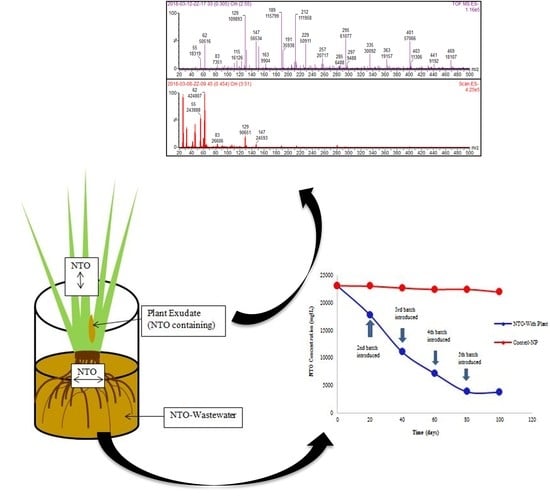
Evidence for Phytoremediation and Phytoexcretion of NTO from Industrial Wastewater by Vetiver Grass
Abstract
1. Introduction
2. Results and Discussion
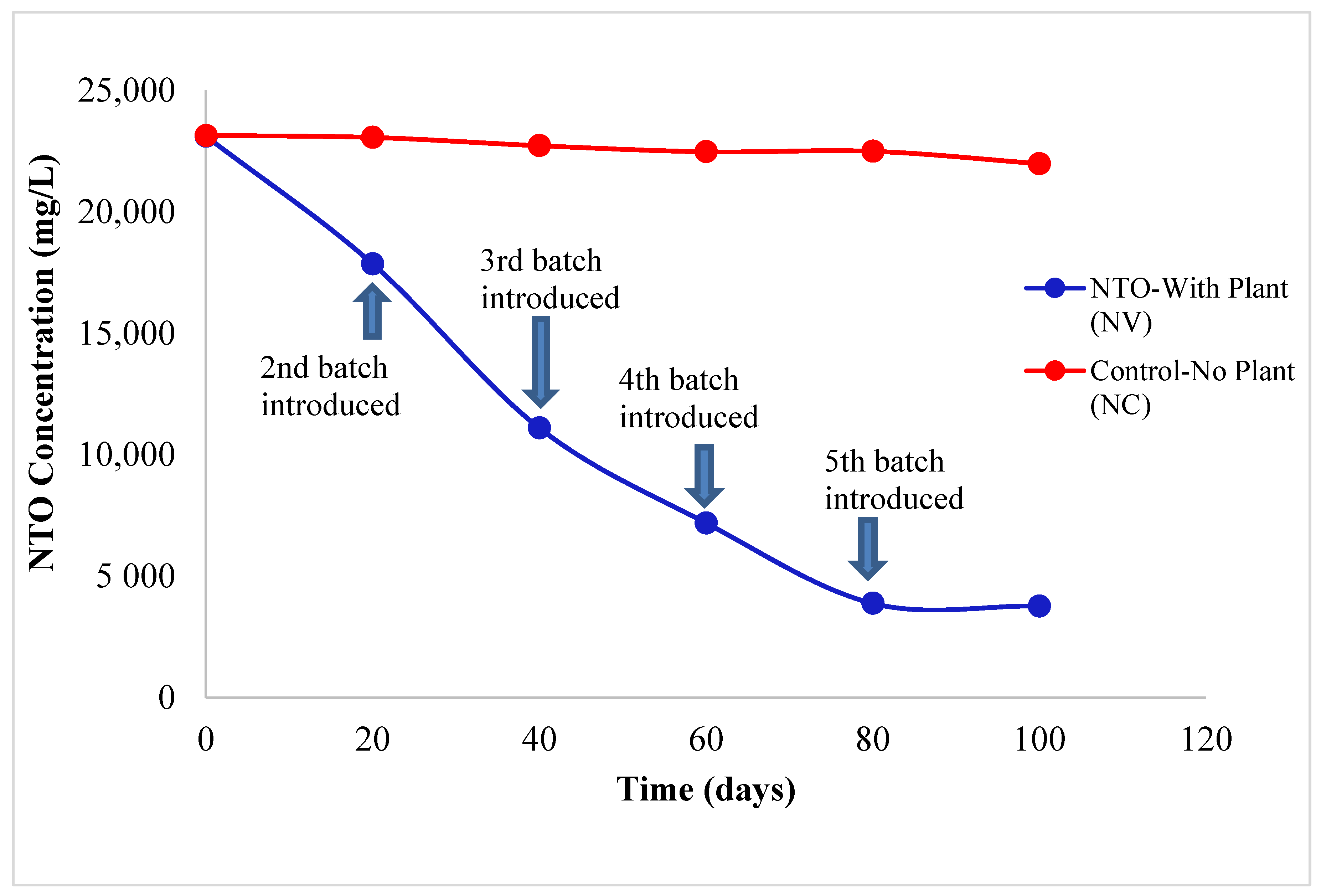
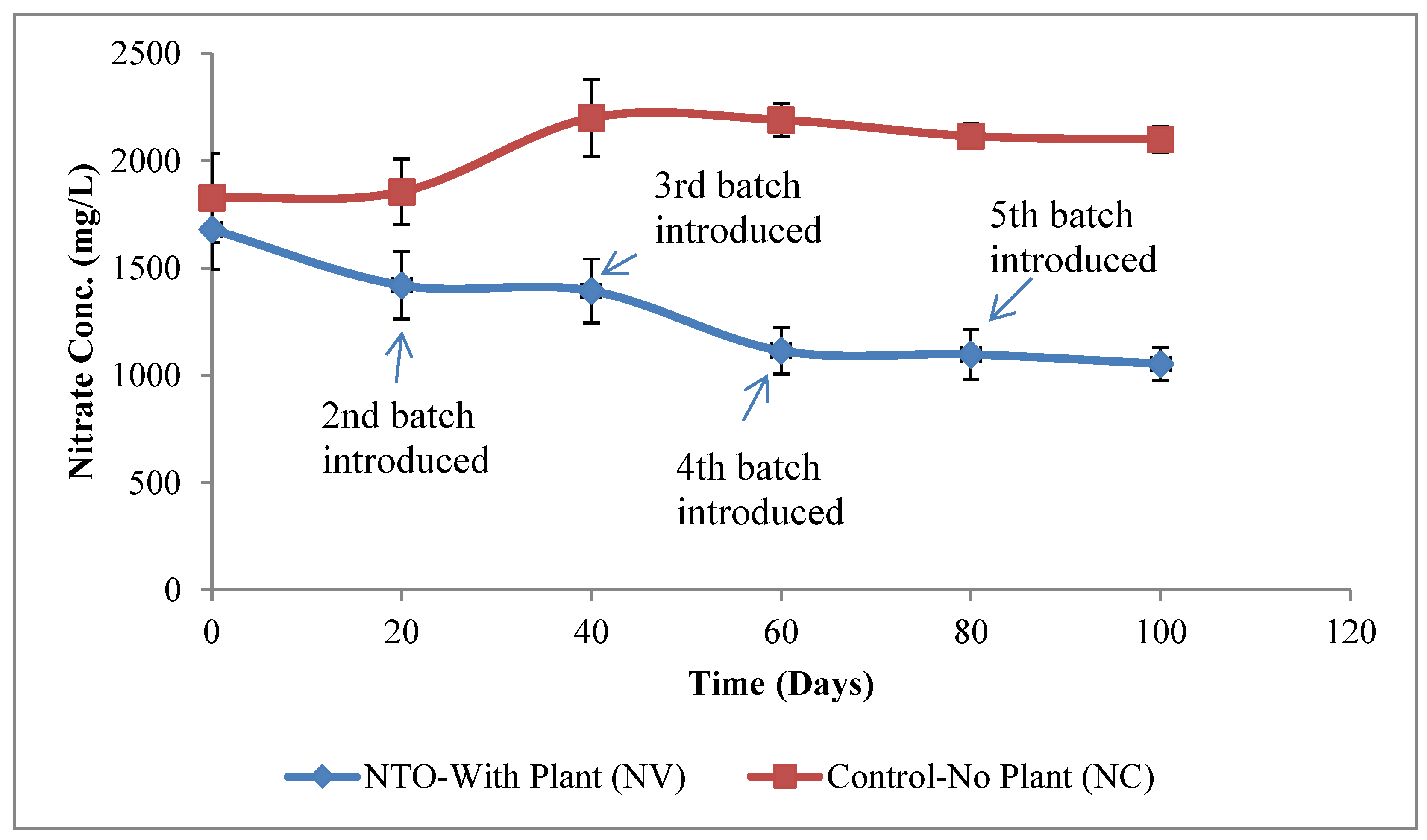
2.1. Uptake of NTO by Vetiver Grass
2.2. NTO Phytotoxicity Analysis
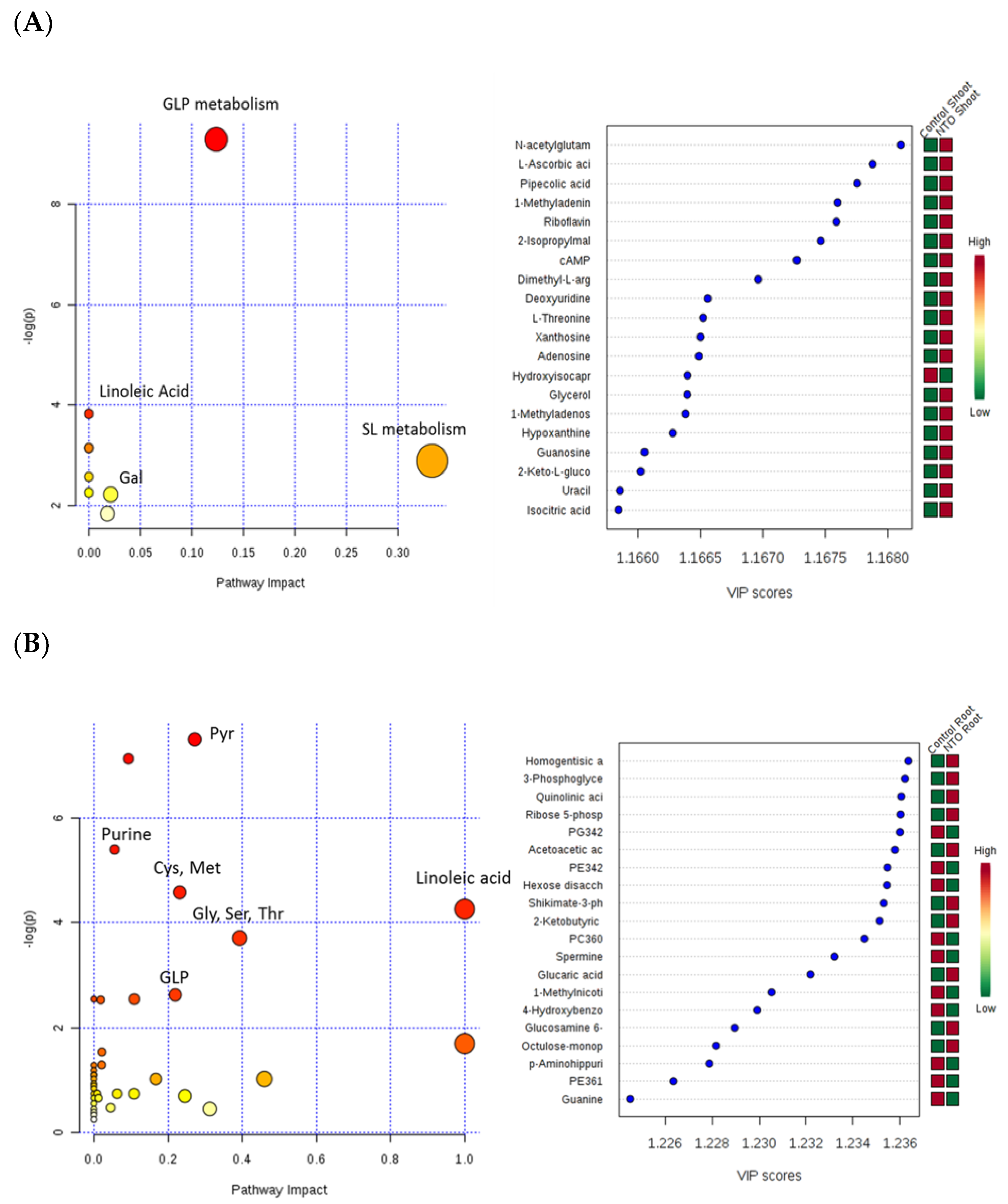
2.3. Plant Exudates Analysis
2.4. Optical Microscopy and Scanning Electron Microscope (SEM) Analysis
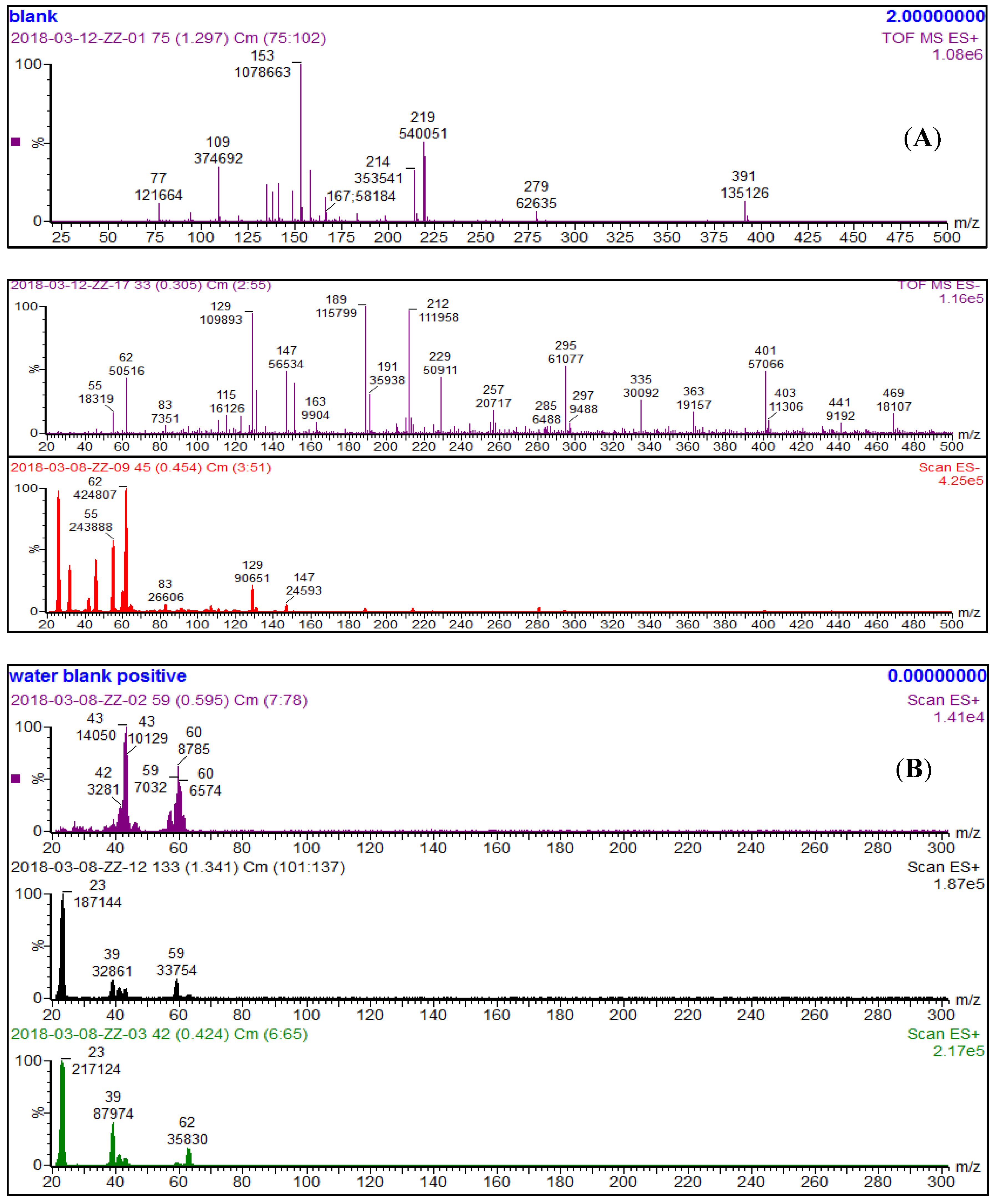
2.5. Electrospray Ionization Mass Spectrometry (ESI-MS) Analysis
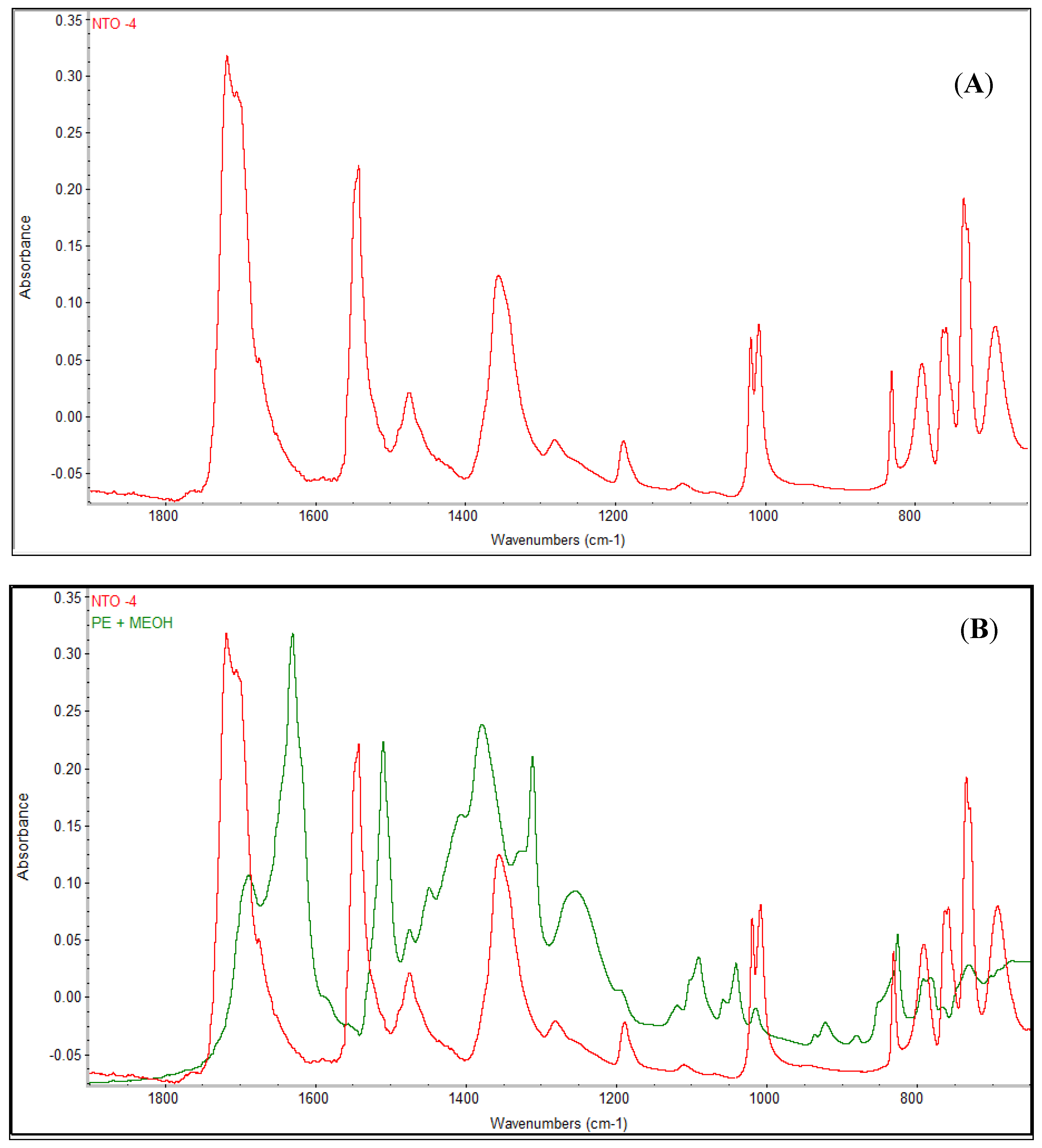
2.6. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3. Materials and Methods
3.1. Wastewater Characterization
3.2. Experimental Setup and Analyses
3.3. Phytotoxicity Analysis
3.4. Plant Metabolomics Study
3.5. Plant Exudates Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felt, D.; Johnson, J.; Larson, S.; Hubbard, B.; Henry, K.; Nestler, C.; Ballard, J. Evaluation of Treatment Technologies for Wastewater from Insensitive Munitions Production. Phase 1: Technology Down-Selection; ERDC/EL TR-13-20; Technical Report; US Army, Armament Research, Development and Engineering Center (ARDEC): Picatinny Arsenal, NJ, USA, 2013. [Google Scholar]
- Richard, T.; Weidhass, J. Biodegradation of IMX-101 explosive formulation constituents: 2,4-Dinitroanisole (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and nitroguanidine. J. Hazard. Mater. 2014, 280, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Jangid, S.K.; Sarangapani, R.; Solanki, V.J.; Singh, M.K.; Pandit, G.; Vijayalakshmi, R.; Sinha, R.K. Evaluation studies on partial replacement of RDX by spherical NTO in HTPB-based insensitive sheet explosive formulation. J. Energetic Mater. 2019, 37, 320–330. [Google Scholar] [CrossRef]
