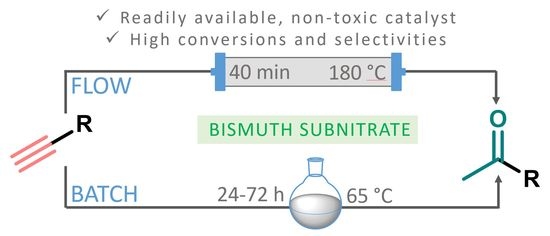
Bismuth Subnitrate-Catalyzed Markovnikov-Type Alkyne Hydrations under Batch and Continuous Flow Conditions
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Batch Reactions
3.3. General Procedure for Flow Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bryan, M.C.; Dunn, P.J.; Entwistle, D.; Gallou, F.; Koenig, S.G.; Hayler, J.D.; Hickey, M.R.; Hughes, S.; Kopach, M.E.; Moine, G.; et al. Key Green Chemistry research areas from a pharmaceutical manufacturers' perspective revisited. Green Chem. 2018, 20, 5082–5103. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Which Metals are Green for Catalysis? Comparison of the Toxicities of Ni, Cu, Fe, Pd, Pt, Rh, and Au Salts. Angew. Chem. Int. Ed. 2016, 55, 12150–12162. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Zimmerman, J.B.; de Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.H.; Lounsbury, A.W.; Mellor, K.E.; Janković, N.Z.; Tu, Q.; et al. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Ollevier, T. Bismuth-Mediated Organic Reactions. Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ollevier, T. New trends in bismuth-catalyzed synthetic transformations. Org. Biomol. Chem. 2013, 11, 2740–2755. [Google Scholar] [CrossRef]
- Nordberg, G.F. Handbook on the Toxicology of Metals, 4th ed.; Academic Press: San Diego, CA, USA, 2015. [Google Scholar]
- Matano, Y. Organobismuth Chemistry; Elsevier Science: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Mohan, R. Green bismuth. Nat. Chem. 2010, 2, 336. [Google Scholar] [CrossRef]
- Ondet, P.; Lemière, G.; Duñach, E. Cyclisations Catalysed by Bismuth(III) Triflate. Eur. J. Org. Chem. 2017, 2017, 761–780. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Ppinto, R.M.A.; Silvestre, S.M. Recent Advances of Bismuth(III) Salts in Organic Chemistry: Application to the Synthesis of Aliphatics, Alicyclics, Aromatics, Amino Acids and Peptides, Terpenes and Steroids of Pharmaceutical Interest. Mini-Rev. Org. Chem. 2009, 6, 241–274. [Google Scholar] [CrossRef]
- Ruimao, H. Recent Advances in Bismuth-Catalyzed Organic Synthesis. Curr. Org. Synth. 2008, 5, 1–27. [Google Scholar] [CrossRef]
- Gaspard-Iloughmane, H.; Le Roux, C. Bismuth(III) Triflate in Organic Synthesis. Eur. J. Org. Chem. 2004, 2004, 2517–2532. [Google Scholar] [CrossRef]
- Leonard, N.M.; Wieland, L.C.; Mohan, R.S. Applications of bismuth(III) compounds in organic synthesis. Tetrahedron 2002, 58, 8373–8397. [Google Scholar] [CrossRef]
- Bothwell, J.M.; Krabbe, S.W.; Mohan, R.S. Applications of bismuth(III) compounds in organic synthesis. Chem. Soc. Rev. 2011, 40, 4649–4707. [Google Scholar] [CrossRef]
- Rueping, M.; Nachtsheim, B.J. Bismuth Salts in Catalytic Alkylation Reactions. In Bismuth-Mediated Organic Reactions; Ollevier, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 115–141. [Google Scholar]
- Rueping, M.; Nachtsheim, B.J.; Ieawsuwan, W. An Effective Bismuth-Catalyzed Benzylation of Arenes and Heteroarenes. Adv. Synth. Catal. 2006, 348, 1033–1037. [Google Scholar] [CrossRef]
