The RNA-Binding Protein HuD Regulates Alternative Splicing and Alternative Polyadenylation in the Mouse Neocortex
Abstract
:1. Introduction
2. Results
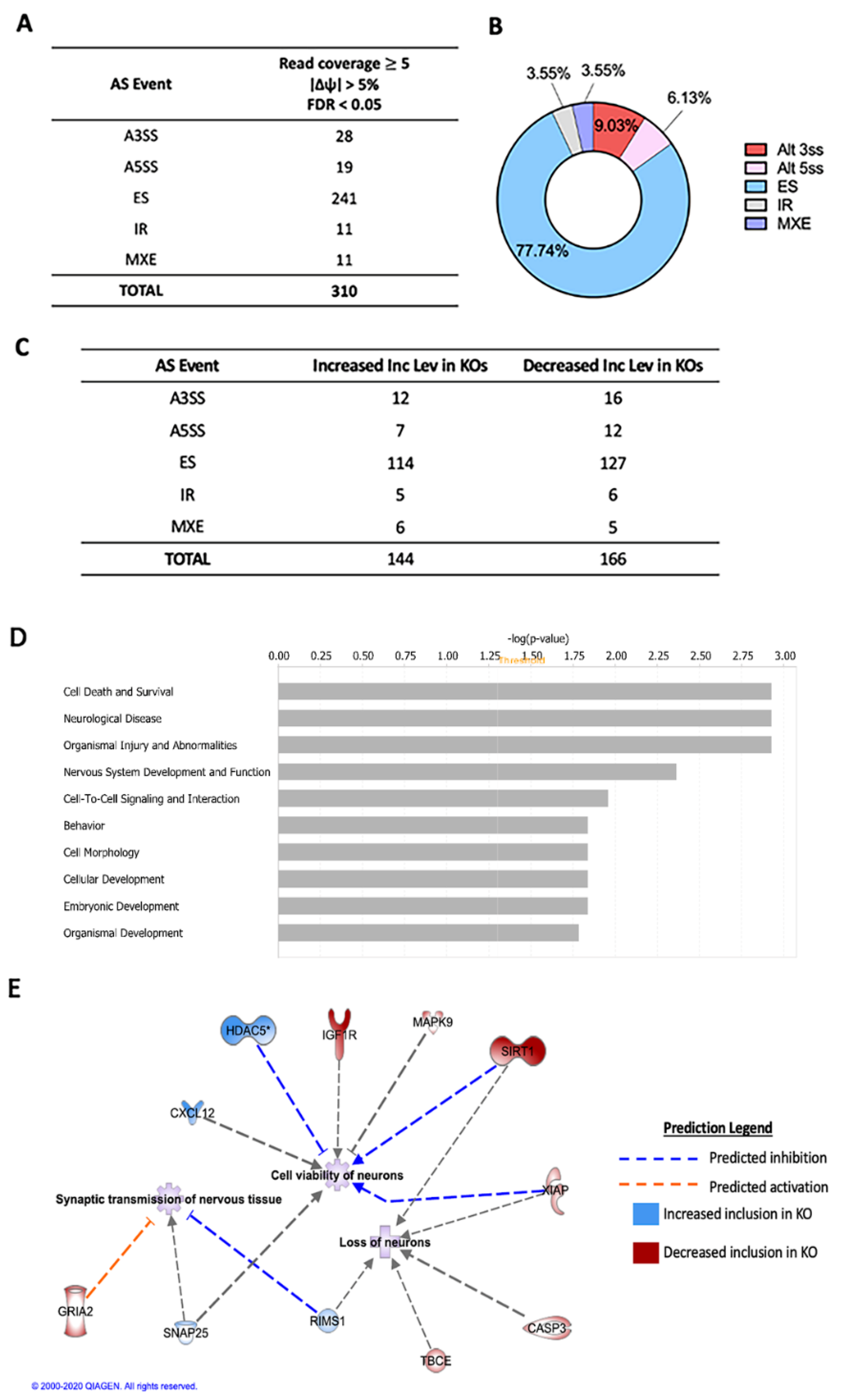
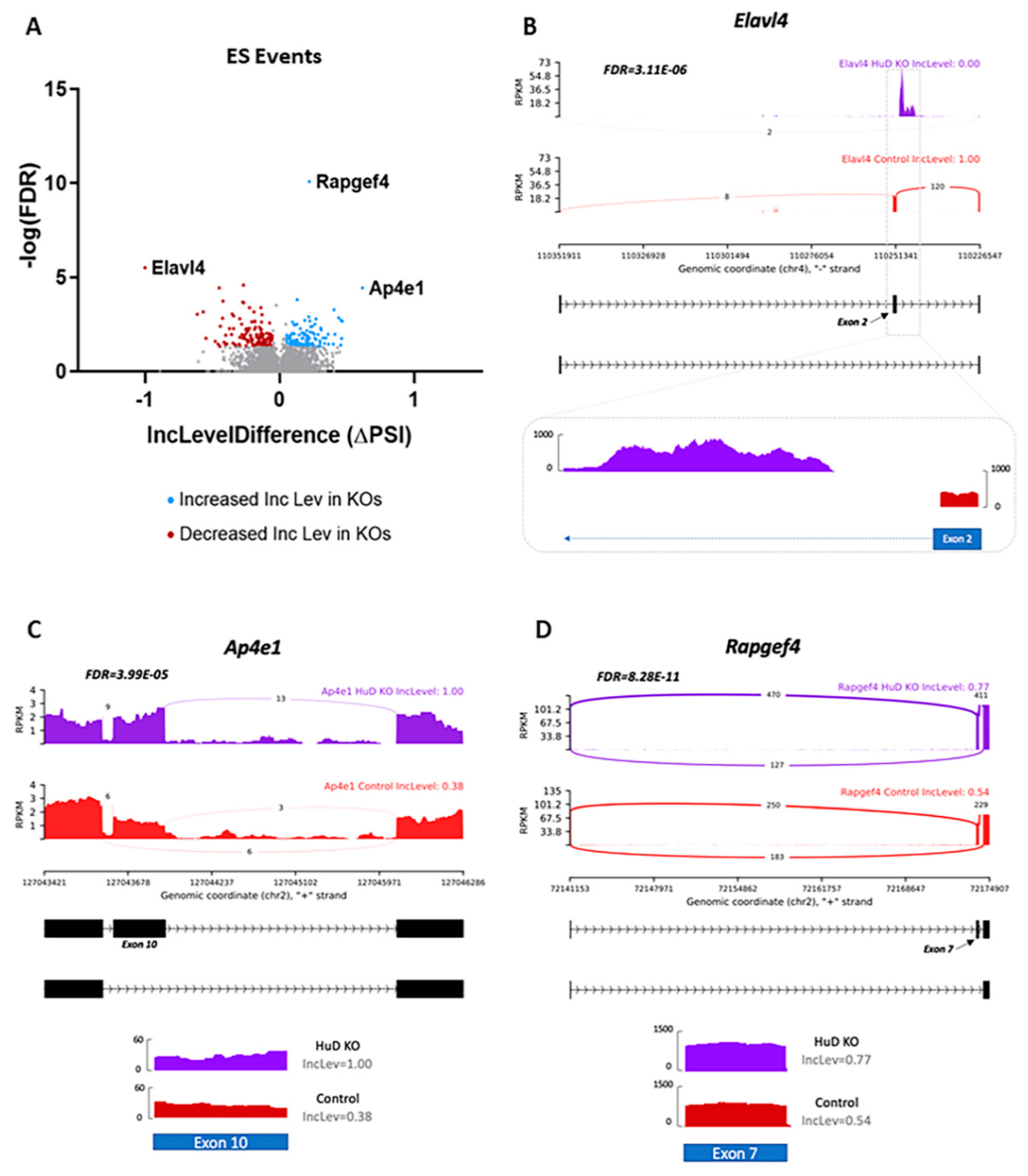
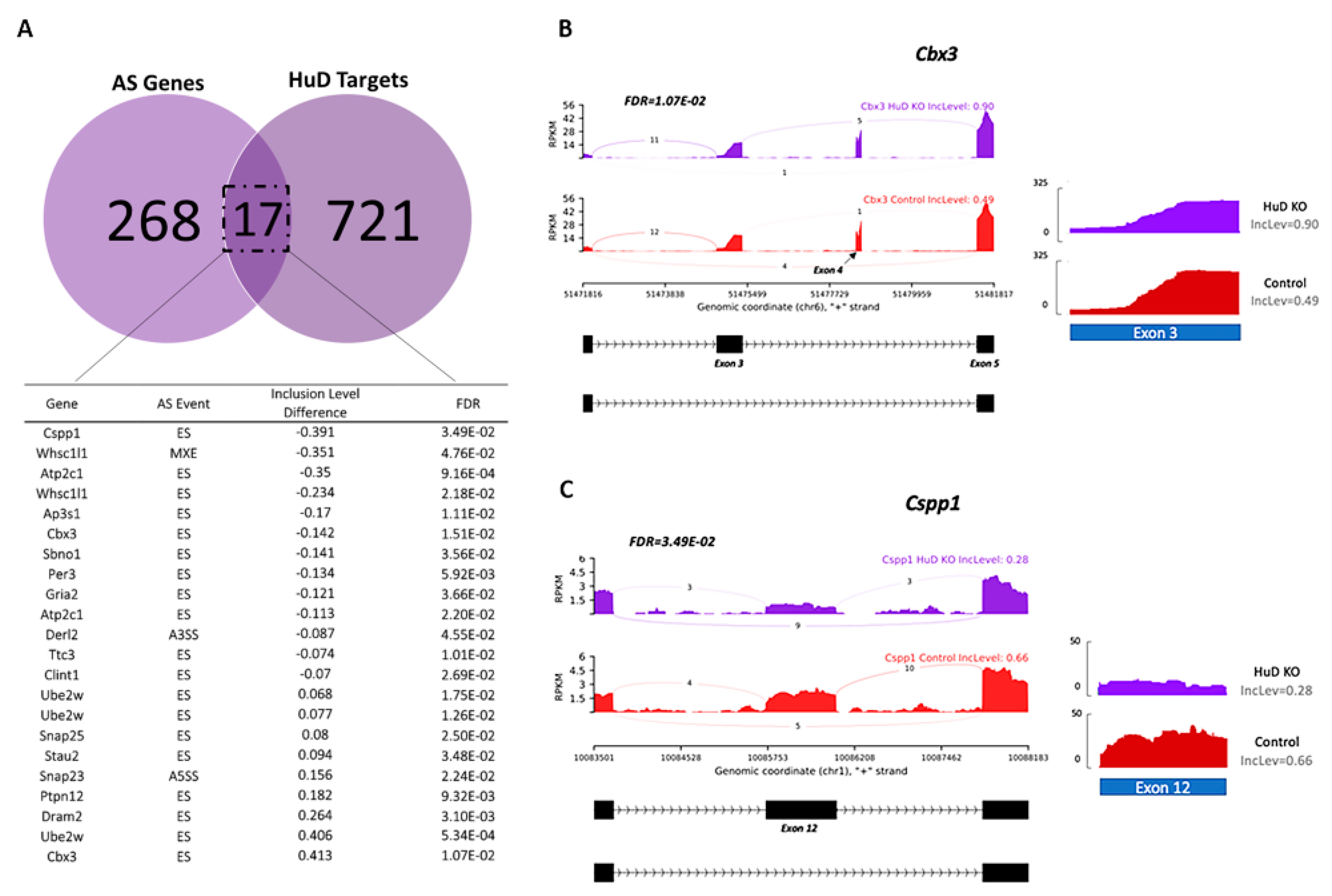
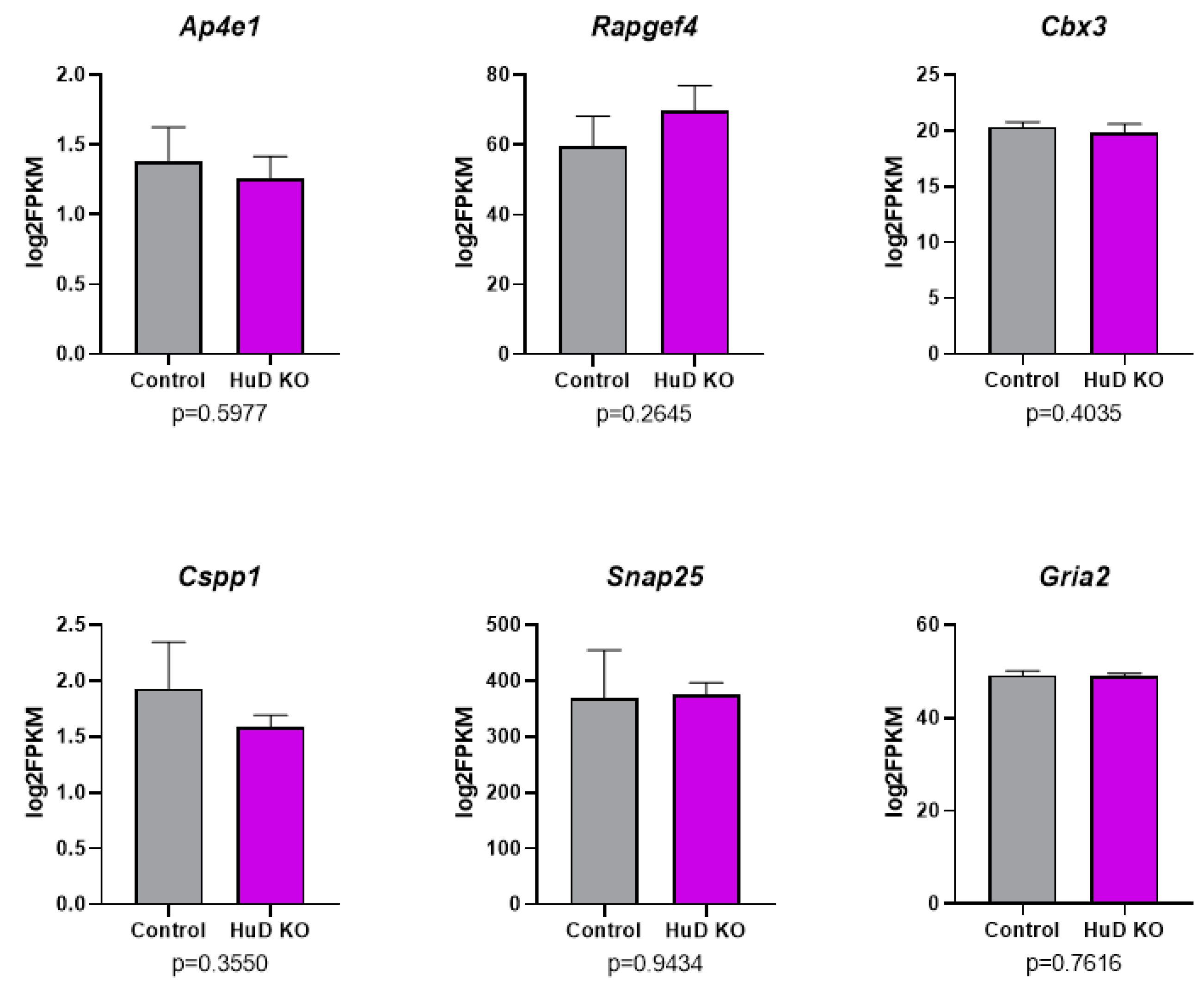
2.1. Alternative Splicing of Transcripts in HuD KO Cortex
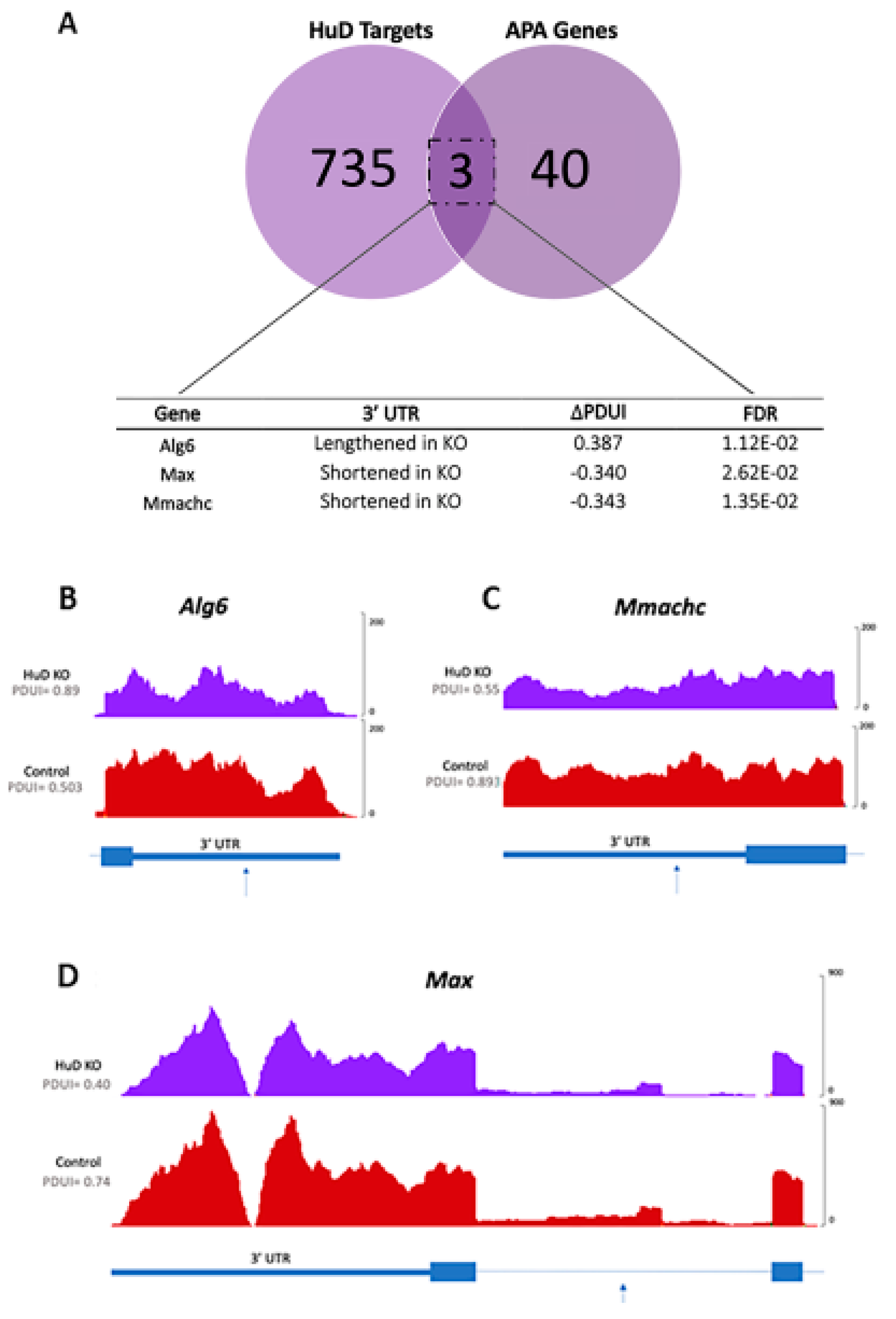
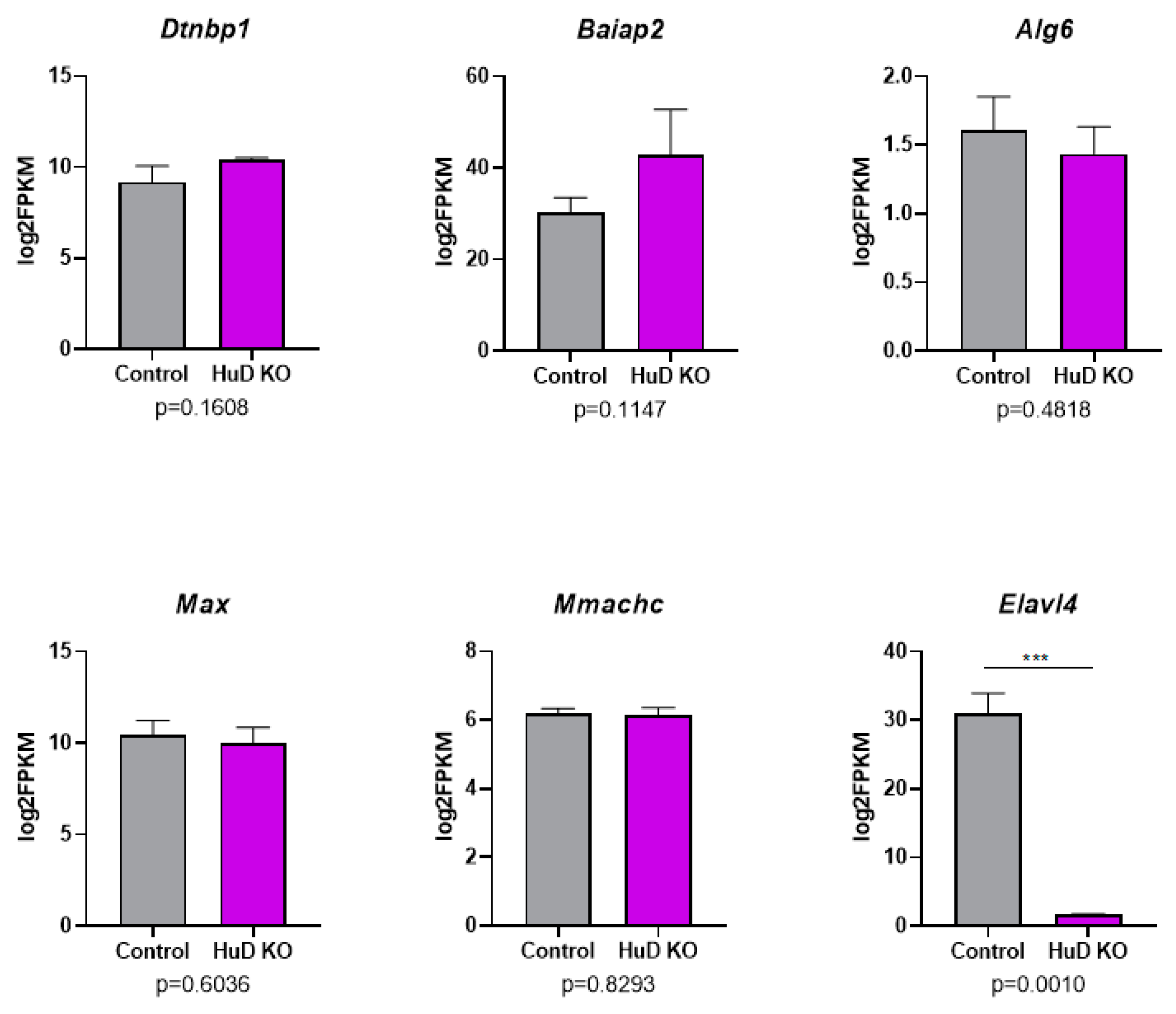
2.2. Alternative Polyadenylation of Transcripts in HuD KO Cortex
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RNA sequencing (RNA-seq)
4.3. Quality Check of Sequencing Reads and Alignment
4.4. Alternative Splicing Analysis
4.4.1. rMATS
4.4.2. Visualization of rMATS Results
4.5. Alternative Polyadenylation Analysis
4.5.1. BAM to BedGraph Conversion
4.5.2. DaPars Analysis
4.5.3. Visualization of DaPars Results
4.6. Pathway Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glisovic, T.; Bachorik, J.L.; Yong, J.; Dreyfuss, G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008, 582, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Bolognani, F.; Perrone-Bizzozero, N.I. RNA-protein interactions and control of MRNA stability in neurons. J. Neurosci. Res. 2008, 86, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Modrek, B.; Lee, C. Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res. 2002, 30, 3754–3766. [Google Scholar] [CrossRef]
- Vuong, C.K.; Black, D.L.; Zheng, S. The neurogenetics of alternative splicing. Nat. Rev. Neurosci. 2016, 17, 265–281. [Google Scholar] [CrossRef]
- Dredge, B.K.; Polydorides, A.D.; Darnell, R.B. The splice of life: Alternative splicing and neurological disease. Nat. Rev. Neurosci. 2001, 2, 43–50. [Google Scholar] [CrossRef]
- Reble, E.; Dineen, A.; Barr, C.L. The contribution of alternative splicing to genetic risk for psychiatric disorders. Genes Brain Behav. 2018, 17, e12430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatt, S.J.; Cohen, O.S.; Faraone, S.V.; Tsuang, M.T. Dysfunctional gene splicing as a potential contributor to neuropsychiatric disorders. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2011, 156B, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derti, A.; Garrett-Engele, P.; MacIsaac, K.D.; Stevens, R.C.; Sriram, S.; Chen, R.; Rohl, C.A.; Johnson, J.M.; Babak, T. A quantitative atlas of polyadenylation in five mammals. Genome Res. 2012, 22, 1173–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, C.C. Tissue-specific mechanisms of alternative polyadenylation: Testis, brain, and beyond (2018 update). Wiley Interdiscip. Rev. RNA 2019, 10, e1526. [Google Scholar] [CrossRef] [Green Version]
- Miura, P.; Sanfilippo, P.; Shenker, S.; Lai, E.C. Alternative polyadenylation in the nervous system: To what lengths will 3′ UTR extensions take us? Bioessays News Rev. Mol. Cell. Dev. Biol. 2014, 36, 766–777. [Google Scholar] [CrossRef] [Green Version]
