Tunable Properties of Nature-Inspired N,N′-Alkylated Riboflavin Semiconductors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Thermal Properties
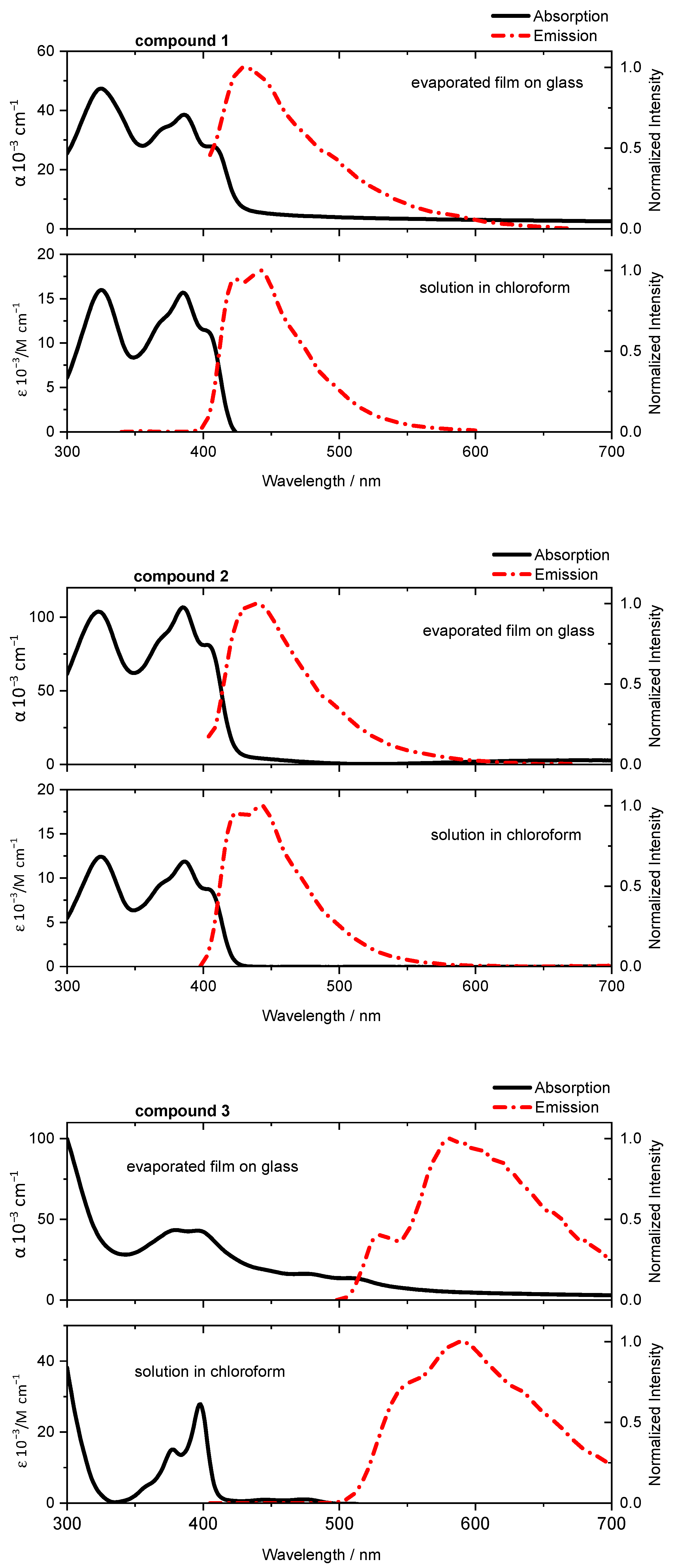
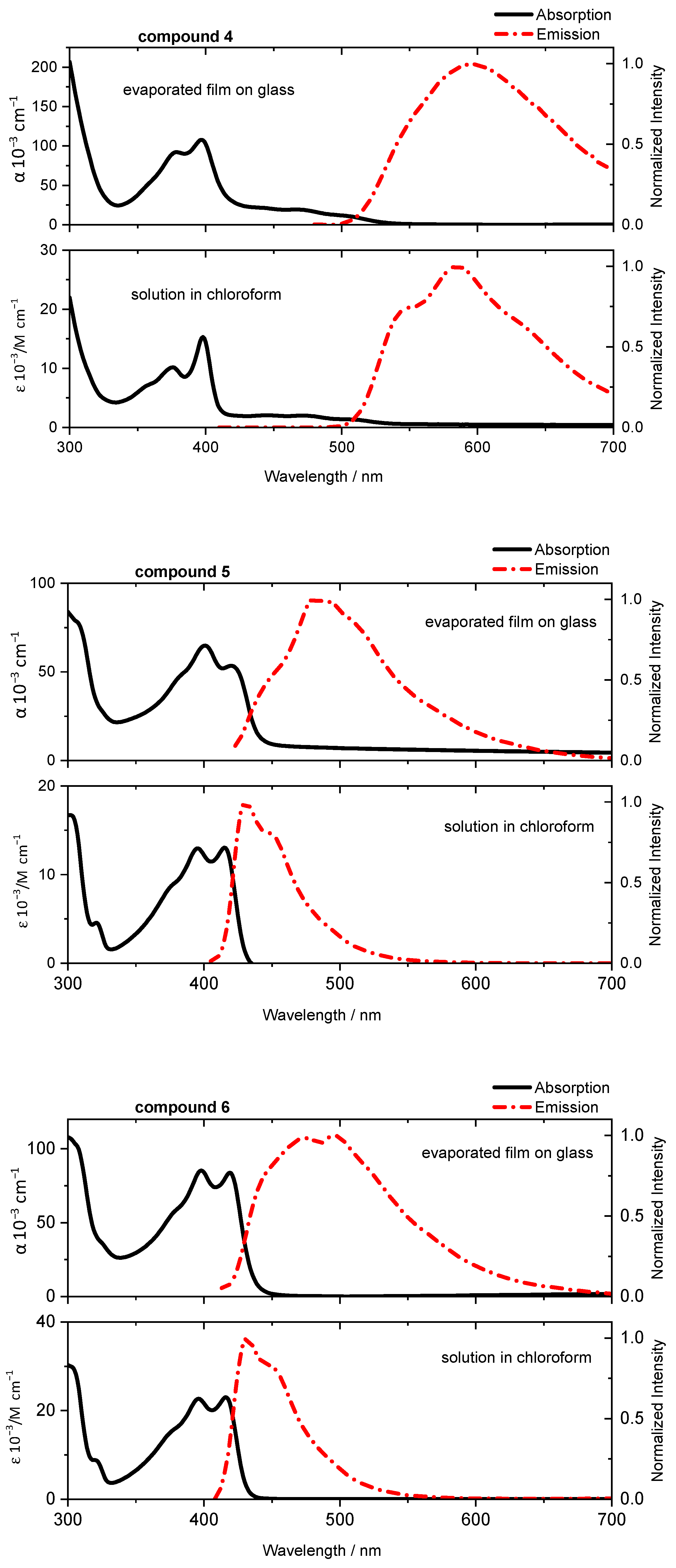
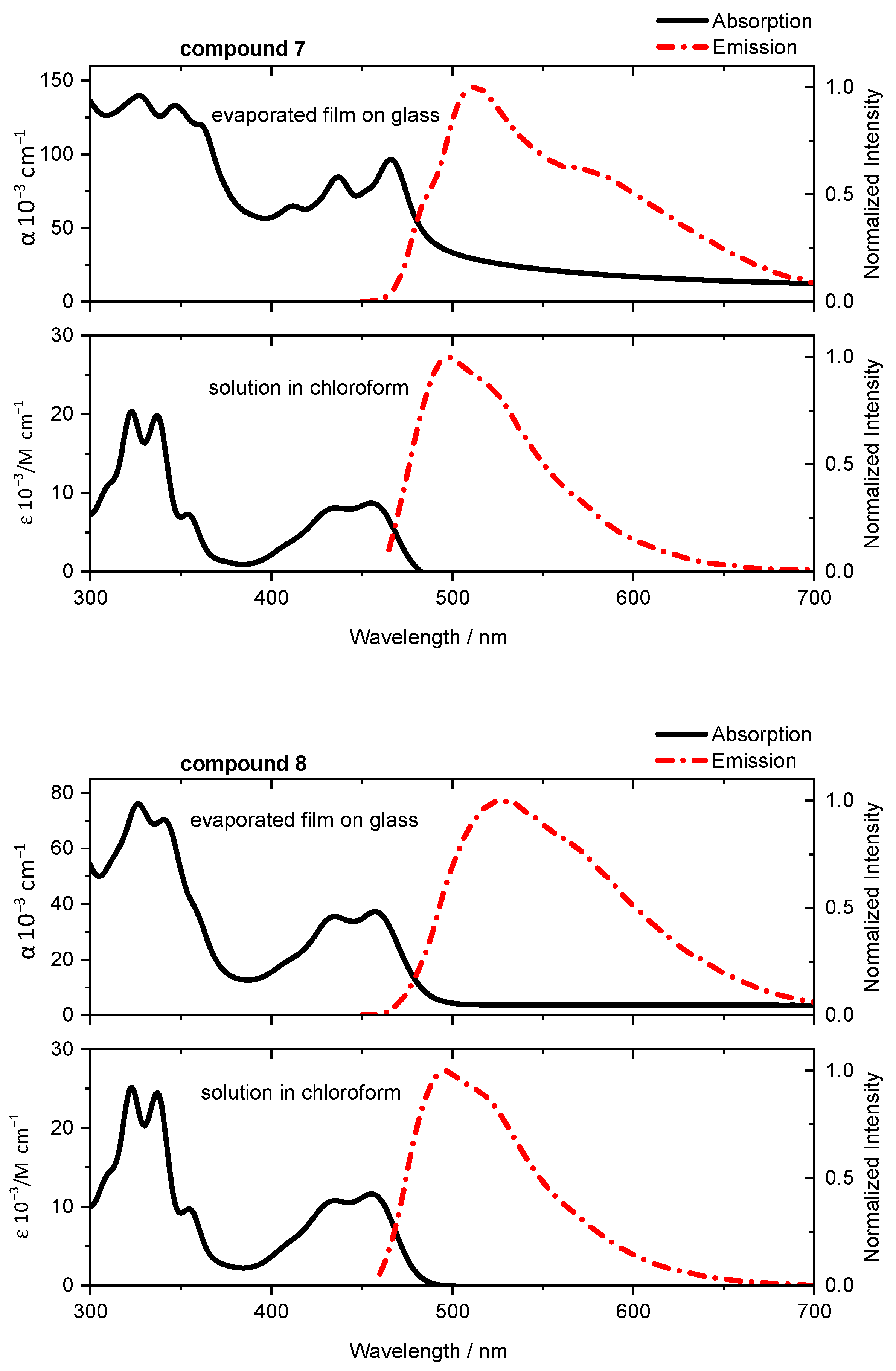
2.3. Optical and Electrical Properties
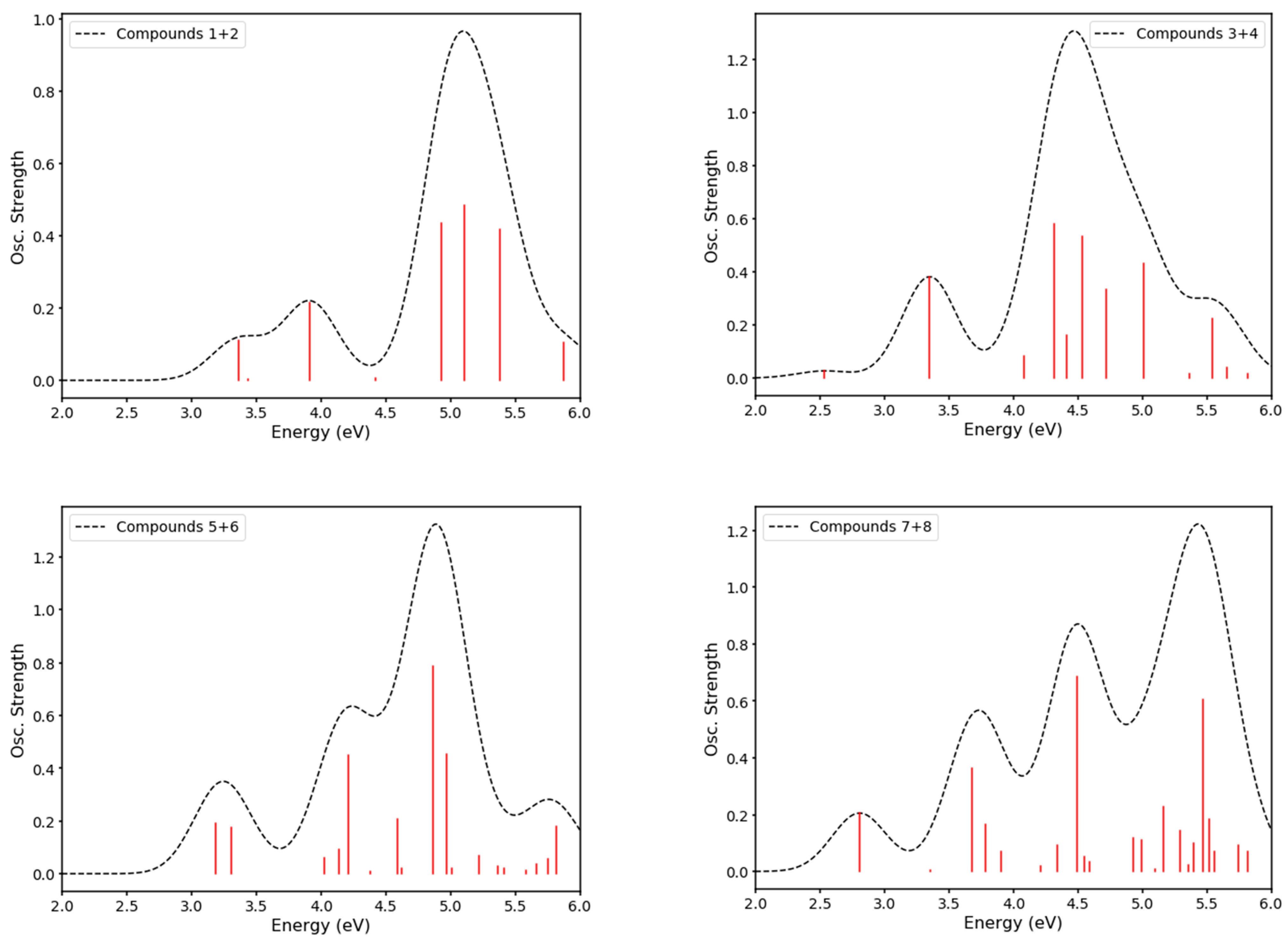
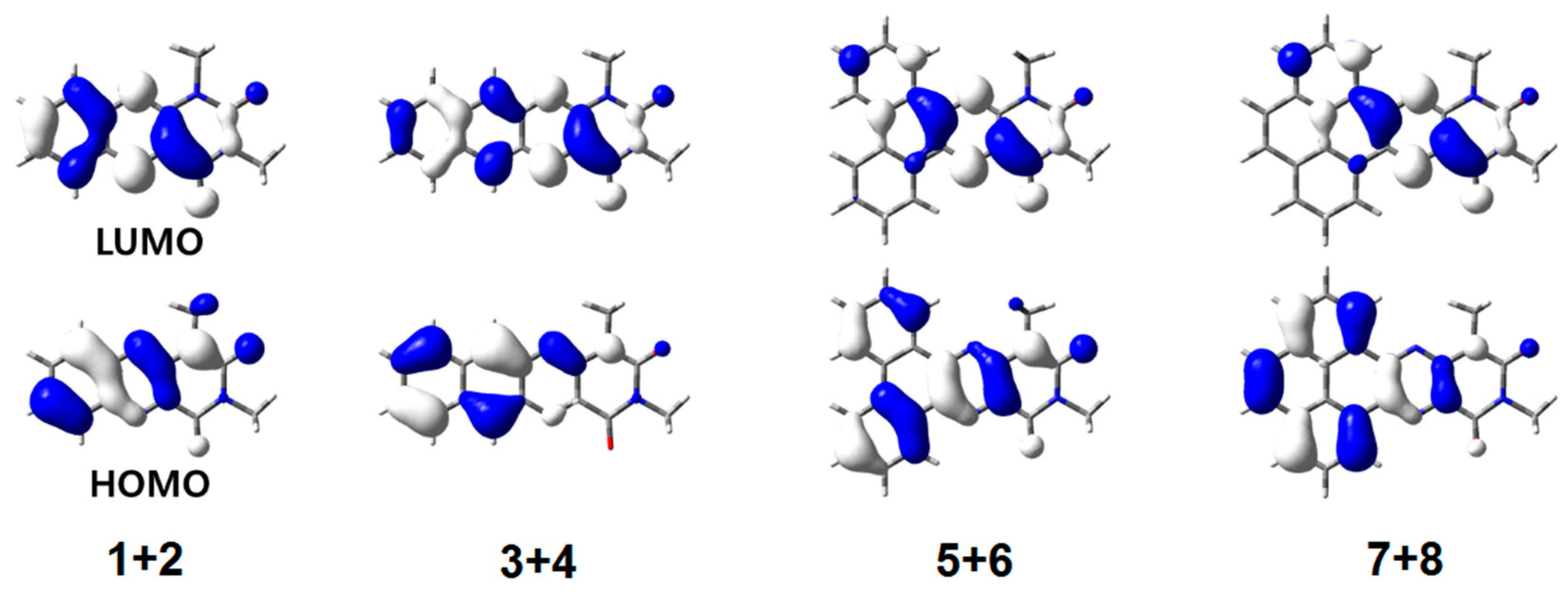
2.4. DFT Modeling
2.5. Electrochemical Measurements
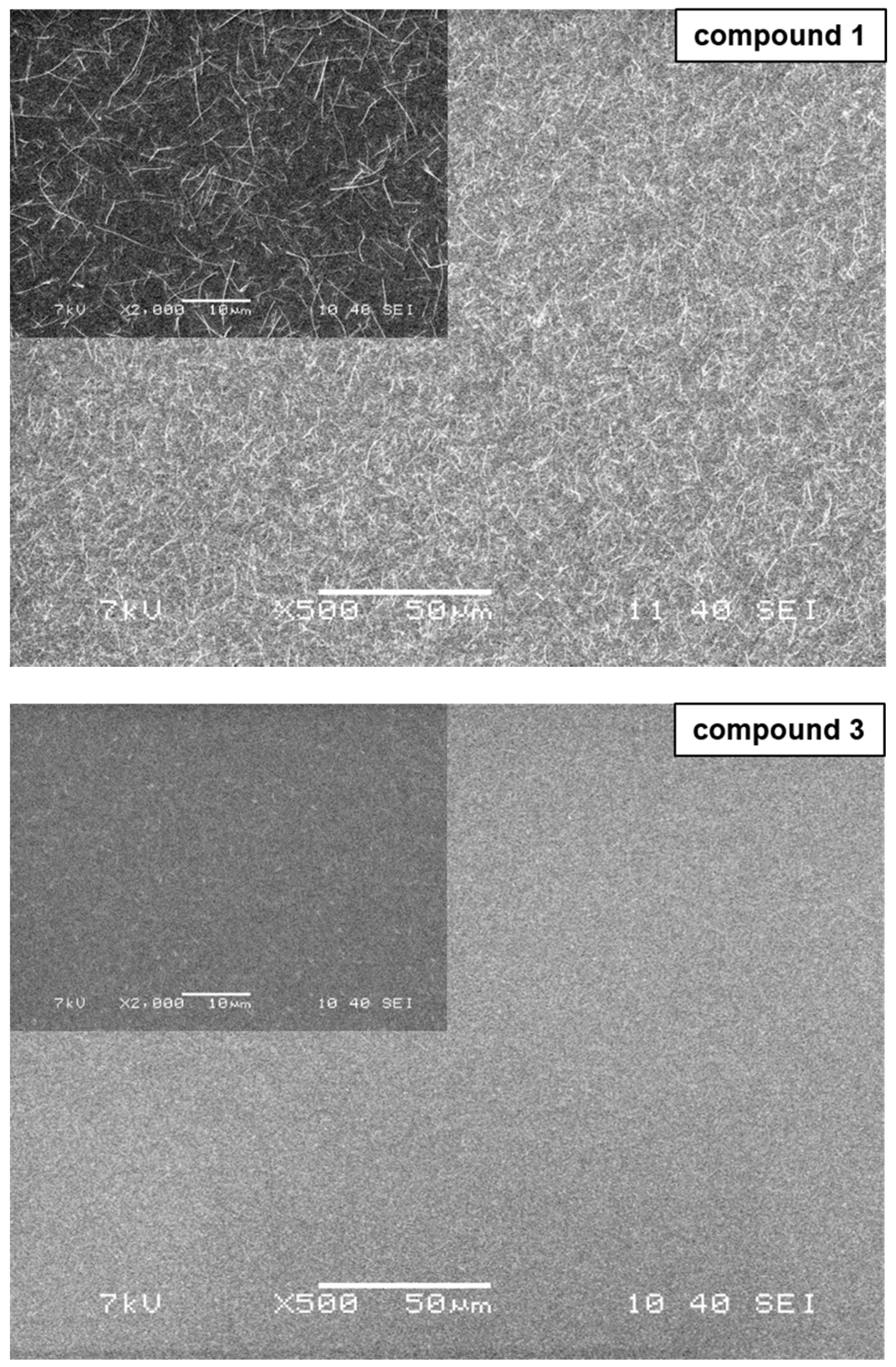
2.6. Thin Film Microscopy
2.7. Thin Film Aging
3. Conclusions
4. Experimental Section
4.1. Materials and Synthesis
4.2. General Procedure for the Synthesis of N,N′-Dialkylated Flavins
4.3. 1,3-Dibutylbenzo[g]pteridine-2,4(1H,3H)-dione (1)
4.4. 1,3-Bis(2-(-adamantan-1-yl)ethyl)benzo[g]pteridine-2,4(1H,3H)- dione (2)
4.5. 1,3-Dibutylnaphtho[2,3-g]pteridine-2,4(1H,3H)-dione (3)
4.6. 1,3-Bis(2-(adamantan-1-yl)ethyl)naphtho[2,3-g]pteridine2,4(1H,3H)-dione (4)
4.7. 10,12-dibutylphenanthro[9,10-g]pteridine-11,13(10H,12H)-dione (5)
4.8. 10,12-Bis(2-(adamantan-1-yl)ethyl)phenanthro[9,10-g]pteridine11,13(10H,12H)-dione (6)
4.9. 10,12-Dibutyl-5a1,10-dihydropyreno[4,5-g]pteridine11,13(3a1H,12H)-dione (7)
4.10. 10,12-Bis(2-(adamantan-1-yl)ethyl)-5a1,10-dihydropyreno[4,5-g]pteridine-11,13(3a1H,12H)-dione (8)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irimia-Vladu, M.; Glowacki, E.D.; Sariciftci, N.S.; Bauer, S. Green Materials for Electronics; Wiley-VCH: Weinheim, Germany, 2017; p. 39. [Google Scholar]
- Glowacki, E.D.; Tangorra, R.R.; Coskun, H.; Farka, D.; Operamolla, A.; Kanbur, Y.; Milano, F.; Giotta, L.; Farinola, G.M.; Sariciftci, N.S. Bioconjugation of hydrogen-bonded organic semiconductors with functional proteins. J. Mater. Chem. C 2015, 3, 6554. [Google Scholar] [CrossRef] [Green Version]
