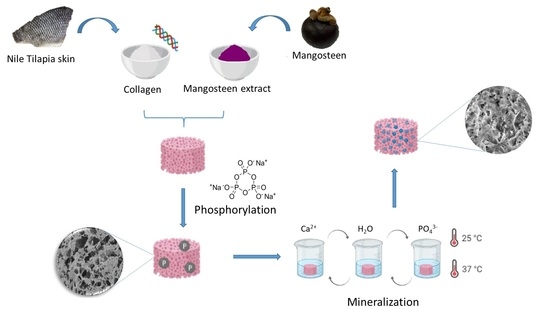
Mineralization of Phosphorylated Fish Skin Collagen/Mangosteen Scaffolds as Potential Materials for Bone Tissue Regeneration
Abstract
1. Introduction
2. Results and Discussion
2.1. Collagen SDS-Page
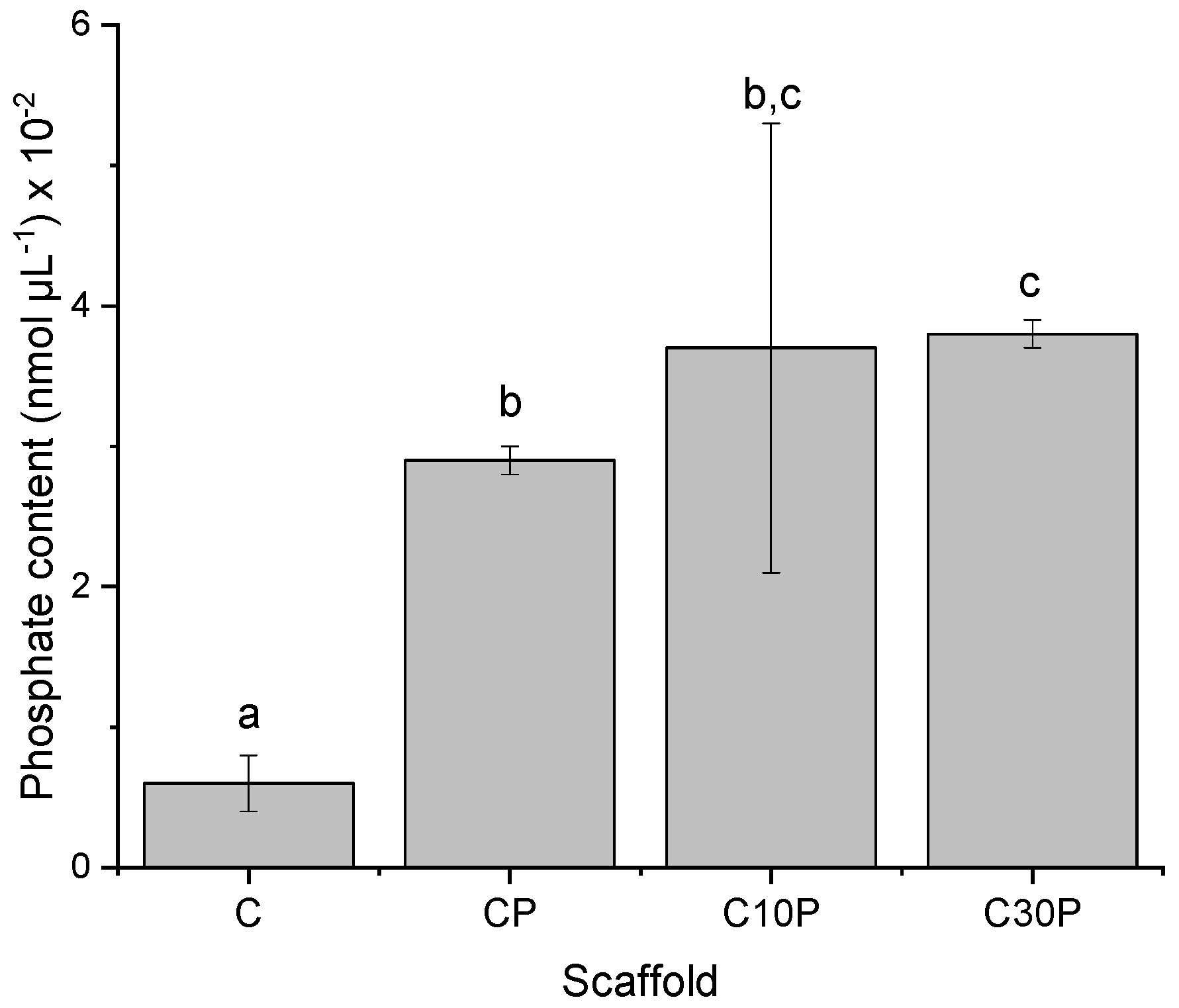
2.2. Phosphate Determination
2.3. ATR-FTIR
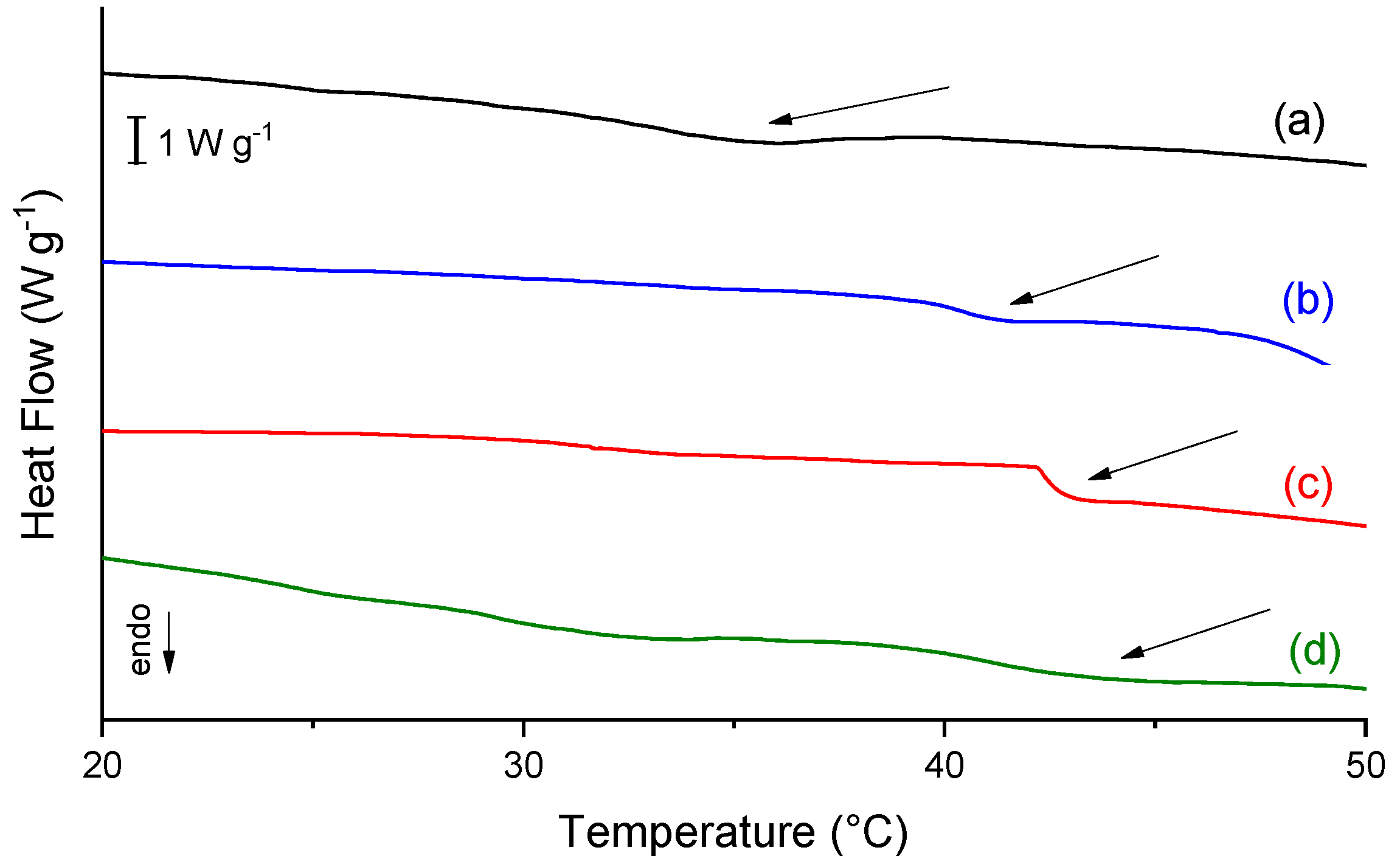
2.4. Differential Scanning