- Taliaferro, J.M.; Vidaki, M.; Oliveira, R.; Olson, S.; Zhan, L.; Saxena, T.; Wang, E.T.; Graveley, B.R.; Gertler, F.B.; Swanson, M.S.; et al. Distal alternative last exons localize mRNAs to neural projections. Mol. Cell 2016, 61, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, A.G.; Irier, H.A.; Gu, J.; Tian, D.; Ku, L.; Liu, G.; Xia, M.; Fritsch, B.; Zheng, J.Q.; Dingledine, R.; et al. Distinct 3′ UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc. Natl. Acad. Sci. USA 2010, 107, 15945–15950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.J.; Gharami, K.; Liao, G.-Y.; Woo, N.H.; Lau, A.G.; Vanevski, F.; Torre, E.R.; Jones, K.R.; Feng, Y.; Lu, B.; et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 2008, 134, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Campos, A.R.; And, D.G.; White, K. Mutant alleles at the locus Elav in drosophila melanogaster lead to nervous system defects. A developmental-genetic analysis. J. Neurogenet. 1985, 2, 197–218. [Google Scholar] [CrossRef]
- King, P.H.; Levine, T.D.; Fremeau, R.T., Jr.; Keene, J.D. Mammalian homologs of drosophila ELAV localized to a neuronal subset can bind in vitro to the 3′ UTR of mRNA encoding the Id transcriptional repressor. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 1943–1952. [Google Scholar] [CrossRef]
- Pascale, A.; Amadio, M.; Quattrone, A. Defining a neuron: Neuronal ELAV proteins. Cell. Mol. Life Sci. 2008, 65, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Perrone-Bizzozero, N.; Bolognani, F. Role of HuD and other RNA-binding proteins in neural development and plasticity. J. Neurosci. Res. 2002, 68, 121–126. [Google Scholar] [CrossRef]
- Bronicki, L.M.; Jasmin, B.J. Emerging complexity of the HuD/ELAVl4 gene; implications for neuronal development, function, and dysfunction. RNA 2013, 19, 1019–1037. [Google Scholar] [CrossRef] [Green Version]
- Mobarak, C.D.; Anderson, K.D.; Morin, M.; Beckel-Mitchener, A.; Rogers, S.L.; Furneaux, H.; King, P.; Perrone-Bizzozero, N.I. The RNA-binding protein HuD is required for GAP-43 mRNA stability, GAP-43 gene expression, and PKC-dependent neurite outgrowth in PC12 cells. Mol. Biol. Cell 2000, 11, 3191–3203. [Google Scholar] [CrossRef] [Green Version]
- Beckel-Mitchener, A.C.; Miera, A.; Keller, R.; Perrone-Bizzozero, N.I. Poly(A) tail length-dependent stabilization of GAP-43 mRNA by the RNA-binding protein HuD. J. Biol. Chem. 2002, 277, 27996–28002. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.S.-Y. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998, 17, 3461–3470. [Google Scholar] [CrossRef]
- Zhang, Z.; So, K.; Peterson, R.; Bauer, M.; Ng, H.; Zhang, Y.; Kim, J.H.; Kidd, T.; Miura, P. Elav-mediated exon skipping and alternative polyadenylation of the dscam1 gene are required for axon outgrowth. Cell Rep. 2019, 27, 3808–3817.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Lee, S.; Majumdar, S.; Zhang, B.; Sanfilippo, P.; Joseph, B.; Miura, P.; Soller, M.; Lai, E.C. Overlapping activities of ELAV/Hu family RNA binding proteins specify the extended neuronal 3′ UTR landscape in drosophila. Mol. Cell 2020, 80, 140–155.e6. [Google Scholar] [CrossRef]
- Carrasco, J.; Rauer, M.; Hummel, B.; Grzejda, D.; Alfonso-Gonzalez, C.; Lee, Y.; Wang, Q.; Puchalska, M.; Mittler, G.; Hilgers, V. ELAV and FNE determine neuronal transcript signatures through exon-activated rescue. Mol. Cell 2020, 80, 156–163.e6. [Google Scholar] [CrossRef]
- Soller, M.; White, K. ELAV inhibits 3′-end processing to promote neural splicing of Ewg pre-mRNA. Genes Dev. 2003, 17, 2526–2538. [Google Scholar] [CrossRef] [Green Version]