- Simon, D.T.; Gabrielsson, E.O.; Tybrandt, K.; Berggren, M. Organic Bioelectronics: Bridging the Signaling Gap between Biology and Technology. Chem. Rev. 2016, 116, 13009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbarella, G.; Di Maria, F. Supramolecular Oligothiophene Microfibers Spontaneously Assembled on Surfaces or Coassembled with Proteins inside Live Cells. Acc. Chem. Res. 2015, 48, 2230. [Google Scholar] [CrossRef] [PubMed]
- Kanbur, Y.; Coskun, H.; Głowacki, E.D.; Irimia-Vladu, M.; Sariciftci, N.S.; Yumusak, C. High temperature-stability of organic thin-film transistors based on quinacridone pigments. Org. Electron. 2019, 66, 53. [Google Scholar] [CrossRef]
- Jürgensen, N.; Ackermann, M.; Marszalek, T.; Zimmermann, J.; Morfa, A.J.; Pisula, W.; Bunz, U.H.F.; Hinkel, F.; Hernandez-Sosa, G. Solution-Processed Bio-OLEDs with a Vitamin-Derived Riboflavin Tetrabutyrate Emission Layer. ACS Sustain. Chem. Eng. 2017, 5, 5368. [Google Scholar] [CrossRef]
- Glowacki, E.D.; Irimia-Vladu, M.; Kaltenbrunner, M.; Gasiorowski, J.; White, M.S.; Monkowius, U.; Romanazzi, G.; Suranna, G.P.; Mastrorilli, P.; Sekitani, T.; et al. Hydrogen-Bonded Semiconducting Pigments for Air-Stable Field-Effect Transistors. Adv. Mater. 2013, 25, 1563. [Google Scholar] [CrossRef]
- Glowacki, E.D.; Apaydin, D.H.; Bozkurt, Z.; Monkowius, U.; Demirak, K.; Tordin, E.; Himmelsbach, M.; Schwarzinger, C.; Burian, M.; Lechner, R.; et al. Air-stable organic semiconductors based on 6,6′-dithienylindigo and polymers thereof. J. Mater. Chem. C 2014, 2, 8089. [Google Scholar] [CrossRef]