- Rueping, M.; Nachtsheim, B.J.; Kuenkel, A. Efficient Metal-Catalyzed Direct Benzylation and Allylic Alkylation of 2,4-Pentanediones. Org. Lett. 2007, 9, 825–828. [Google Scholar] [CrossRef]
- Ötvös, S.B.; Szécsényi, Z.; Fülöp, F. Bismuth(III)-Catalyzed Hydration of Terminal Alkynes: Sustainable Synthesis of Methyl Ketones in Batch and Flow. ACS Sustain. Chem. Eng. 2019, 7, 13286–13293. [Google Scholar] [CrossRef]
- Sun, H.-B.; Li, B.; Hua, R.; Yin, Y. An Efficient and Selective Hydroarylation of Styrenes with Electron-Rich Arenes, Catalyzed by Bismuth(III) Chloride and Affording Markovnikov Adducts. Eur. J. Org. Chem. 2006, 2006, 4231–4236. [Google Scholar] [CrossRef]
- Rueping, M.; Nachtsheim, B.J.; Sugiono, E. Direct Catalytic Benzylation of Hydroxycoumarin—Efficient Synthesis of Warfarin Derivatives and Analogues. Synlett 2010, 2010, 1549–1553. [Google Scholar] [CrossRef]
- Qin, H.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. Bismuth-Catalyzed Intermolecular Hydroamination of 1,3-Dienes with Carbamates, Sulfonamides, and Carboxamides. J. Am. Chem. Soc. 2006, 128, 1611–1614. [Google Scholar] [CrossRef]
- Wei, H.; Qian, G.; Xia, Y.; Li, K.; Li, Y.; Li, W. BiCl3-Catalyzed Hydroamination of Norbornene with Aromatic Amines. Eur. J. Org. Chem. 2007, 2007, 4471–4474. [Google Scholar] [CrossRef]
- Ötvös, S.B.; Mészáros, R.; Varga, G.; Kocsis, M.; Kónya, Z.; Kukovecz, Á.; Pusztai, P.; Sipos, P.; Pálinkó, I.; Fülöp, F. A mineralogically-inspired silver–bismuth hybrid material: An efficient heterogeneous catalyst for the direct synthesis of nitriles from terminal alkynes. Green Chem. 2018, 20, 1007–1019. [Google Scholar] [CrossRef]
- Beller, M.; Seayad, J.; Tillack, A.; Jiao, H. Catalytic Markovnikov and anti-Markovnikov Functionalization of Alkenes and Alkynes: Recent Developments and Trends. Angew. Chem. Int. Ed. 2004, 43, 3368–3398. [Google Scholar] [CrossRef]
- Hintermann, L.; Labonne, A. Catalytic Hydration of Alkynes and Its Application in Synthesis. Synthesis 2007, 2007, 1121–1150. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Gonzalez-Rodriguez, E.; Kawade, R.K.; Stepanov, A.A.; Vasilevsky, S.F. Alkynes as Synthetic Equivalents of Ketones and Aldehydes: A Hidden Entry into Carbonyl Chemistry. Molecules 2019, 24, 1036. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef]
- Fang, G.; Bi, X. Silver-catalysed reactions of alkynes: Recent advances. Chem. Soc. Rev. 2015, 44, 8124–8173. [Google Scholar] [CrossRef]
- Marion, N.; Ramón, R.S.; Nolan, S.P. [(NHC)AuI]-Catalyzed Acid-Free Alkyne Hydration at Part-per-Million Catalyst Loadings. J. Am. Chem. Soc. 2009, 131, 448–449. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Shao, J.; Yang, G.; Wu, Y.; Zhang, Z. Hydration of alkynes at room temperature catalyzed by gold(I) isocyanide compounds. Green Chem. 2015, 17, 532–537. [Google Scholar] [CrossRef]
- Rao, K.T.V.; Prasad, P.S.S.; Lingaiah, N. Solvent-free hydration of alkynes over a heterogeneous silver exchanged silicotungstic acid catalyst. Green Chem. 2012, 14, 1507–1514. [Google Scholar] [CrossRef]