- Hinman, M.N.; Lou, H. Diverse molecular functions of Hu proteins. Cell. Mol. Life Sci. CMLS 2008, 65, 3168–3181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Hasman, R.A.; Barron, V.A.; Luo, G.; Lou, H. A nuclear function of hu proteins as neuron-specific alternative RNA processing regulators. Mol. Biol. Cell 2006, 17, 5105–5114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Zhou, H.-L.; Hasman, R.A.; Lou, H. Hu Proteins regulate polyadenylation by blocking sites containing U-rich sequences. J. Biol. Chem. 2007, 282, 2203–2210. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Hinman, M.N.; Hasman, R.A.; Mehta, P.; Lou, H. Regulation of neuron-specific alternative splicing of neurofibromatosis type 1 pre-mRNA. Mol. Cell. Biol. 2008, 28, 1240–1251. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-L.; Hinman, M.N.; Barron, V.A.; Geng, C.; Zhou, G.; Luo, G.; Siegel, R.E.; Lou, H. Hu proteins regulate alternative splicing by inducing localized histone hyperacetylation in an RNA-dependent manner. Proc. Natl. Acad. Sci. USA 2011, 108, E627–E635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheckel, C.; Drapeau, E.; Frias, M.A.; Park, C.Y.; Fak, J.; Zucker-Scharff, I.; Kou, Y.; Haroutunian, V.; Ma’ayan, A.; Buxbaum, J.D.; et al. Regulatory consequences of neuronal ELAV-like protein binding to coding and non-coding RNAs in human brain. eLife 2016, 5, e10421. [Google Scholar] [CrossRef] [PubMed]
- Ince-Dunn, G.; Okano, H.J.; Jensen, K.B.; Park, W.-Y.; Zhong, R.; Ule, J.; Mele, A.; Fak, J.J.; Yang, C.; Zhang, C.; et al. Neuronal Elav-like (Hu) proteins regulate RNA splicing and abundance to control glutamate levels and neuronal excitability. Neuron 2012, 75, 1067–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardia, C.M.; De Pace, R.; Mattera, R.; Bonifacino, J.S. Neuronal functions of adaptor complexes involved in protein sorting. Curr. Opin. Neurobiol. 2018, 51, 103–110. [Google Scholar] [CrossRef]
- Sugawara, K.; Shibasaki, T.; Takahashi, H.; Seino, S. Structure and functional roles of Epac2 (Rapgef4). Gene 2016, 575, 577–583. [Google Scholar] [CrossRef]
- Hoivik, E.A.; Witsoe, S.L.; Bergheim, I.R.; Xu, Y.; Jakobsson, I.; Tengholm, A.; Doskeland, S.O.; Bakke, M. DNA methylation of alternative promoters directs tissue specific expression of Epac2 isoforms. PLoS ONE 2013, 8, e67925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolognani, F.; Contente-Cuomo, T.; Perrone-Bizzozero, N.I. Novel recognition motifs and biological functions of the RNA-binding protein HuD revealed by genome-wide identification of its targets. Nucleic Acids Res. 2010, 38, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Smallwood, A.; Hon, G.C.; Jin, F.; Henry, R.E.; Espinosa, J.M.; Ren, B. CBX3 regulates efficient RNA processing genome-wide. Genome Res. 2012, 22, 1426–1436. [Google Scholar] [CrossRef] [Green Version]
- Patzke, S.; Redick, S.; Warsame, A.; Murga-Zamalloa, C.A.; Khanna, H.; Doxsey, S.; Stokke, T. CSPP is a ciliary protein interacting with nephrocystin 8 and required for cilia formation. Mol. Biol. Cell 2010, 21, 2555–2567. [Google Scholar] [CrossRef] [Green Version]
- Asiedu, M.; Wu, D.; Matsumura, F.; Wei, Q. Centrosome/Spindle pole–associated protein regulates cytokinesis via promoting the recruitment of MyoGEF to the central spindle. Mol. Biol. Cell 2009, 20, 1428–1440. [Google Scholar] [CrossRef]