- Glowacki, E.D.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Hydrogen-bonds in molecular solids—from biological systems to organic electronics. J. Mater. Chem. B 2013, 1, 3742. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.U.; Roberts, M.E.; Johnson, O.; Knoll, W.; Bao, Z. The effect of pH and DNA concentration on organic thin-film transistor biosensors. Org. Electron. 2012, 13, 519. [Google Scholar] [CrossRef]
- Casalini, S.; Leonardi, F.; Cramer, T.; Biscarini, F. Organic field-effect transistor for label-free dopamine sensing. Org. Electron. 2013, 14, 156. [Google Scholar] [CrossRef]
- Jang, M.; Kim, H.; Lee, S.; Kim, H.W.; Khedkar, J.K.; Rhee, Y.M.; Hwang, I.; Kim, K.; Oh, J.H. Highly Sensitive and Selective Biosensors Based on Organic Transistors Functionalized with Cucurbit [6] uril Derivatives. Adv. Funct. Mater. 2015, 25, 4882. [Google Scholar] [CrossRef]
- Cotrone, S.; Ambrico, M.; Toss, H.; Angione, M.D.; Magliulo, M.; Mallardi, A.; Berggren, M.; Palazzo, G.; Horowitz, G.; Ligonzo, T.; et al. Phospholipid film in electrolyte-gated organic field-effect transistors. Org. Electron. 2012, 13, 638. [Google Scholar] [CrossRef] [Green Version]
- Whitesides, G.M. Soft Robotics. Angew. Chem. Int. Ed. 2018, 57, 4258. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Shang, L.; Chen, Z.; Yu, Y.; Zhao, Y. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3, 8580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, M.J.; Owh, C.; Chee, P.L.; Kyaw, A.K.K.; Kai, D.; Loh, X.J. Biodegradable electronics: Cornerstone for sustainable electronics and transient applications. J. Mater. Chem. 2016, 4, 5531. [Google Scholar] [CrossRef]
- Feig, V.F.; Tran, H.; Bao, Z. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4, 337. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Uhrich, K.E. Biodegradable and biocompatible polymers for electronic applications: A review. J. Bioact. Compat. Polym. 2019, 34, 3. [Google Scholar] [CrossRef]