- Kitcher, E.; Braida, W.; Koutsospyros, A.; Pavlov, J.; Su, T.-L. Characteristics and products of the reductive degradation of 3-nitro-1,2,4-triazol-5-one (NTO) and 2,4-dinitroanisole (DNAN) in a Fe-Cu bimetal system. Environ. Sci. Pollut. Res. 2016, 24, 2744–2753. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Kim, M.J.; Lee, J.-M.; Kim, S.-H.; Kim, H.-S.; Park, B.-S. Solubility, Density, and Metastable Zone Width of the 3-Nitro-1,2,4-triazol-5-one + Water System. J. Chem. Eng. Data 1998, 43, 65–68. [Google Scholar] [CrossRef]
- Abraham, J.; Lin, Y.; Roychowdhury, A.; Christodoulatos, C.; Conway, M.; Smolinski, B.; Braida, W. Algae toxicological assessment and valorization of energetic-laden wastewater streams using Scenedesmus obliquus. J. Clean. Prod. 2018, 202, 838–845. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Abraham, J.; Abimbola, T.; Lin, Y.; Christodoulatos, C.; Lawal, A.; Arienti, P.; Smolinski, B.; Braida, W. From waste to energy: Optimizing growth of microalgae Scenedesmus obliquus in untreated ener-getic-laden wastewater streams from an ammunition facility for bioenergy production. Protect. Restor. Environ. 2018, 14, 1085–1094. [Google Scholar]
- Roychowdhury, A.; Abraham, J.; Abimbola, T.; Lin, Y.; Christodoulatos, C.; Lawal, A.; Koutsospyros, A.; Braida, W. Assessing Oil Content of Microalgae Grown in Industrial Energetic-Laden Wastewater. Environ. Process. 2019, 6, 969–983. [Google Scholar] [CrossRef]
- Lin, Y.; Abraham, J.; Roychowdhury, A.; Su, T.-L.; Braida, W.; Christodoulatos, C. Ecotoxicological response of Scenedesmus obliquus to pure energetic compounds and metal ions found in wastewater streams from munitions manufacturing. Algal Res. 2020, 48, 101927. [Google Scholar] [CrossRef]