- Thuong, M.B.T.; Mann, A.; Wagner, A. Mild chemo-selective hydration of terminal alkynes catalysed by AgSbF6. Chem. Commun. 2012, 48, 434–436. [Google Scholar] [CrossRef]
- Trentin, F.; Chapman, A.M.; Scarso, A.; Sgarbossa, P.; Michelin, R.A.; Strukul, G.; Wass, D.F. Platinum(II) Diphosphinamine Complexes for the Efficient Hydration of Alkynes in Micellar Media. Adv. Synth. Catal. 2012, 354, 1095–1104. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Wang, Z.; Fu, X. Visible light promoted hydration of alkynes catalyzed by rhodium(III) porphyrins. Chem. Commun. 2015, 51, 11896–11898. [Google Scholar] [CrossRef] [PubMed]
- Tachinami, T.; Nishimura, T.; Ushimaru, R.; Noyori, R.; Naka, H. Hydration of Terminal Alkynes Catalyzed by Water-Soluble Cobalt Porphyrin Complexes. J. Am. Chem. Soc. 2013, 135, 50–53. [Google Scholar] [CrossRef]
- Mainkar, P.S.; Chippala, V.; Chegondi, R.; Chandrasekhar, S. Ruthenium(II)-Catalyzed Hydration of Terminal Alkynes in PEG-400. Synlett 2016, 27, 1969–1972. [Google Scholar] [CrossRef]
- Keogan, D.M.; Griffith, D.M. Current and Potential Applications of Bismuth-Based Drugs. Molecules 2014, 19, 15258–15297. [Google Scholar] [CrossRef] [PubMed]
- Lazarini, F. Thermal dehydration of some basic bismuth nitrates. Thermochim. Acta 1981, 46, 53–55. [Google Scholar] [CrossRef]
- Reddy, Y.T.; Rajitha, B.; Reddy, P.N.; Kumar, B.S.; Rao, V.P. Bismuth Subnitrate Catalyzed Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones: An Improved Protocol for the Biginelli Reaction. Synth. Commun. 2004, 34, 3821–3825. [Google Scholar] [CrossRef]
- Wu, S.; Dai, W.; Yin, S.; Li, W.; Au, C.-T. Bismuth Subnitrate as an Efficient Heterogeneous Catalyst for Acetalization and Ketalization of Carbonyl Compounds with Diols. Catal. Lett. 2008, 124, 127–132. [Google Scholar] [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M.; Luque, R. Heterogeneous catalysis under flow for the 21st century fine chemical industry. Green Energy Environ. 2021. [Google Scholar] [CrossRef]
- Liu, X.; Unal, B.; Jensen, K.F. Heterogeneous catalysis with continuous flow microreactors. Catal. Sci. Technol. 2012, 2, 2134–2138. [Google Scholar] [CrossRef]
- Frost, C.G.; Mutton, L. Heterogeneous catalytic synthesis using microreactor technology. Green Chem. 2010, 12, 1687–1703. [Google Scholar] [CrossRef]
- Munirathinam, R.; Huskens, J.; Verboom, W. Supported Catalysis in Continuous-Flow Microreactors. Adv. Synth. Catal. 2015, 357, 1093–1123. [Google Scholar] [CrossRef]
- Yoo, W.-J.; Ishitani, H.; Saito, Y.; Laroche, B.; Kobayashi, S. Reworking Organic Synthesis for the Modern Age: Synthetic Strategies Based on Continuous-Flow Addition and Condensation Reactions with Heterogeneous Catalysts. J. Org. Chem. 2020, 85, 5132–5145. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Ichitsuka, T.; Koumura, N.; Sato, K.; Kobayashi, S. Flow fine synthesis with heterogeneous catalysts. Tetrahedron 2018, 74, 1705–1730. [Google Scholar] [CrossRef]