- Martin, D.J.; Reardon, P.J.T.; Moniz, S.J.A.; Tang, J. Visible Light-Driven Pure Water Splitting by a Nature-Inspired Organic Semiconductor-Based System. J. Am. Chem. Soc. 2014, 136, 12568. [Google Scholar] [CrossRef]
- Huynh, T.; Sonar, P.; Haick, H. Advanced Materials for Use in Soft Self-Healing Devices. Adv. Mater. 2017, 29, 1604973. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.; Edwards, A.M. Flavins: Photochemistry and Photobiology; RSC Publishing: Cambridge, UK, 2006. [Google Scholar]
- Massey, V. The Chemical and Biological Versatility of Riboflavin. Biochem. Soc. Trans. 2000, 28, 283. [Google Scholar] [CrossRef]
- Zanetti, G.; Aliverti, A. Chemistry and Biochemistry of Flavoenzymes, Vol. II; Muller, F., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 305–351. [Google Scholar]
- Joms, M.S.; Wang, B.; Jordan, S.P. DNA repair catalyzed by Escherichia coli DNA photolyase containing only reduced flavin: Elimination of the enzyme’s second chromophore by reduction with sodium borohydride. Biochemistry 1987, 26, 6810. [Google Scholar]
- Dagley, S. Lessons From Biodegradation. Annu. Rev. Microbiol. 1987, 41, 1. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.K.; Ceccarelli, E.A.; Carrillo, N. Plant-type ferredoxin-NADP+ reductases: A basal structural framework and a multiplicity of functions. FASEB J. 1997, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lee, M.; Lee, B.; Seo, D.H.; Park, C.B.; Kang, K. Biologically inspired pteridine redox centres for rechargeable batteries. Nat. Commun. 2014, 5, 5335. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Hong, J.; Seo, D.H.; Dong, H.N.; Ki, T.N.; Kang, K.; Chan, B.P. Redox Cofactor from Biological Energy Transduction as Molecularly Tunable Energy-Storage Compound. Angew. Chem. Int. Ed. 2013, 52, 8322. [Google Scholar] [CrossRef]
