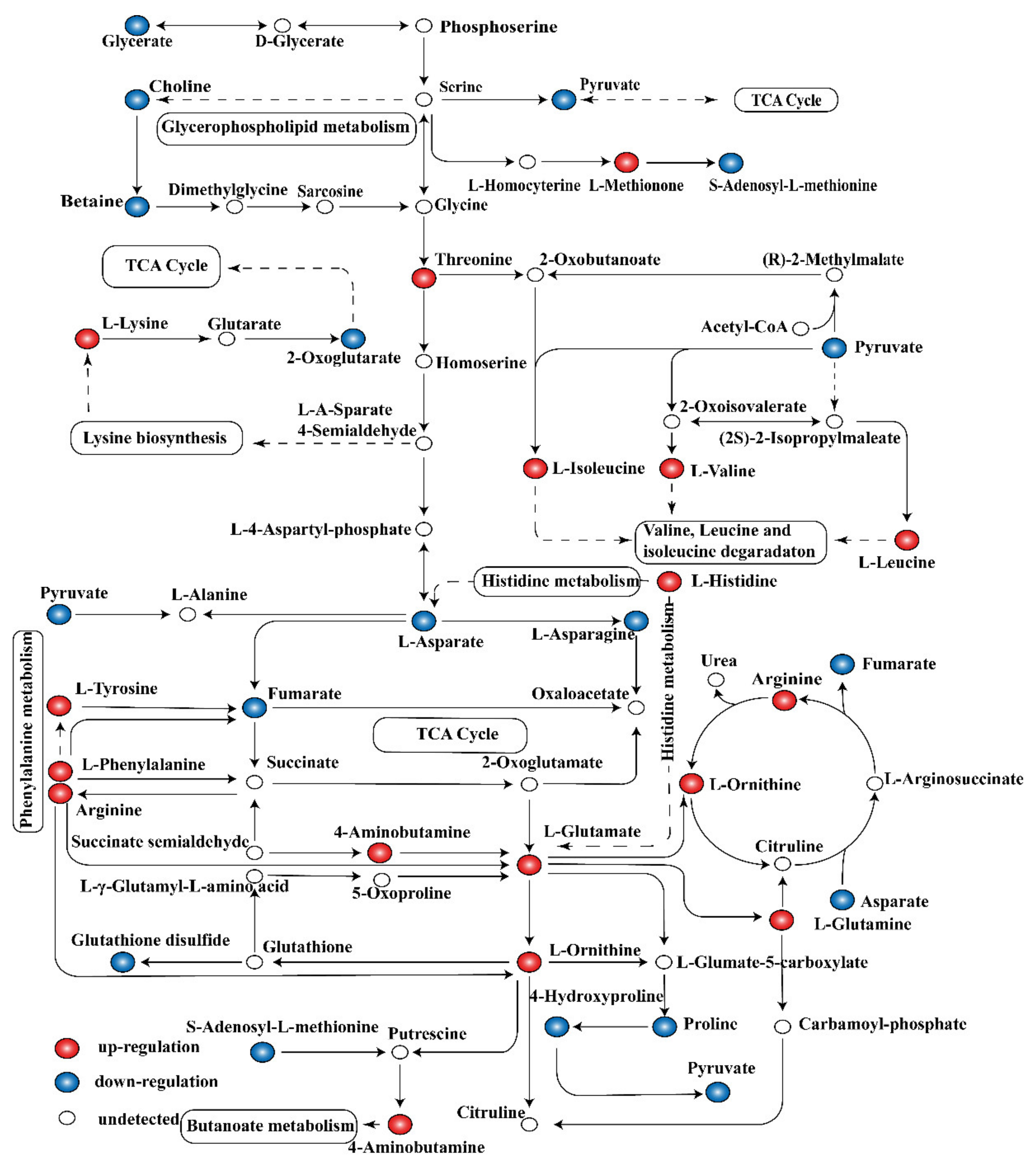
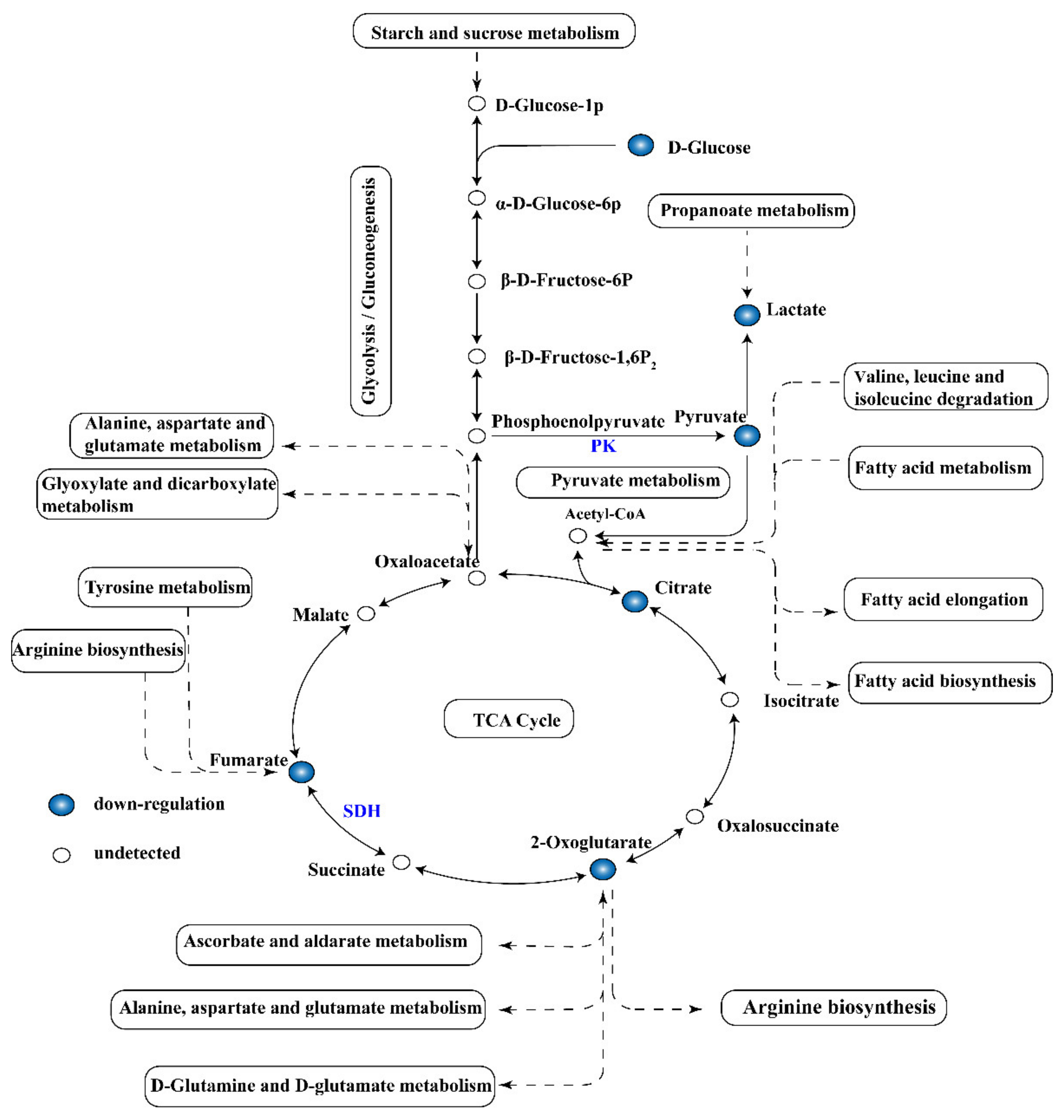
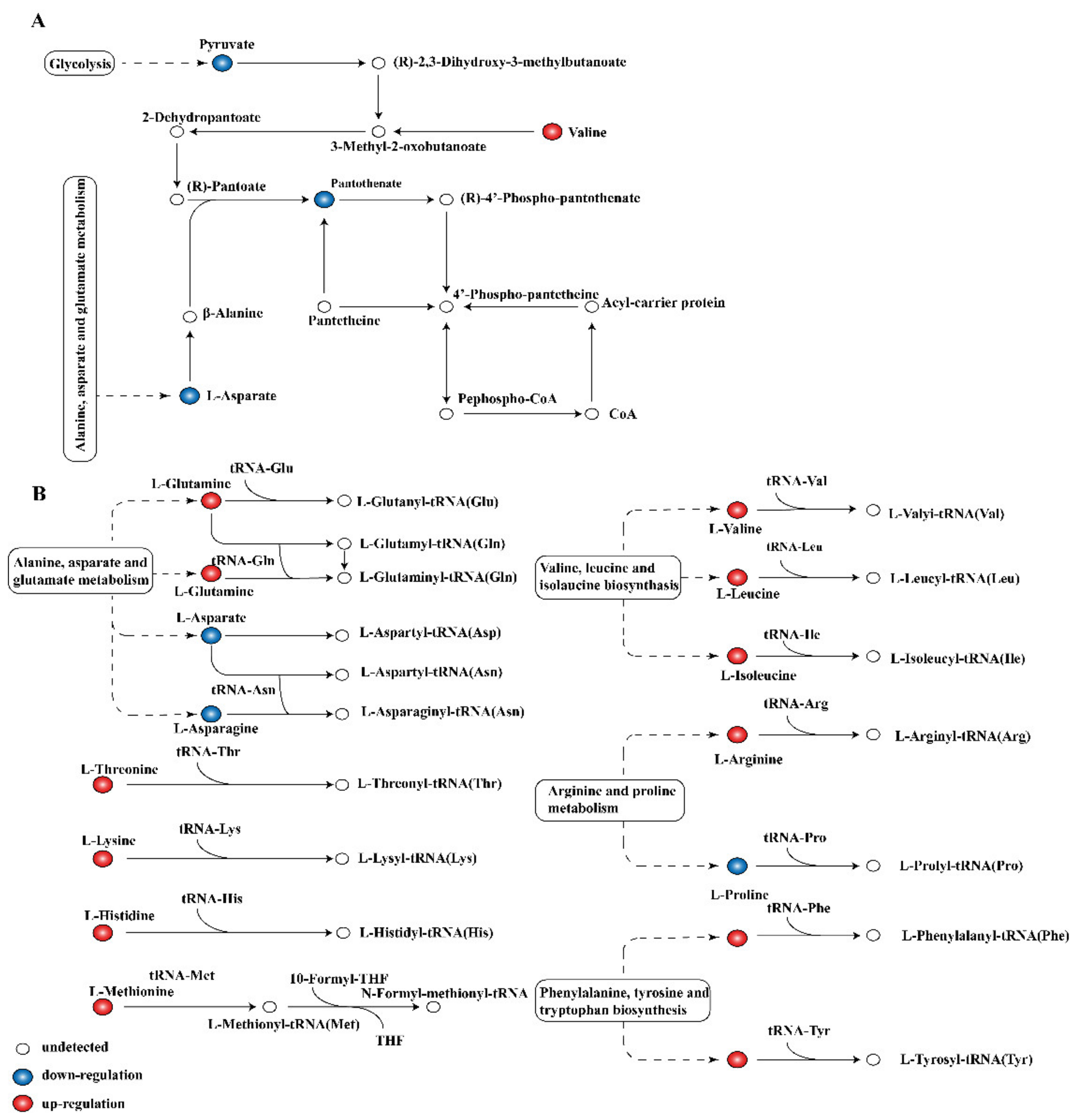
2.6. Global Analysis of Metabolomic Response
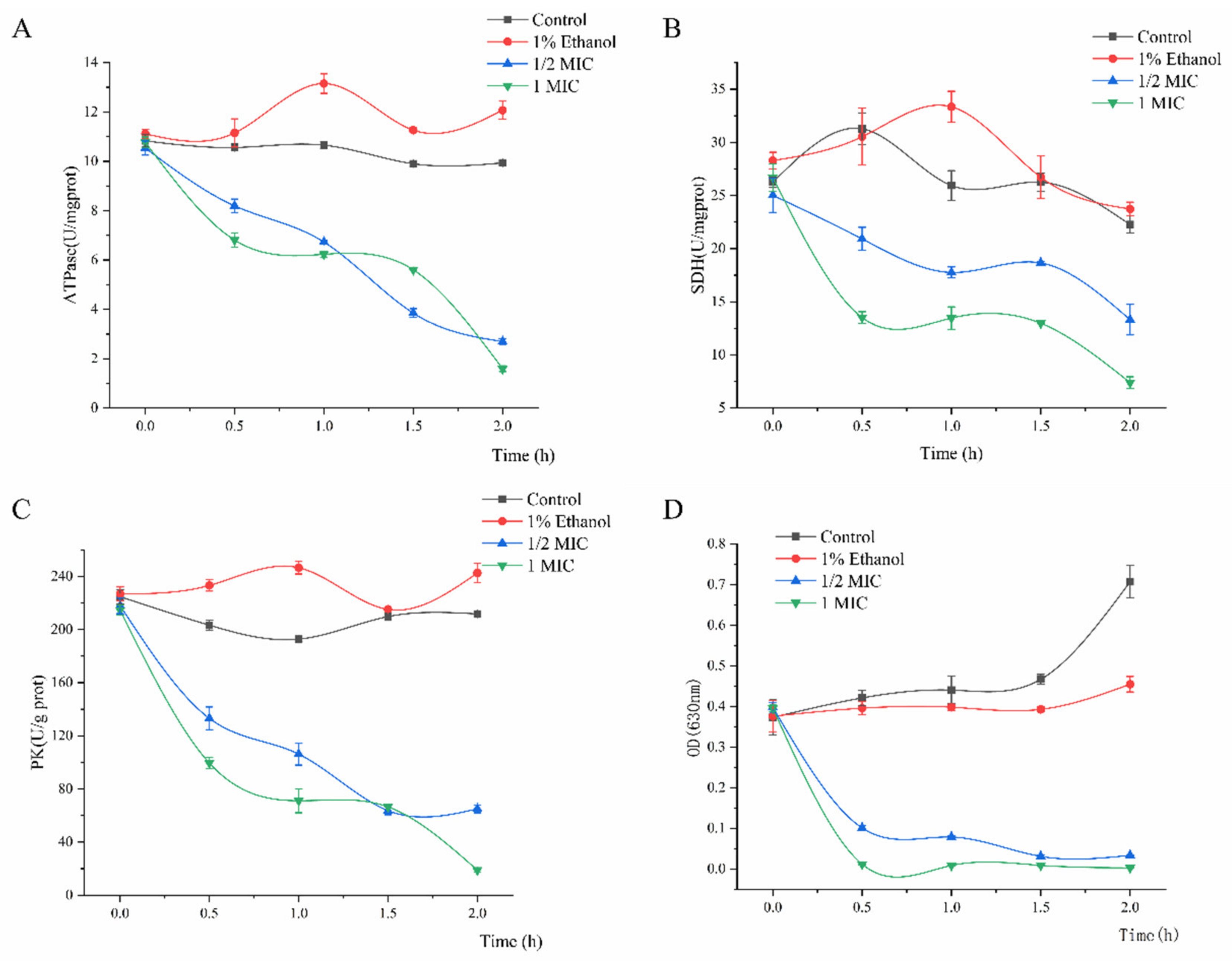
2.7. Activity of Key Enzymes and Respiratory Metabolism
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. MIC Determination
4.3. S. putrefaciens Growth Curve
4.4. MP Determination
4.5. Alkaline Phosphatase (AKP) Activity Determination
4.6. Loss of DNA and RNA
4.7. Protein Leakage
4.8. Metabolomics Analysis
4.9. Activity Determination for Adenosine Triphosphatase (ATPase), Succinic Acid Dehydrogenase (SDH), and Pyruvate Kinase (PK)
4.10. Activity of Respiratory Chain Dehydrogenase
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diao, W.R.; Hu, Q.P.; Feng, S.S.; Li, W.Q.; Xu, J.G. Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens. J. Agric. Food Chem. 2013, 61, 6044–6049. [Google Scholar] [CrossRef]
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; He, Q. Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara). J. Food Sci. 2012, 77, C824–C829. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.N.; Khan, I.; Kang, S.C. Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub. Asian Pac. J. Trop. Med. 2015, 8, 694–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.F.; Ye, J.X.; Yang, S.P.; Lin, Z.Q.; Cao, W.; Xie, J. Evaluation of the spoilage potential of Shewanella putrefaciens, Aeromonas hydrophila, and Aeromonas sobria isolated from spoiled Pacific white shrimp (Litopenaeus vannamei) during cold storage. J. Food Saf. 2018. [Google Scholar] [CrossRef]
- Bernshteyn, M.; Ashok Kumar, P.; Joshi, S. Shewanella algae—A Novel Organism Causing Bacteremia: A Rare Case and Literature Review. Cureus 2020, 12, 10676. [Google Scholar] [CrossRef]