- Park, S.M.; Jang, H.J.; Lee, J.H. Roles of primary cilia in the developing brain. Front. Cell. Neurosci. 2019, 13, 218. [Google Scholar] [CrossRef] [Green Version]
- Patzke, S.; Stokke, T.; Aasheim, H.-C. CSPP and CSPP-L Associate with centrosomes and microtubules and differently affect microtubule organization. J. Cell. Physiol. 2006, 209, 199–210. [Google Scholar] [CrossRef]
- Delgado-Martinez, I.; Nehring, R.B.; Sorensen, J.B. Differential abilities of SNAP-25 homologs to support neuronal function. J. Neurosci. 2007, 27, 9380–9391. [Google Scholar] [CrossRef] [Green Version]
- Nagy, G.; Milosevic, I.; Mohrmann, R.; Wiederhold, K.; Walter, A.M.; Sørensen, J.B. The SNAP-25 linker as an adaptation toward fast exocytosis. Mol. Biol. Cell 2008, 19, 3769–3781. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.; Vissel, B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front. Mol. Neurosci. 2012, 5. [Google Scholar] [CrossRef] [Green Version]
- Balik, A.; Penn, A.C.; Nemoda, Z.; Greger, I.H. Activity-regulated RNA editing in select neuronal subfields in hippocampus. Nucleic Acids Res. 2013, 41, 1124–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, W.; Huang, Z.; Wang, C.; Han, Y.; Park, J.S.; Niu, L. Flip and flop: A molecular determinant for AMPA Receptor channel opening. Biochemistry 2009, 48, 3767–3777. [Google Scholar] [CrossRef]
- Koike, M.; Tsukada, S.; Tsuzuki, K.; Kijima, H.; Ozawa, S. Regulation of kinetic properties of GluR2 AMPA receptor channels by alternative splicing. J. Neurosci. 2000, 20, 2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Z.; Donehower, L.A.; Cooper, T.A.; Neilson, J.R.; Wheeler, D.A.; Wagner, E.J.; Li, W. Dynamic analyses of alternative polyadenylation from RNA-Seq reveal a 3′-UTR landscape across seven tumour types. Nat. Commun. 2014, 5, 5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masamha, C.P.; Xia, Z.; Yang, J.; Albrecht, T.R.; Li, M.; Shyu, A.-B.; Li, W.; Wagner, E.J. CFIm25 links alternative polyadenylation to glioblastoma tumour suppression. Nature 2014, 510, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Sammeth, M.; Foissac, S.; Guigó, R. A general definition and nomenclature for alternative splicing events. PLoS Comput. Biol. 2008, 4, e1000147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-De-Luca, A.; Helmers, S.L.; Mao, H.; Burns, T.G.; Melton, A.M.A.; Schmidt, K.R.; Fernhoff, P.M.; Ledbetter, D.H.; Martin, C.L. Adaptor protein complex-4 (AP-4) deficiency causes a novel autosomal recessive cerebral palsy syndrome with microcephaly and intellectual disability. J. Med. Genet. 2011, 48, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.H.; Mattera, R.; Morell, R.; Sainz, E.; Rahn, R.; Gutierrez, J.; Paris, E.; Root, J.; Solomon, B.; Brewer, C.; et al. Association between rare variants in AP4E1, a component of intracellular trafficking, and persistent stuttering. Am. J. Hum. Genet. 2015, 97, 715–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamra, R.A.; Philippe, O.; Raas-Rothschild, A.; Eck, S.H.; Graf, E.; Buchert, R.; Borck, G.; Ekici, A.; Brockschmidt, F.F.; Nöthen, M.M.; et al. Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature. Am. J. Hum. Genet. 2011, 88, 788–795. [Google Scholar] [CrossRef] [Green Version]