- Ötvös, S.B.; Pericàs, M.A.; Kappe, C.O. Multigram-scale flow synthesis of the chiral key intermediate of (−)-paroxetine enabled by solvent-free heterogeneous organocatalysis. Chem. Sci. 2019, 10, 11141–11146. [Google Scholar] [CrossRef] [PubMed]
- Ötvös, S.B.; Llanes, P.; Pericàs, M.A.; Kappe, C.O. Telescoped Continuous Flow Synthesis of Optically Active γ-Nitrobutyric Acids as Key Intermediates of Baclofen, Phenibut, and Fluorophenibut. Org. Lett. 2020, 22, 8122–8126. [Google Scholar] [CrossRef]
- Mándity, I.M.; Ötvös, S.B.; Fülöp, F. Strategic Application of Residence-Time Control in Continuous-Flow Reactors. ChemistryOpen 2015, 4, 212–223. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Akwi, F.M.; Watts, P. Continuous flow chemistry: Where are we now? Recent applications, challenges and limitations. Chem. Commun. 2018, 54, 13894–13928. [Google Scholar] [CrossRef]
- Rogers, L.; Jensen, K.F. Continuous manufacturing—The Green Chemistry promise? Green Chem. 2019, 21, 3481–3498. [Google Scholar] [CrossRef]
- Hou, S.; Yang, H.; Cheng, B.; Zhai, H.; Li, Y. Cobaloxime-catalyzed hydration of terminal alkynes without acidic promoters. Chem. Commun. 2017, 53, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G.; Russo, N.; Sicilia, E. Homogeneous Gold Catalysis: Hydration of 1,2-Diphenylacetylene with Methanol in Aqueous Media. A Theoretical Viewpoint. Organometallics 2012, 31, 3074–3080. [Google Scholar] [CrossRef]
- Wang, H.; Hussain, A.A.; Pyrek, J.S.; Goodman, J.; Wedlund, P.J. Assay for nipecotic acid in small blood samples by gas chromatography–mass spectroscopy. J. Pharmaceut. Biomed. 2004, 34, 1063–1070. [Google Scholar] [CrossRef]
- Sanderson, K. Big interest in heavy drugs. Nature 2009, 458, 269. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.M.; McLachlan, K.A.; Wildman, M.A.; Ehresmann, J.O.; Kletnieks, P.W.; Haw, J.F. Experimental Evidence from H/D Exchange Studies for the Failure of Direct C-C Coupling Mechanisms in the Methanol-to-Olefin Process Catalyzed by HSAPO-34. Angew. Chem. Int. Ed. 2006, 45, 3133–3136. [Google Scholar] [CrossRef]
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. Int. Ed. 2018, 57, 1758–1784. [Google Scholar] [CrossRef] [PubMed]
- Atzrodt, J.; Derdau, V.; Fey, T.; Zimmermann, J. The Renaissance of H/D Exchange. Angew. Chem. Int. Ed. 2007, 46, 7744–7765. [Google Scholar] [CrossRef] [PubMed]
- Coumbarides, G.S.; Dingjan, M.; Eames, J.; Flinn, A.; Northen, J. An efficient laboratory synthesis of α-deuteriated profens. J. Label. Compd. Radiopharm. 2006, 49, 903–914. [Google Scholar] [CrossRef]
- Erdogan, G.; Grotjahn, D.B. Mild and Selective Deuteration and Isomerization of Alkenes by a Bifunctional Catalyst and Deuterium Oxide. J. Am. Chem. Soc. 2009, 131, 10354–10355. [Google Scholar] [CrossRef]
- Zhan, M.; Zhang, T.; Huang, H.; Xie, Y.; Chen, Y. A simple method for α-position deuterated carbonyl compounds with pyrrolidine as catalyst. J. Label. Compd. Radiopharm. 2014, 57, 533–539. [Google Scholar] [CrossRef] [PubMed]