- Shokri, S.; Parastouei, K.; Taghdir, M.; Abbaszadeh, S. Application an edible active coating based on chitosan- Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4 degrees C. Int. J. Biol. Macromol. 2020, 153, 846–854. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, Y.; Li, T.; Zhao, H.; Li, X.; Li, J.; Sun, T. Effects of lysozyme combined with cinnamaldehyde on storage quality of olive flounder (Paralichthys olivaceus) fillets. J. Food Sci. 2020, 85, 1037–1044. [Google Scholar] [CrossRef]
- Markley, J.L.; Bruschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Makenzi, N.G.; Mbinda, W.M.; Okoth, R.O.; Ngugi, M.P. In Vitro Plant Regeneration of Sweetpotato Through Direct Shoot Organogenesis. J. Plant Biochem. Physiol. 2018, 5, 207. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, L.; Laserna, A.K.C.; He, Y.; Feng, X.; Yang, H. Synergistic action of electrolyzed water and mild heat for enhanced microbial inactivation of Escherichia coli O157:H7 revealed by metabolomics analysis. Food Control 2020. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Wang, X.; Yu, J.; Li, Z.; Lin, W.; Lin, X. Systematically integrated metabonomic-proteomic studies of Escherichia coli under ciprofloxacin stress. J. Proteomics 2018, 179, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Xu, L.; Lin, Y.; Cai, X.; Wang, S. The preservative potential of Octopus scraps peptides−Zinc chelate against Staphylococcus aureus: Its fabrication, antibacterial activity and action mode. Food Control 2019, 98, 24–33. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Zhang, L.-F.; Hu, Q.-P.; Hao, D.-L.; Xu, J.-G. Chemical composition, antibacterial activity of Cyperus rotundus rhizomes essential oil against Staphylococcus aureus via membrane disruption and apoptosis pathway. Food Control 2017, 80, 290–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Wang, H.; Zou, D.; Xie, K.; Xie, M. Antibacterial mechanism of fraxetin against Staphylococcus aureus. Mol. Med. Rep. 2014, 10, 2341–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, M.; Qi, D.; Xu, M.; Lu, Z.; Lv, F.; Bie, X.; Zhang, C.; Zhao, H. Antibacterial activity and mechanism of monolauroyl-galactosylglycerol against Bacillus cereus. Food Control 2018, 85, 339–344. [Google Scholar] [CrossRef]