- Orita, A.; Verde, M.G.; Sakai, M.; Meng, Y.S. A biomimetic redox flow battery based on flavin mononucleotide. Nat. Commun. 2016, 7, 13230. [Google Scholar] [CrossRef]
- Yu, X.; Eymur, S.; Singh, V.; Yang, B.; Tonga, M.; Bheemaraju, A.; Cooke, G.; Subramani, C.; Venkataraman, D.; Stanley, R.J.; et al. Flavin as a photo-active acceptor for efficient energy and charge transfer in a model donor–acceptor system. Phys. Chem. Chem. Phys. 2012, 14, 6749. [Google Scholar] [CrossRef]
- Jortner, J.; Ratner, M.A. Molecular Electronics; Blackwell: Oxford, UK, 1997. [Google Scholar]
- Carter, F.L.; Siatkowski, R.F.; Wohltjen, J. Molecular Electronic Devices; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Mojr, V.; Svobodova, E.; Strakova, K.; Nevesely, T.; Chudoba, J.; Dvorakova, H.; Cibulka, R. Tailoring flavins for visible light photocatalysis: Organocatalytic [2+2] cycloadditions mediated by a flavin derivative and visible light. Chem. Commun. 2015, 51, 12036. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, N.; Wiles, A.A.; Belsley, M.; Fernandes, S.S.M.; Cariello, M.; Rotello, V.M.; Raposo, M.M.M.; Cooke, G. Synthesis and characterisation of push–pull flavin dyes with efficient second harmonic generation (SHG) properties. RSC Adv. 2017, 7, 24462. [Google Scholar] [CrossRef] [Green Version]
- Prongjit, M.; Sucharitakul, J.; Palfey, B.A.; Chaiyen, P. Oxidation Mode of Pyranose 2-Oxidase Is Controlled by pH. Biochemistry 2013, 52, 1437. [Google Scholar] [CrossRef] [Green Version]
- Nandwana, V.; Samuel, I.; Cooke, G.; Rotello, V.M. Aromatic Stacking Interactions in Flavin Model Systems. Acc. Chem. Res. 2013, 46, 1000. [Google Scholar] [CrossRef] [PubMed]