- Madeira, C.L.; Speet, S.A.; Nieto, C.A.; Abrell, L.; Chorover, J.; Sierra-Alvarez, R.; Field, J.A. Sequential anaerobic-aerobic biodegradation of emerging insensitive munitions compound 3-nitro-1,2,4-triazol-5-one (NTO). Chemosphere 2017, 167, 478–484. [Google Scholar] [CrossRef]
- Madeira, C.L.; Jog, K.V.; Vanover, E.T.; Brooks, M.D.; Taylor, D.K.; Sierra-Alvarez, R.; Waidner, L.A.; Spain, J.C.; Krzmarzick, M.J.; Field, J.A. Microbial Enrichment Culture Responsible for the Complete Oxidative Biodegradation of 3-Amino-1,2,4-triazol-5-one (ATO), the Reduced Daughter Product of the Insensitive Munitions Compound 3-Nitro-1,2,4-triazol-5-one (NTO). Environ. Sci. Technol. 2019, 53, 12648–12656. [Google Scholar] [CrossRef]
- Fawcett-Hirst, W.; Temple, T.; Ladyman, M.K.; Coulon, F. Adsorption behaviour of 1,3,5-trinitroperhydro-1,3,5-triazine, 2,4-dinitroanisole and 3-nitro-1,2,4-triazol-5-one on commercial activated carbons. Chemosphere 2020, 255, 126848. [Google Scholar] [CrossRef]
- Koutsospyros, A.; Pavlov, J.; Fawcett, J.; Strickland, D.; Smolinski, B.; Braida, W. Degradation of high energetic and insensitive munitions compounds by Fe/Cu bimetal reduction. J. Hazard. Mater. 2012, 219, 75–81. [Google Scholar] [CrossRef]
- Becher, J.B.; Beal, S.A.; Taylor, S.; Dontsova, K.; Wilcox, D.E. Photo-transformation of aqueous nitroguanidine and 3-nitro-1,2,4-triazol-5-one: Emerging munitions compounds. Chemosphere 2019, 228, 418–426. [Google Scholar] [CrossRef]
- Jog, K.V.; Sierra-Alvarez, R.; Field, J.A. Rapid biotransformation of the insensitive munitions compound, 3-nitro-1,2,4-triazol-5-one (NTO), by wastewater sludge. World J. Microbiol. Biotechnol. 2020, 36, 67. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.S.; Datta, R.; Sarkar, D.; Makris, K.C.; Mullens, C.P.; Sahi, S.V.; Bach, S.B. Induction of lead-binding phytochelatins in vetiver grass [Vetiveria zizanioides (L.)]. J. Environ. Qual. 2009, 38, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, A.; Sarkar, D.; Datta, R. Remediation of Acid Mine Drainage-Impacted Water. Curr. Pollut. Rep. 2015, 1, 131–141. [Google Scholar] [CrossRef]
- Roychowdhury, A.; Datta, R.; Sarkar, D. Heavy Metal Pollution and Remediation. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–373. [Google Scholar]
- Roychowdhury, A.; Sarkar, D.; Datta, R. A combined chemical and phytoremediation method for reclamation of acid mine drainage–impacted soils. Environ. Sci. Pollut. Res. 2019, 26, 14414–14425. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Sarkar, D.; Das, P.; Panja, S.; Parikh, C.; Ramanathan, D.; Bagley, S.; Datta, R. Tetracycline uptake and metabolism by vetiver grass (Chrysopogon zizanioides L. Nash). Environ. Sci. Pollut. Res. 2016, 23, 24880–24889. [Google Scholar] [CrossRef]
- Panz, K.; Miksch, K. Phytoremediation of explosives (TNT, RDX, HMX) by wild-type and transgenic plants. J. Environ. Manag. 2012, 113, 85–92. [Google Scholar] [CrossRef]
- Kiiskila, J.D.; Das, P.; Sarkar, D.; Datta, R. Phytoremediation of Explosive-Contaminated Soils. Curr. Pollut. Rep. 2015, 1, 23–34. [Google Scholar] [CrossRef]
- Makris, K.C.; Shakya, K.M.; Datta, R.; Sarkar, D.; Pachanoor, D. High uptake of 2,4,6-trinitrotoluene by vetiver grass-Potential for phytoremediation? Environ. Pollut. 2007, 146, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Doskey, C.M. Uptake and Fate of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine by Chrysopogon zizanioides. Master’s Thesis, Michigan Technological University, Houghton, MI, USA, 2012. [Google Scholar]