- Pei, Q.; Li, Y.; Ge, X.; Tian, P. Multipath effects of berberine on peach Brown rot fungus Monilinia fructicola. Crop Prot. 2019, 116, 92–100. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, N.; Liu, S.; Chen, M.; Xie, J. epsilon-Polylysine Inhibits Shewanella putrefaciens with Membrane Disruption and Cell Damage. Molecules 2019, 24, 3727. [Google Scholar] [CrossRef] [Green Version]
- Shu, H.; Zhang, W.; Yun, Y.; Chen, W.; Zhong, Q.; Hu, Y.; Chen, H.; Chen, W. Metabolomics study on revealing the inhibition and metabolic dysregulation in Pseudomonas fluorescens induced by 3-carene. Food Chem. 2020, 329, 127220. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Hou, Q.; Tian, X.; Fan, X.; Zhang, W.; Zhou, G. Comparison of activity, expression and S-nitrosylation of glycolytic enzymes between pale, soft and exudative and red, firm and non-exudative pork during post-mortem aging. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-h.; Zhou, T.-t.; Wei, C.-h.; Lan, W.-q.; Zhao, Y.; Pan, Y.-j.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver nanoparticles as an antimicrobial agent: A case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Planson, A.G.; Sauveplane, V.; Dervyn, E.; Jules, M. Bacterial growth physiology and RNA metabolism. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194502. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, T.; Tamaoka, K.; Furusawa, C.; Ono, N.; Suzuki, S.; Hirasawa, T.; Yomo, T.; Shimizu, H. Transcriptome analysis of parallel-evolved Escherichia coli strains under ethanol stress. BMC Genomics 2010, 11, 579. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-Y.; Jo, S.-K.; Park, K.