- Gozem, S.; Mirzakulova, E.; Schapiro, I.; Melaccio, F.; Glusac, K.D.; Olivucci, M. A Conical Intersection Controls the Deactivation of the Bacterial Luciferase Fluorophore. Angew. Chem. Int. Ed. 2014, 53, 9870. [Google Scholar] [CrossRef] [PubMed]
- Szymański, M.; Maciejewski, A.; Steer, R.P. Photophysics of thione triplets in solution: Factors controlling the rates of radiationless decay. Chem. Phys. 1988, 124, 143. [Google Scholar] [CrossRef]
- Kiyama, T.; Simeno, F.; Murakami, M.; Yoneda, F. Flavin-6-carboxylic acids as novel and simple flavoenzyme models. Nonenzymatic stabilization of the flavin semiquinone radical and the 4a-hydroperoxyflavin by intramolecular hydrogen bonding. J. Am. Chem. Soc. 1992, 114, 6613. [Google Scholar]
- Zoltowski, D.; Nash, A.I.; Gardner, K.H. Variations in Protein–Flavin Hydrogen Bonding in a Light, Oxygen, Voltage Domain Produce Non-Arrhenius Kinetics of Adduct Decay. Biochemistry 2011, 50, 8771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marian, C.M.; Nakagawa, S.; Rai-Constapel, V.; Karasulu, B.; Thiel, W. Photophysics of Flavin Derivatives Absorbing in the Blue-Green Region: Thioflavins As Potential Cofactors of Photoswitches. J. Phys. Chem. B 2014, 118, 1743. [Google Scholar] [CrossRef] [Green Version]
- Richtar, J.; Heinrichova, P.; Apaydin, D.H.; Schmiedova, V.; Yumusak, C.; Kovalenko, A.; Weiter, M.; Sariciftci, N.S.; Krajcovic, J. Novel Riboflavin-Inspired Conjugated Bio-Organic Semiconductors. Molecules 2018, 23, 2271. [Google Scholar] [CrossRef] [Green Version]
- Mataranga-Popa, L.N.; Torje, I.; Ghosh, T.; Leitl, M.J.; Spath, A.; Novianti, M.L.; Webster, R.D.; Konig, B. Synthesis and electronic properties of π-extended flavins. Org. Biomol. Chem. 2015, 13, 10198. [Google Scholar] [CrossRef] [Green Version]