- Panja, S.; Sarkar, D.; Datta, R. Vetiver grass (Crysopogon zizanioides) is capable of removing insensitive high explosives from munition industry wastewater. Chemosphere 2018, 209, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Datta, R.; Makris, K.C.; Sarkar, D. Vetiver grass is capable of removing TNT from soil in the presence of urea. Environ. Pollut. 2010, 158, 1980–1983. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Sarkar, D.; Makris, K.C.; Datta, R. Urea-facilitated uptake and nitroreductase-mediated transformation of 2,4,6-trinitrotoluene in soil using vetiver grass. J. Environ. Chem. Eng. 2015, 3, 445–452. [Google Scholar] [CrossRef]
- Rylott, E.L.; Lorenz, A.; Bruce, N.C. Biodegradation and biotransformation of explosives. Curr. Opin. Biotechnol. 2011, 22, 434–440. [Google Scholar] [CrossRef]
- Richard, T.; Weidhaas, J. Dissolution, sorption, and phytoremediation of IMX-101 explosive formulation constituents: 2,4-dinitroanisole (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and nitroguanidine. J. Hazard. Mater. 2014, 280, 561–569. [Google Scholar] [CrossRef]
- Price, R.; Pennington, J.C.; Neumann, D.; Hayes, C.L. Plant Uptake of Explosives from Contaminated Soil and Irrigation Water at the Former Nebraska Ordnance Plant, Mead, Nebraska; Technical Report; EL-97–11; US Army Corps of Engineers, Waterways Experiment Station: Washington, DC, USA, 1997. [Google Scholar]
- Makris, K.C.; Sarkar, D.; Datta, R. Coupling indigenous biostimulation and phytoremediation for the restoration of 2,4,6-trinitrotoluene-contaminated sites. J. Environ. Monit. 2010, 12, 399–403. [Google Scholar] [CrossRef]