-M.; Yu, H.; Bai, J.; Ryu, S.; Chang, P.-S. Transcriptomic analysis of Staphylococcus aureus under the stress condition of antibacterial erythorbyl laurate by RNA sequencing. Food Control 2019, 96, 1–8. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, G.; Leng, C.; Zhang, Y.; Lv, X.; Chen, H.; Sun, J.; Feng, Z. Metabolic profiles of cysteine, methionine, glutamate, glutamine, arginine, aspartate, asparagine, alanine and glutathione in Streptococcus thermophilus during pH-controlled batch fermentations. Sci. Rep. 2018, 8, 12441. [Google Scholar] [CrossRef]
- Cabral, M.P.; Soares, N.C.; Aranda, J.; Parreira, J.R.; Rumbo, C.; Poza, M.; Valle, J.; Calamia, V.; Lasa, I.; Bou, G. Proteomic and functional analyses reveal a unique lifestyle for Acinetobacter baumannii biofilms and a key role for histidine metabolism. J. Proteome. Res. 2011. [Google Scholar] [CrossRef]
- Jang, I.A.; Kim, J.; Park, W. Endogenous hydrogen peroxide increases biofilm formation by inducing exopolysaccharide production in Acinetobacter oleivorans DR1. Sci. Rep. 2016, 6, 21121. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Gao, L.; Jiang, W.; Lin, W.; Niu, C.; Yuan, K.; Ma, R.; Huang, Z. The Impairment of Methyl Metabolism From luxS Mutation of Streptococcus mutans. Front. Microbiol. 2018, 9, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, M.; Zuniga, M. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 2006, 32, 155–183. [Google Scholar] [CrossRef] [PubMed]
- Gillner, D.; Armoush, N.; Holz, R.C.; Becker, D.P. Inhibitors of bacterial N-succinyl-L,L-diaminopimelic acid desuccinylase (DapE) and demonstration of in vitro antimicrobial activity. Bioorg. Med. Chem. Lett. 2009, 19, 6350–6352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usha, V.; Lloyd, A.J.; Lovering, A.L.; Besra, G.S. Structure and function of Mycobacterium tuberculosis meso-diaminopimelic acid (DAP) biosynthetic enzymes. FEMS Microbiol. Lett. 2012, 330, 10–16. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L.; Shin, H.-d.; Chen, R.R.; Zhang, J.; Li, J.; Du, G.; Shi, Z.; Chen, J. Systems-level understanding of how Propionibacterium acidipropionici respond to propionic acid stress at the microenvironment levels: Mechanism and application. J. Biotechnol. 2013, 167, 56–63. [Google Scholar] [CrossRef]
- Teixeira, J.S.; Seeras, A.; Sanchez-Maldonado, A.F.; Zhang, C.; Su, M.S.-W.; Gänzle, M.G. Glutamine, glutamate, and arginine-based acid resistance in Lactobacillus reuteri. Food Microbiol. 2014, 42, 172–180. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Branched-Chain Amino Acids: Metabolism, Physiological Function, and Application. J. Nutr. 2006, 136, 319S–323S. [Google Scholar]
- Yuan, H.; Xu, Y.; Chen, Y.; Zhan, Y.; Wei, X.; Li, L.; Wang, D.; He, P.; Li, S.; Chen, S. Metabolomics analysis reveals global acetoin stress response of Bacillus licheniformis. Metabolomics 2019, 15, 25. [Google Scholar] [CrossRef]
- Zhai, Q.; Xiao, Y.; Narbad, A.; Chen, W. Comparative metabolomic analysis reveals global cadmium stress response of Lactobacillus plantarum strains. Metallomics 2018, 10, 1065–1077. [Google Scholar] [CrossRef]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Liu, M.; Feng, M.; Yang, K.; Cao, Y.; Zhang, J.; Xu, J.; Hernández, S.H.; Wei, X.; Fan, M. Transcriptomic and metabolomic analyses reveal antibacterial mechanism of astringent persimmon tannin against Methicillin-resistant Staphylococcus aureus isolated from pork. Food Chem. 2020, 309, 125692. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, L.; Li, D.; Xia, H.; Su, X.; Peng, L.; Pan, S. Use of active extracts of poplar buds against Penicillium italicum and possible modes of action. Food Chem. 2016, 196, 610–618. [Google Scholar] [CrossRef]