- Penzkofer, A. Absorption Spectroscopic Determination of Solubility of Alloxazine in Aqueous Solutions. J. Anal. Sci. Methods Instrum. 2015, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Giovannitti, A.; Maria, I.P.; Hanifi, D.; Donahue, M.J.; Bryant, D.; Barth, K.J.; Makdah, B.E.; Savva, A.; Moia, D.; Zetek, M.; et al. The Role of the Side Chain on the Performance of N-type Conjugated Polymers in Aqueous Electrolytes. Chem. Mater. 2018, 30, 2945. [Google Scholar] [CrossRef] [Green Version]
- Krajčovič, J.; Kovalenko, A.; Heinrichová, P.; Vala, M.; Weiter, M. Adamantyl side groups boosting the efficiency and thermal stability of organic solid-state fluorescent dyes. J. Lumin. 2016, 175, 94. [Google Scholar] [CrossRef]
- Kovalenko, A.; Yumusak, C.; Heinrichova, P.; Stritesky, S.; Fekete, L.; Vala, M.; Weiter, M.; Sariciftci, N.S.; Krajcovic, J. Adamantane substitutions: A path to high-performing, soluble, versatile and sustainable organic semiconducting materials. J. Mater. Chem. C 2017, 5, 4716. [Google Scholar] [CrossRef]
- Edwards, A.M. Structure and general properties of flavins. Methods Mol. Biol. 2014, 1146, 3. [Google Scholar] [PubMed]

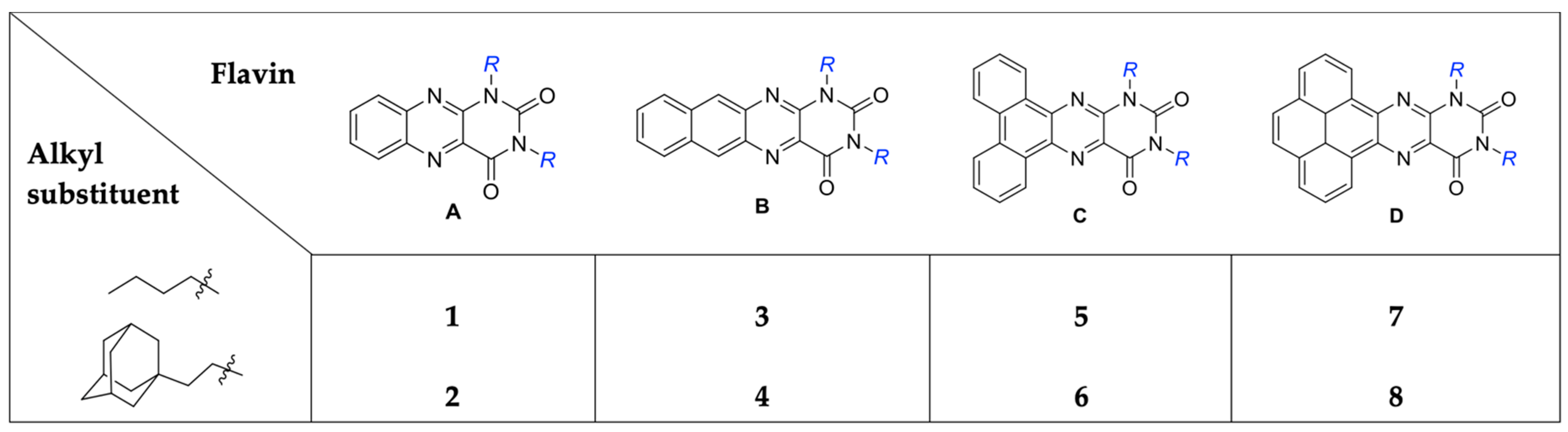

| Flavin | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | A | B | C | D |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decomposition temperature (°C) | 274 | 380 | 338 | 422 | 385 | 475 | 364 | 475 | 377 | 393 | 398 | 421 |
| Melting point (°C) | 135–137 | 251–252 | 210–211 | 308–309 | 197–198 | >350 | 240–241 | >350 | >350 | >350 | >350 | >350 |
| Solution | Solid State | ||||
|---|---|---|---|---|---|
| # | absmax*/nm | emmax/nm | absmax*/nm | emmax/nm | ϕF |
| 1 | 385 | 423,442 | 386 | 434 | 0.01 |
| 2 | 386 | 424,442 | 385 | 438 | 0.01 |
| 3 | 398 | 589 | 397 | 579 | 0.02 |
| 4 | 398 | 584 | 397 | 597 | 0.02 |
| 5 | 415 | 431 | 420 | 480 | 0.02 |
| 6 | 416 | 430 | 419 | 475,495 | 0.02 |