- International Molecular Genetic Study of Autism Consortium (IMGSAC); Bacchelli, E.; Blasi, F.; Biondolillo, M.; Lamb, J.A.; Bonora, E.; Barnby, G.; Parr, J.; Beyer, K.S.; Klauck, S.M.; et al. Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the CAMP-GEFII Gene. Mol. Psychiatry 2003, 8, 916–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, D.P.; Jones, K.A.; Woolfrey, K.M.; Burgdorf, J.; Russell, T.A.; Kalmbach, A.; Lee, H.; Yang, C.; Bradberry, M.M.; Wokosin, D.; et al. Social, Communication, and cortical structural impairments in Epac2-deficient mice. J. Neurosci. 2012, 32, 11864–11878. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, D.P.; Woolfrey, K.M.; Jones, K.A.; Anderson, C.T.; Smith, K.R.; Russell, T.A.; Lee, H.; Yasvoina, M.V.; Wokosin, D.L.; Ozdinler, P.H.; et al. An autism-associated variant of Epac2 reveals a role for Ras/Epac2 signaling in controlling basal dendrite maintenance in mice. PLoS Biol. 2012, 10, e1001350. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.A.; Sumiya, M.; Woolfrey, K.M.; Srivastava, D.P.; Penzes, P. Loss of EPAC2 alters dendritic spine morphology and inhibitory synapse density. Mol. Cell. Neurosci. 2019, 98, 19–31. [Google Scholar] [CrossRef]
- DeBoer, E.M.; Azevedo, R.; Vega, T.A.; Brodkin, J.; Akamatsu, W.; Okano, H.; Wagner, G.C.; Rasin, M.-R. Prenatal deletion of the RNA-binding protein HuD disrupts postnatal cortical circuit maturation and behavior. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 3674–3686. [Google Scholar] [CrossRef]
- Bark, I.C. Structure of the chicken gene for SNAP-25 reveals duplicated exons encoding distinct isoforms of the protein. J. Mol. Biol. 1993, 233, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bark, I.C.; Wilson, M.C. Human CDNA clones encoding two different isoforms of the nerve terminal protein SNAP-25. Gene 1994, 139, 291–292. [Google Scholar] [CrossRef]
- Irfan, M.; Gopaul, K.R.; Miry, O.; Hökfelt, T.; Stanton, P.K.; Bark, C. SNAP-25 isoforms differentially regulate synaptic transmission and long-term synaptic plasticity at central synapses. Sci. Rep. 2019, 9, 6403. [Google Scholar] [CrossRef] [Green Version]
- Prescott, G.R.; Chamberlain, L.H. Regional and developmental brain expression patterns of SNAP25 splice variants. BMC Neurosci. 2011, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, J.B.; Nagy, G.; Varoqueaux, F.; Nehring, R.B.; Brose, N.; Wilson, M.C.; Neher, E. Differential control of the releasable vesicle pools by SNAP-25 splice variants and SNAP-23. Cell 2003, 114, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Penn, A.C.; Balik, A.; Wozny, C.; Cais, O.; Greger, I.H. Activity-mediated AMPA receptor remodeling, driven by alternative splicing in the ligand-binding domain. Neuron 2012, 76, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Oliver, R.J.; Brigman, J.L.; Bolognani, F.; Allan, A.M.; Neisewander, J.L.; Perrone-Bizzozero, N.I. Neuronal RNA-binding protein HuD regulates addiction-related gene expression and behavior. Genes Brain Behav. 2018, 17, e12454. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.M.; Sower, A.C.; Perrone-Bizzozero, N.I. Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol. Psychiatry 1998, 43, 239–243. [Google Scholar] [CrossRef]