- Vinayavekhin, N.; Mahipant, G.; Vangnai, A.S.; Sangvanich, P. Untargeted metabolomics analysis revealed changes in the composition of glycerolipids and phospholipids in Bacillus subtilis under 1-butanol stress. Appl. Microbiol. Biotechnol. 2015, 99, 5971–5983. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wu, J.; Wu, M. Enhanced acetoin production by Bacillus amyloliquefaciens through improved acetoin tolerance. Process Biochem. 2014, 49, 1223–1230. [Google Scholar] [CrossRef]
- Anaokar, S.; Kodali, R.; Jonik, B.; Renne, M.F.; Brouwers, J.; Lager, I.; de Kroon, A.; Patton-Vogt, J. The glycerophosphocholine acyltransferase Gpc1 is part of a phosphatidylcholine (PC)-remodeling pathway that alters PC species in yeast. J. Biol. Chem. 2019, 294, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Khakbaz, P.; Chen, Y.; Lombardo, J.; Yoon, J.M.; Shanks, J.V.; Klauda, J.B.; Jarboe, L.R. Engineering Escherichia coli membrane phospholipid head distribution improves tolerance and production of biorenewables. Metab. Eng. 2017, 44, 1–12. [Google Scholar] [CrossRef]
- Zheng, G.; Li, W. Profiling membrane glycerolipids during gamma-ray-induced membrane injury. BMC Plant Biol. 2017, 17, 203. [Google Scholar] [CrossRef] [Green Version]
- Hussein, M.; Schneider-Futschik, E.K.; Paulin, O.K.A.; Allobawi, R.; Crawford, S.; Zhou, Q.T.; Hanif, A.; Baker, M.; Zhu, Y.; Li, J.; et al. Effective Strategy Targeting Polymyxin-Resistant Gram-Negative Pathogens: Polymyxin B in Combination with the Selective Serotonin Reuptake Inhibitor Sertraline. ACS Infect. Dis. 2020, 6, 1436–1450. [Google Scholar] [CrossRef]
- Shimada, Y.J.; Batra, J.; Kochav, S.M.; Patel, P.; Jung, J.; Maurer, M.S.; Hasegawa, K.; Reilly, M.P.; Fifer, M.A. Difference in Metabolomic Response to Exercise between Patients with and without Hypertrophic Cardiomyopathy. J. Cardiovasc. Transl. Res. 2020, 1–10. [Google Scholar] [CrossRef]