- Segonzac, C.; Boyer, J.-C.; Ipotesi, E.; Szponarski, W.; Tillard, P.; Touraine, B.; Sommerer, N.; Rossignol, M.; Gibrat, R. Nitrate Efflux at the Root Plasma Membrane: Identification of an Arabidopsis Excretion Transporter. Plant Cell 2007, 19, 3760–3777. [Google Scholar] [CrossRef]
- Ali, N.A.; Dewez, D.; Robidoux, P.Y.; Popovic, R. Photosynthetic parameters as indicators of trinitrotoluene (TNT) inhibitory effect: Change in chlorophyll a fluorescence induction upon exposure of Lactuca sativa to TNT. Ecotoxicology 2006, 15, 437–441. [Google Scholar] [CrossRef]
- Peterson, M.; Horst, G.; Shea, P.; Comfort, S. Germination and seedling development of switchgrass and smooth bromegrass exposed to 2,4,6-trinitrotoluene. Environ. Pollut. 1998, 99, 53–59. [Google Scholar] [CrossRef]
- Krishnan, G.; Horst, G.L.; Darnell, S.; Powers, W.L. Growth and development of smooth bromegrass and tall fescue in TNT-contaminated soil. Environ. Pollut. 2000, 107, 109–116. [Google Scholar] [CrossRef]
- Best, E.P.H.; Smith, T.; Hagen, F.L.; Dawson, J.; Torrey, A.J. Candidate Herbaceous Plants for Phytoremediation of Energetics on Ranges; ERDC Report TR-07-11; Engineer Research and Development Center: Vicksburg, MS, USA, 2007. [Google Scholar]
- Via, S.M.; Zinnert, J. Impacts of explosive compounds on vegetation: A need for community scale investigations. Environ. Pollut. 2016, 208, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Lorber-Pascal, S.; Laurent, F. Fate of RDX and TNT in agronomic plants. Environ. Pollut. 2007, 148, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant, Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.C.H.; Moco, S.; Lommen, A.; Keurentjes, J.J.B.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, B.J. Accumulation of Inorganic and Organic Osmolytes and Their Role in Osmotic Adjustment in NaCl-Stressed Vetiver Grass Seedlings. Russ. J. Plant Physiol. 2009, 56, 678–685. [Google Scholar] [CrossRef]