- Johansson, J.U.; Ericsson, J.; Janson, J.; Beraki, S.; Stanić, D.; Mandic, S.A.; Wikström, M.A.; Hökfelt, T.; Ogren, S.O.; Rozell, B.; et al. An ancient duplication of exon 5 in the Snap25 gene is required for complex neuronal development/function. PLoS Genet. 2008, 4, e1000278. [Google Scholar] [CrossRef] [Green Version]
- Barakauskas, V.E.; Moradian, A.; Barr, A.M.; Beasley, C.L.; Rosoklija, G.; Mann, J.J.; Ilievski, B.; Stankov, A.; Dwork, A.J.; Falkai, P.; et al. Quantitative mass spectrometry reveals changes in SNAP-25 isoforms in schizophrenia. Schizophr. Res. 2016, 177, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, P.M.; Cruz, D.A.; Fucich, E.A.; Olukotun, D.Y.; Takahashi, M.; Itakura, M. SNAP-25a/b Isoform levels in human brain dorsolateral prefrontal cortex and anterior cingulate cortex. Mol. Neuropsychiatry 2015, 1, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Acosta, G.; Freidman, D.P.; Grant, K.A.; Hemby, S.E. Alternative splicing of AMPA subunits in prefrontal cortical fields of cynomolgus monkeys following chronic ethanol self-administration. Front. Psychiatry 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Su, T.; Xue, Y.; Cheng, C.; Lay, F.D.; McKee, R.A.; Li, M.; Vashisht, A.; Wohlschlegel, J.; Novitch, B.G.; et al. Cbx3 maintains lineage specificity during neural differentiation. Genes Dev. 2017, 31, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Mathison, A.; De Assuncao, T.M.; Dsouza, N.R.; Williams, M.; Zimmermann, M.T.; Urrutia, R.; Lomberk, G. Discovery, expression, cellular localization, and molecular properties of a novel, alternative spliced HP1γ isoform, lacking the chromoshadow domain. PLoS ONE 2020, 15, e0217452. [Google Scholar] [CrossRef] [Green Version]
- Oshiro, H.; Hirabayashi, Y.; Furuta, Y.; Okabe, S.; Gotoh, Y. Up-regulation of HP1γ expression during neuronal maturation promotes axonal and dendritic development in mouse embryonic neocortex. Genes Cells 2015, 20, 108–120. [Google Scholar] [CrossRef]
- Mladinov, M.; Sedmak, G.; Fuller, H.R.; Leko, M.B.; Mayer, D.; Kirincich, J.; Štajduhar, A.; Borovečki, F.; Hof, P.R.; Šimić, G. Gene expression profiling of the dorsolateral and medial orbitofrontal cortex in schizophrenia. Transl. Neurosci. 2016, 7, 139–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Shin, J.-Y.; Kim, J.-I.; Seo, J.-S.; Webster, M.J.; Lee, D.; Kim, S. Somatic deletions implicated in functional diversity of brain cells of individuals with schizophrenia and unaffected controls. Sci. Rep. 2014, 4, 3807. [Google Scholar] [CrossRef] [Green Version]
- Akizu, N.; Silhavy, J.L.; Rosti, R.O.; Scott, E.; Fenstermaker, A.G.; Schroth, J.; Zaki, M.S.; Sanchez, H.; Gupta, N.; Kabra, M.; et al. Mutations in CSPP1 lead to classical joubert syndrome. Am. J. Hum. Genet. 2014, 94, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, R.; Shamseldin, H.E.; Loucks, C.M.; Seidahmed, M.Z.; Ansari, S.; Khalil, M.I.; Al-Yacoub, N.; Davis, E.E.; Mola, N.A.; Szymanska, K.; et al. Mutations in CSPP1, encoding a core centrosomal protein, cause a range of ciliopathy phenotypes in humans. Am. J. Hum. Genet. 