- Kuncha, S.K.; Kruparani, S.P.; Sankaranarayanan, R. Chiral checkpoints during protein biosynthesis. J. Biol. Chem. 2019, 294, 16535–16548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, C.; Huang, X.; Wu, Y.; Zhan, J.; Zhang, X.; Liu, Q.; Huang, Y. Untargeted metabolomics study and pro-apoptotic properties of B-norcholesteryl benzimidazole compounds in ovarian cancer SKOV3 cells. J. S. B. Mol. Biol. 2020, 202. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Chen, H.; Wang, X.; Hu, Y.; Yun, Y.; Zhong, Q.; Chen, W.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, W.C.; Zhu, R.X.; Jia, L.R.; Gao, H.; Zheng, Y.; Sun, Q. Chemical composition, antimicrobial and antioxidant activities of essential oil from Gnaphlium affine. Food Chem. Toxicol. 2011, 49, 1322–1328. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Lin, L. The specific antibacterial activity of liposome-encapsulated Clove oil and its application in tofu. Food Control 2015, 56, 128–134. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.W.; Kim, S.S.; Kang, D.H. Inactivation dynamics of 222nm krypton-chlorine excilamp irradiation on Gram-positive and Gram-negative foodborne pathogenic bacteria. Food Res. Int. 2018, 109, 325–333. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens. Molecules 2021, 26, 245. https://doi.org/10.3390/molecules26010245
Guo F, Liang Q, Zhang M, Chen W, Chen H, Yun Y, Zhong Q, Chen W. Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens. Molecules. 2021; 26(1):245. https://doi.org/10.3390/molecules26010245
Chicago/Turabian StyleGuo, Fengyu, Qiong Liang, Ming Zhang, Wenxue Chen, Haiming Chen, Yonghuan Yun, Qiuping Zhong, and Weijun Chen. 2021. "Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens" Molecules 26, no. 1: 245. https://doi.org/10.3390/molecules26010245
APA StyleGuo, F., Liang, Q., Zhang, M., Chen, W., Chen, H., Yun, Y., Zhong, Q., & Chen, W. (2021). Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens. Molecules, 26(1), 245. https://doi.org/10.3390/molecules26010245