| 7 | 455 | 497 | 466 | 514 | 0.02 |
| 8 | 456 | 494 | 457 | 528 | 0.02 |
| Compound | LUMO/V vs. SHE | HOMO/V vs. SHE | LUMO/eV | HOMO/eV | Eg(echem)/eV |
|---|---|---|---|---|---|
| 1 | −0.91 | - | −3.84 | - | - |
| 2 | −0.86 | - | −3.89 | - | - |
| 3 | −0.72 | +1.79 | −4.03 | −6.54 | 2.51 |
| 4 | −0.55 | +1.87 | −4.20 | −6.62 | 2.42 |
| 5 | −1.03 | +1.71 | −3.72 | −6.46 | 2.74 |
| 6 | −1.00 | - | −3.75 | - | - |
| 7 | −0.98 | +1.76 | −3.77 | −6.51 | 2.74 |
| 8 | −0.97 | +1.78 | −3.78 | −6.53 | 2.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richtar, J.; Ivanova, L.; Whang, D.R.; Yumusak, C.; Wielend, D.; Weiter, M.; Scharber, M.C.; Kovalenko, A.; Sariciftci, N.S.; Krajcovic, J. Tunable Properties of Nature-Inspired N,N′-Alkylated Riboflavin Semiconductors. Molecules 2021, 26, 27. https://doi.org/10.3390/molecules26010027
Richtar J, Ivanova L, Whang DR, Yumusak C, Wielend D, Weiter M, Scharber MC, Kovalenko A, Sariciftci NS, Krajcovic J. Tunable Properties of Nature-Inspired N,N′-Alkylated Riboflavin Semiconductors. Molecules. 2021; 26(1):27. https://doi.org/10.3390/molecules26010027
Chicago/Turabian StyleRichtar, Jan, Lucia Ivanova, Dong Ryeol Whang, Cigdem Yumusak, Dominik Wielend, Martin Weiter, Markus Clark Scharber, Alexander Kovalenko, Niyazi Serdar Sariciftci, and Jozef Krajcovic. 2021. "Tunable Properties of Nature-Inspired N,N′-Alkylated Riboflavin Semiconductors" Molecules 26, no. 1: 27. https://doi.org/10.3390/molecules26010027
APA StyleRichtar, J., Ivanova, L., Whang, D. R., Yumusak, C., Wielend, D., Weiter, M., Scharber, M. C., Kovalenko, A., Sariciftci, N. S., & Krajcovic, J. (2021). Tunable Properties of Nature-Inspired N,N′-Alkylated Riboflavin Semiconductors. Molecules, 26(1), 27. https://doi.org/10.3390/molecules26010027






