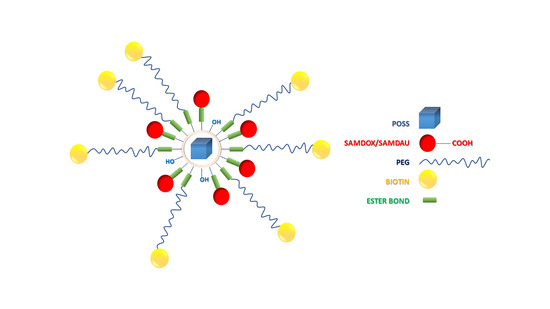
Novel Polyhedral Silsesquioxanes [POSS(OH)32] as Anthracycline Nanocarriers—Potential Anticancer Prodrugs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Model Reaction
2.2. Determination of Total Drug Content in Nanoconjugates 4–9
2.3. Drugs Release Study
2.4. Nuclear Magnetic Resonance (NMR)—1H-13C HSQC
2.5. Diffusion NMR Spectroscopy
2.6. Fourier Transform Infrared Spectroscopy
2.7. Hydrodynamic Diameters of Conjugates 4–9
3. Materials and Methods
3.1. Materials
3.2. General Remarks
3.3. General Synthesis of Nanoconjugates 4–9
3.4. Model Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Piorecka, K.; Kurjata, J.; Stanczyk, M.; Stanczyk, W.A. Synthetic routes to nanomaterials containing anthracyclines: Noncovalent systems. Biomater. Sci. 2018, 6, 2552–2565. [Google Scholar] [CrossRef] [PubMed]
- Piorecka, K.; Smith, D.; Kurjata, J.; Stanczyk, M.; Stanczyk, W.A. Synthetic routes to nanoconjugates of anthracyclines. Bioorg. Chem. 2020, 96, 103617. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Mumper, R.J. Anthracycline Nano-Delivery Systems to Overcome Multiple Drug Resistance: A Comprehensive Review. Nano Today 2013, 8, 313–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, U.M.; Jacobs, G.; Yokel, R.A.; Davis, B.H.; Dozier, A.K.; Birch, M.E.; Tseng, M.T.; Oberdörster, G.; Elder, A.; DeLouise, L. From Dose to Response: In Vivo Nanoparticle Processing and Potential Toxicity. Adv. Exp. Med. Biol. 2017, 947, 71–100. [Google Scholar] [PubMed]
- Janaszewska, A.; Gradzinska, K.; Marcinkowska, M.; Klajnert-Maculewicz, B.; Stanczyk, W.A. In Vitro Studies of Polyhedral Oligo Silsesquioxanes: Evidence for Their Low Cytotoxicity. Materials 2015, 8, 6062–6070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobierajska, E.; Konopka, M.; Janaszewska, A.; Piorecka, K.; Blauz, A.; Klajnert-Maculewicz, B.; Stanczyk, M.; Stanczyk, W.A. Unusual Enhancement of Doxorubicin Activity on Co-Delivery with Polyhedral Oligomeric Silsesquioxane (POSS). Materials 2017, 10, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piorecka, K.; Radzikowska, E.; Kurjata, J.; Rozga-Wijas, K.; Stanczyk, W.A.; Wielgus, E. Synthesis of the first POSS cage—Anthracycline conjugates via amide bonds. New J. Chem. 2016, 40, 5997–6000. [Google Scholar] [CrossRef]
- Piorecka, K.; Kurjata, J.; Bak-Sypien, I.; Cypryk, M.; Steinke, U.; Stanczyk, W.A. Reasons for enhanced activity of doxorubicin on co-delivery with octa(3-aminopropyl)silsesquioxane. RSC Adv. 2020, 10, 15579–15585. [Google Scholar] [CrossRef] [Green Version]
- Shanmugan, S.; Cani, D.; Pescarmona, P.P. The design and synthesis of an innovative octacarboxy-silsesquioxane building block. Chem. Commun. 2014, 50, 11008–11011. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, H.; de Mel, A.; Seifalian, A. Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: A glimpse into prospective horizons. Int. J. Nanomed. 2011, 6, 775. [Google Scholar]
- Rosenholm, J.M.; Linden, M. Towards establishing structure-activity relationships for mesoporous silica in drug delivery applications. J. Control. Release 2008, 28, 996. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Lanzendorfer, M.G.; Muller, A.H.E. Silsesquioxane-Based Nanoparticles Formed via Hydrolytic Condensation of Organotriethoxysilane Containing Hydroxy Groups. Macromolecules 2004, 37, 5228–5238. [Google Scholar] [CrossRef]
- Piorecka, K.; Stanczyk, W.; Florczak, M. NMR analysis of antitumor drugs: Doxorubicin, daunorubicin and their functionalized derivatives. Tetrahedron Lett. 2017, 58, 152–155. [Google Scholar] [CrossRef]
- Piorecka, K.; Janaszewska, A.; Majkowska, M.; Marcinkowska, M.; Kurjata, J.; Kazmierski, S.; Radzikowska-Cieciura, E.; Kost, B.; Klajnert-Maculewicz, B.; Stanczyk, W.A. Hydrophilic polyhedral oligomeric silsesquioxane—POSS(OH)32 as a complexing nanocarrier for doxorubicin and daunorubicin. Materials 2020, 13, 5512. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Ni, C.; Wu, G.; Zhu, C.; Yao, B. The Preparation and Characterization of Amphiphilic Star Block Copolymer Nano Micelles Using Silsesquioxane as the Core. J. Phys. Chem. C 2010, 114, 13471–13476. [Google Scholar] [CrossRef]













| Type of Nanoconjugate | Total Drug Content (wt%) | Calculated Maximum Drug Contents (wt%) | Drug Attachment Efficiency (wt%) |
|---|---|---|---|
| PossDoxPEG1 (4) | 42.88% | 48.70% | 88.0% |
| PossDoxPEG2 (5) | 17.54% | 37.02% | 47.4% |
| PossDoxPEGB3 (6) | 16.38% | 27.00% | 60.7% |
| PossDauPEG1 (7) | 42.33% | 54.50% | 77.7% |
| PossDauPEG2 (8) | 19.20% | 33.93% | 56.6% |
| PossDauPEGB3 (9) | 19.52% | 27.18% | 71.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piorecka, K.; Kurjata, J.; Stanczyk, W.A. Novel Polyhedral Silsesquioxanes [POSS(OH)32] as Anthracycline Nanocarriers—Potential Anticancer Prodrugs. Molecules 2021, 26, 47. https://doi.org/10.3390/molecules26010047
Piorecka K, Kurjata J, Stanczyk WA. Novel Polyhedral Silsesquioxanes [POSS(OH)32] as Anthracycline Nanocarriers—Potential Anticancer Prodrugs. Molecules. 2021; 26(1):47. https://doi.org/10.3390/molecules26010047
Chicago/Turabian StylePiorecka, Kinga, Jan Kurjata, and Wlodzimierz A. Stanczyk. 2021. "Novel Polyhedral Silsesquioxanes [POSS(OH)32] as Anthracycline Nanocarriers—Potential Anticancer Prodrugs" Molecules 26, no. 1: 47. https://doi.org/10.3390/molecules26010047
APA StylePiorecka, K., Kurjata, J., & Stanczyk, W. A. (2021). Novel Polyhedral Silsesquioxanes [POSS(OH)32] as Anthracycline Nanocarriers—Potential Anticancer Prodrugs. Molecules, 26(1), 47. https://doi.org/10.3390/molecules26010047