2014, 94, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansfield, K.D.; Keene, J.D. Neuron-specific ELAV/Hu proteins suppress HuR mRNA during neuronal differentiation by alternative polyadenylation. Nucleic Acids Res. 2012, 40, 2734–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkon, R.; Ugalde, A.P.; Agami, R. Alternative cleavage and polyadenylation: Extent, regulation and function. Nat. Rev. Genet. 2013, 14, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Bird, C.; Feng, W.; Liu, G.; Li, W.; Perrone-Bizzozero, N.I.; Feng, Y. HuD promotes BDNF expression in brain neurons via selective stabilization of the BDNF long 3′ UTR mRNA. PLoS ONE 2013, 8, e55718. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, H.; Kim, E. IRSp53/BAIAP2 in dendritic spine development, NMDA receptor regulation, and psychiatric disorders. Neuropharmacology 2016, 100, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyahara, A.; Okamura-Oho, Y.; Miyashita, T.; Hoshika, A.; Yamada, M. Genomic structure and alternative splicing of the insulin receptor tyrosine kinase substrate of 53-KDa protein. J. Hum. Genet. 2003, 48, 410–414. [Google Scholar] [CrossRef] [Green Version]
- Orozco, I.J.; Koppensteiner, P.; Ninan, I.; Arancio, O. The schizophrenia susceptibility gene DTNBP1 modulates AMPAR synaptic transmission and plasticity in the hippocampus of juvenile DBA/2J mice. Mol. Cell. Neurosci. 2014, 58, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Weickert, C.S.; Straub, R.E.; McClintock, B.W.; Matsumoto, M.; Hashimoto, R.; Hyde, T.M.; Herman, M.M.; Weinberger, D.R.; Kleinman, J.E. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch. Gen. Psychiatry 2004, 61, 544. [Google Scholar] [CrossRef] [Green Version]
- Fallgatter, A.J.; Ehlis, A.-C.; Herrmann, M.J.; Hohoff, C.; Reif, A.; Freitag, C.M.; Deckert, J. DTNBP1 (Dysbindin) gene variants modulate prefrontal brain function in schizophrenic patients—Support for the glutamate hypothesis of schizophrenias. Genes Brain Behav. 2010. [Google Scholar] [CrossRef]
- Fallgatter, A.J.; Herrmann, M.J.; Hohoff, C.; Ehlis, A.-C.; Jarczok, T.A.; Freitag, C.M.; Deckert, J. DTNBP1 (Dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology 2006, 31, 2002–2010. [Google Scholar] [CrossRef] [Green Version]
- Hakak, Y.; Walker, J.R.; Li, C.; Wong, W.H.; Davis, K.L.; Buxbaum, J.D.; Haroutunian, V.; Fienberg, A.A. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 4746–4751. [Google Scholar] [CrossRef] [Green Version]
- Morava, É.; Tiemes, V.; Thiel, C.; Seta, N.; De Lonlay, P.; De Klerk, H.; Mulder, M.; Rubio-Gozalbo, M.; Visser, G.; van Hasselt, P.; et al. ALG6-CDG: A recognizable phenotype with epilepsy, proximal muscle weakness, ataxia and behavioral and limb anomalies. J. Inherit. Metab. Dis. 2016, 39. [Google Scholar] [CrossRef] [Green Version]
- Freeze, H.H.; Eklund, E.A.; Ng, B.G.; Patterson, M.C. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012, 11, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.A.; Sharma, R.P. Modulation of C-myc, max, and mad gene expression during neural differentiation of embryonic stem cells by all-trans-retinoic acid. Gene Expr. 2002, 10, 125–135. [Google Scholar]
- Wang, X.; Sun, W.; Yang, Y.; Jia, J.; Li, C. A clinical and gene analysis of late-onset combined methylmalonic aciduria and homocystinuria, CblC type, in China. J. Neurol. Sci. 2012, 318, 155–159. [Google Scholar] [CrossRef]