 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Conversion (%) a | Selectivity (%) a | |
| 1 | 2 | |||
| 1 | None | 0 | - | - |
| 2 | Bismuth subnitrate | 100 | 100 | 0 |
| 3 | Bi(OTf)3 | 100 | 88 | 12 |
| 4 | BiBr3 | 45 | 92 | 8 |
| 5 | Bi(OAc)3 | 0 | - | - |
| 6 | Bi2O3 | 0 | - | - |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Reaction Time (h) | Catalyst Loading (mol%) | C (M) | T (°C) | Conversion (%) a | Selectivity (%) a | |
| 1 | 2 | ||||||
| 1 | 24 | 15 | 1.0 | 65 | 100 | 100 | 0 |
| 2 | 12 | 15 | 1.0 | 65 | 73 | 100 | 0 |
| 3 | 6 | 15 | 1.0 | 65 | 41 | 100 | 0 |
| 4 | 3 | 15 | 1.0 | 65 | 18 | 100 | 0 |
| 5 | 1 | 15 | 1.0 | 65 | 6 | 100 | 0 |
| 6 | 24 | 10 | 1.0 | 65 | 92 | 100 | 0 |
| 7 | 24 | 5 | 1.0 | 65 | 79 | 88 | 12 |
| 8 | 24 | 2 | 1.0 | 65 | 62 | 71 | 29 |
| 9 | 24 | 15 | 1.0 | 25 | 3 | 100 | 0 |
| 10 | 24 | 15 | 2.0 | 65 | 73 | 92 | 8 |
 | ||||
|---|---|---|---|---|
| Entry | Solvent | Conversion (%) a | Selectivity (%) a | |
| 1 | 2 | |||
| 1 | MeOH | 100 | 100 | 0 |
| 2 | EtOH | 14 | 100 | 0 |
| 3 | iPrOH | traces | - | - |
| 4 | H2O | 69 | 100 | 0 |
| 5 | dry MeOH | 94 | 92 | 8 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Conversion (%) a,b | Sel. (%) a |
| 1 c |  |  | 100 (98) | 100 |
| 2 |  |  | 73 | 100 |
| 3 |  |  | 60 | 100 |
| 4 |  |  | 100 (98) | 100 |
| 5 d |  |  | 20 | 100 |
| 6 |  |  | 81 | 100 |
| 7 |  |  | 82 | 100 |
| 8 |  |  | 74 | 100 |
| 9 c |  |  | 100 | 100 |
| 10 c |  |  | 100 | 100 |
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Product | Conversion (%) a | Sel. (%) a | D (%) a,b |
| 1 |  |  | 70 | 100 | >99 |
| 2 |  |  | 100 | 100 | >99 |
| 3 |  |  | 59 | 100 | >99 |
| 4 |  |  | 78 | 100 | >99 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Conversion (%) a | Sel. (%) a |
| 1 |  |  | 75 | 100 |
| 2 |  |  | 71 | 100 |
| 3 |  |  | 100 | 100 |
| 4 |  |  | 26 | 100 |
| 5 |  |  | 100 | 100 |
| 6 |  |  | 100 | 100 |
| 7 |  |  | 100 | 100 |
| 8 |  |  | 100 | 100 |
| 9 |  |  | 95 | 100 |
| 10 |  |  | 90 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szécsényi, Z.; Fülöp, F.; Ötvös, S.B. Bismuth Subnitrate-Catalyzed Markovnikov-Type Alkyne Hydrations under Batch and Continuous Flow Conditions. Molecules 2021, 26, 2864. https://doi.org/10.3390/molecules26102864
Szécsényi Z, Fülöp F, Ötvös SB. Bismuth Subnitrate-Catalyzed Markovnikov-Type Alkyne Hydrations under Batch and Continuous Flow Conditions. Molecules. 2021; 26(10):2864. https://doi.org/10.3390/molecules26102864
Chicago/Turabian StyleSzécsényi, Zsanett, Ferenc Fülöp, and Sándor B. Ötvös. 2021. "Bismuth Subnitrate-Catalyzed Markovnikov-Type Alkyne Hydrations under Batch and Continuous Flow Conditions" Molecules 26, no. 10: 2864. https://doi.org/10.3390/molecules26102864
APA StyleSzécsényi, Z., Fülöp, F., & Ötvös, S. B. (2021). Bismuth Subnitrate-Catalyzed Markovnikov-Type Alkyne Hydrations under Batch and Continuous Flow Conditions. Molecules, 26(10), 2864. https://doi.org/10.3390/molecules26102864