- Lefévre, I.; Marchal, G.; Meerts, P.; Corréal, E.; Lutts, S. Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environ. Exp. Bot. 2009, 65, 142–152. [Google Scholar] [CrossRef]
- Kadukova, J.; Manousaki, E.; Kalogerakis, N. Pb and Cd Accumulation and Phyto-Excretion by Salt Cedar (Tamarix Smyrnensis Bunge). Int. J. Phytoremediation 2008, 10, 31–46. [Google Scholar] [CrossRef]
- Yang, G.; Nie, F.; Li, J.; Guo, Q.; Qiao, Z. Preparation and Characterization of Nano-NTO Explosive. J. Energetic Mater. 2007, 25, 35–47. [Google Scholar] [CrossRef]
- Ryu, S.R.; Noda, I.; Jung, Y.M. Positional Fluctuation of IR Absorption Peaks: Frequency Shift of a Single Band or Relative Intensity Changes of Overlapped Bands? American Laboratory Technical Article. 2011. Available online: www.americanlaboratory.com/913-Technical-Articles/1244-Positional-Fluctuation-of-IR-Absorption-Peaks-Frequency-Shift-of-a-Single-Band-or-Relative-Intensity-Changes-of-Overlapped-Bands (accessed on 3 November 2020).
- Kannan, P.P.; Karthick, N.K.; Mahendraprabu, A.; Shanmugam, R.; Elangovan, A.; Arivazhagan, G. Red/blue shifting hydrogen bonds in acetonitrile-dimethyl sulphoxide solutions: FTIR and theoretical studies. J. Mol. Struct. 2017, 1139, 196–201. [Google Scholar] [CrossRef]
- Marchiol, L.; Assolari, S.; Sacco, P.; Zerbi, G. Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ. Pollut. 2004, 132, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, X.; Pidatala, V.; Chang, C.-P.; Cao, X. Novel Quantitative Metabolomic Approach for the Study of Stress Responses of Plant Root Metabolism. J. Proteome Res. 2014, 13, 5879–5887. [Google Scholar] [CrossRef] [PubMed]