- Tian, B.; Pan, Z.; Lee, J.Y. Widespread mRNA polyadenylation events in introns indicate dynamic interplay between polyadenylation and splicing. Genome Res. 2007, 17, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, C.J.; Schmidt, R.; Kanitz, A.; Artimo, P.; Gruber, A.J.; Zavolan, M. PolyASite 2.0: A consolidated atlas of polyadenylation sites from 3′ end sequencing. Nucleic Acids Res. 2020, 48. [Google Scholar] [CrossRef] [Green Version]
- Schaum, N.; Karkanias, J.; Neff, N.F.; May, A.P.; Quake, S.R.; Wyss-Coray, T.; Darmani, S. The tabula muris consortium., overall coordination. single-cell transcriptomics of 20 mouse organs creates a tabula muris. Nature 2018, 562, 367–372. [Google Scholar] [CrossRef]
- Akamatsu, W.; Fujihara, H.; Mitsuhashi, T.; Yano, M.; Shibata, S.; Hayakawa, Y.; Okano, H.J.; Sakakibara, S.-I.; Takano, H.; Takano, T.; et al. The RNA-binding protein HuD regulates neuronal cell identity and maturation. Proc. Natl. Acad. Sci. USA 2005, 102, 4625–4630. [Google Scholar] [CrossRef] [Green Version]
- Tanner, D.C.; Qiu, S.; Bolognani, F.; Partridge, L.D.; Weeber, E.J.; Perrone-Bizzozero, N.I. Alterations in mossy fiber physiology and GAP-43 expression and function in transgenic mice overexpressing HuD. Hippocampus 2008, 18, 814–823. [Google Scholar] [CrossRef] [Green Version]
- Wingett, S.W.; Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; [version 2; referees: 4 approved] F1000. Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinforma. Oxf. Engl. 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.; Park, J.W.; Lu, Z.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. RMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga, D.F.T. Maser: Mapping Alternative Splicing Events to pRoteins, R Package Version 1.8.0. 2021. Available online: https://github.com/DiogoVeiga/maser (accessed on 17 March 2021).
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative genomics viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2012, 14, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative Genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [Green Version]
- Karolchik, D.; Hinrichs, A.S.; Furey, T.S.; Roskin, K.M.; Sugnet, C.W.; Haussler, D.; Kent, W.J. The UCSC table browser data retrieval tool. Nucleic Acids Res. 2004, 32, D493–D496. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sena, R.M.; Twiss, J.L.; Gardiner, A.S.; Dell’Orco, M.; Linsenbardt, D.N.; Perrone-Bizzozero, N.I. The RNA-Binding Protein HuD Regulates Alternative Splicing and Alternative Polyadenylation in the Mouse Neocortex. Molecules 2021, 26, 2836. https://doi.org/10.3390/molecules26102836
Sena RM, Twiss JL, Gardiner AS, Dell’Orco M, Linsenbardt DN, Perrone-Bizzozero NI. The RNA-Binding Protein HuD Regulates Alternative Splicing and Alternative Polyadenylation in the Mouse Neocortex. Molecules. 2021; 26(10):2836. https://doi.org/10.3390/molecules26102836
Chicago/Turabian StyleSena, Rebecca M., Jeffery L. Twiss, Amy S. Gardiner, Michela Dell’Orco, David N. Linsenbardt, and Nora I. Perrone-Bizzozero. 2021. "The RNA-Binding Protein HuD Regulates Alternative Splicing and Alternative Polyadenylation in the Mouse Neocortex" Molecules 26, no. 10: 2836. https://doi.org/10.3390/molecules26102836