- Pidatala, V.; Li, K.; Sarkar, D.; Ramakrishna, W.; Datta, R. Identification of Biochemical Pathways Associated with Lead Tolerance and Detoxification in Chrysopogon zizanioides L. Nash (Vetiver) by Metabolic Profiling. Environ. Sci. Technol. 2016, 50, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Hibbs, J.A.; Jariwala, F.B.; Weisbecker, C.S.; Attygalle, A.B. Gas-Phase Fragmentations of Anions Derived from N-Phenyl Benzenesulfonamides. J. Am. Soc. Mass Spectrom. 2013, 24, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Attygalle, A.B. Effect of Electrospray Ionization Source Conditions on the Tautomer Distribution of Deprotonated p-Hydroxybenzoic Acid in the Gas Phase. Anal. Chem. 2016, 88, 6035–6043. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
RoyChowdhury, A.; Mukherjee, P.; Panja, S.; Datta, R.; Christodoulatos, C.; Sarkar, D. Evidence for Phytoremediation and Phytoexcretion of NTO from Industrial Wastewater by Vetiver Grass. Molecules 2021, 26, 74. https://doi.org/10.3390/molecules26010074
RoyChowdhury A, Mukherjee P, Panja S, Datta R, Christodoulatos C, Sarkar D. Evidence for Phytoremediation and Phytoexcretion of NTO from Industrial Wastewater by Vetiver Grass. Molecules. 2021; 26(1):74. https://doi.org/10.3390/molecules26010074
Chicago/Turabian StyleRoyChowdhury, Abhishek, Pallabi Mukherjee, Saumik Panja, Rupali Datta, Christos Christodoulatos, and Dibyendu Sarkar. 2021. "Evidence for Phytoremediation and Phytoexcretion of NTO from Industrial Wastewater by Vetiver Grass" Molecules 26, no. 1: 74. https://doi.org/10.3390/molecules26010074
APA StyleRoyChowdhury, A., Mukherjee, P., Panja, S., Datta, R., Christodoulatos, C., & Sarkar, D. (2021). Evidence for Phytoremediation and Phytoexcretion of NTO from Industrial Wastewater by Vetiver Grass. Molecules, 26(1), 74. https://doi.org/10.3390/molecules26010074