Calorimetry (DSC)
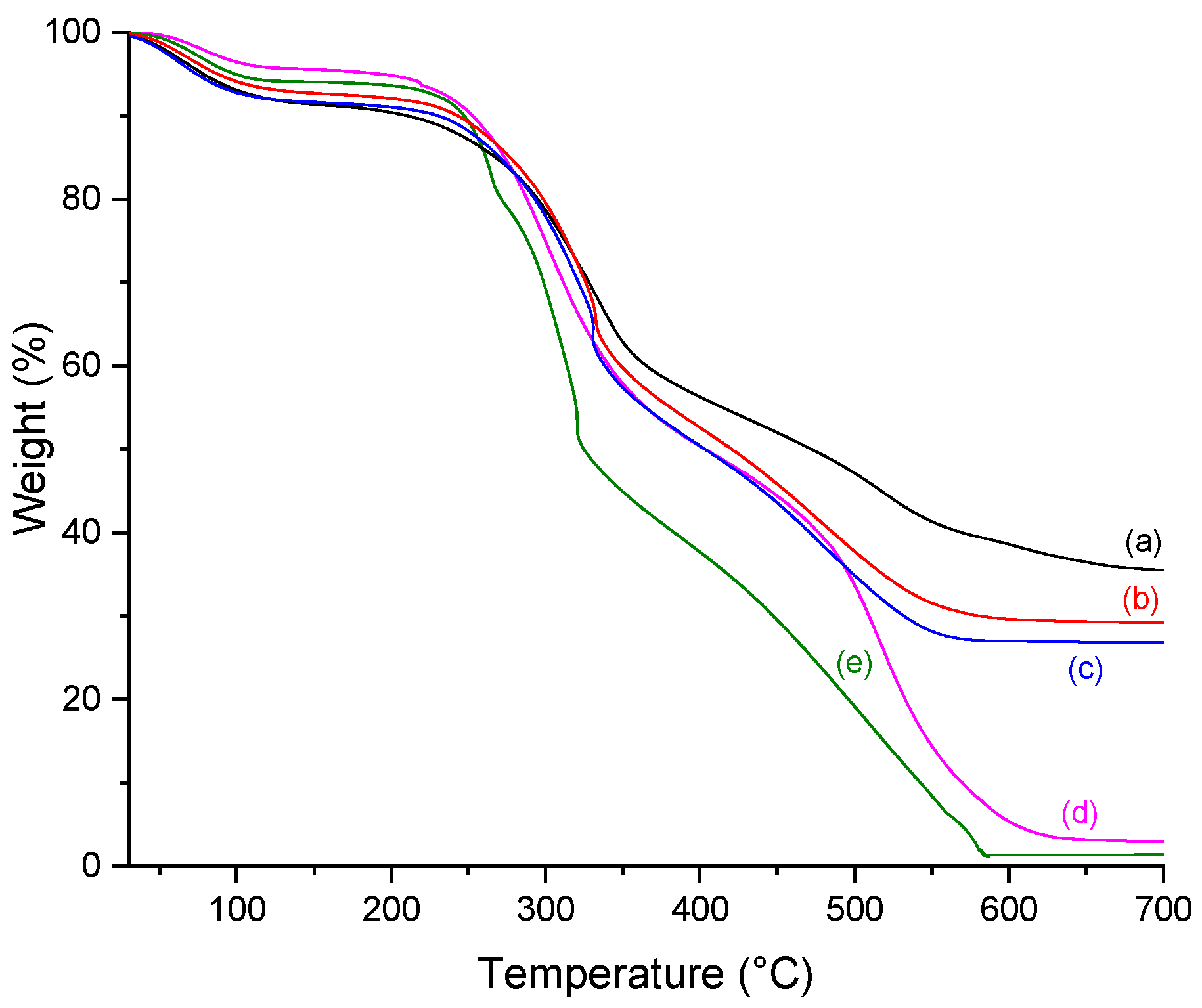
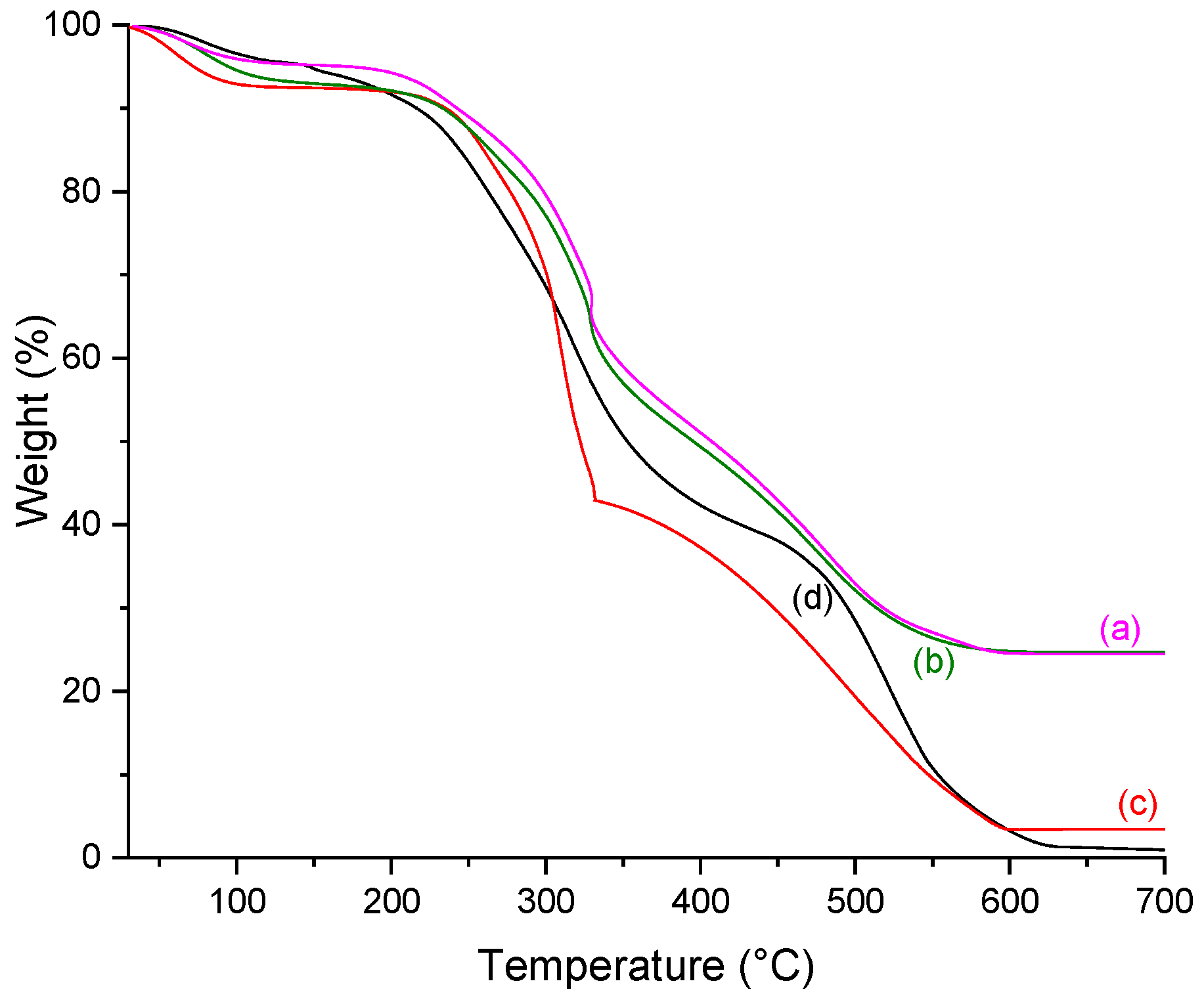
2.5. Thermogravimetry (TGA)
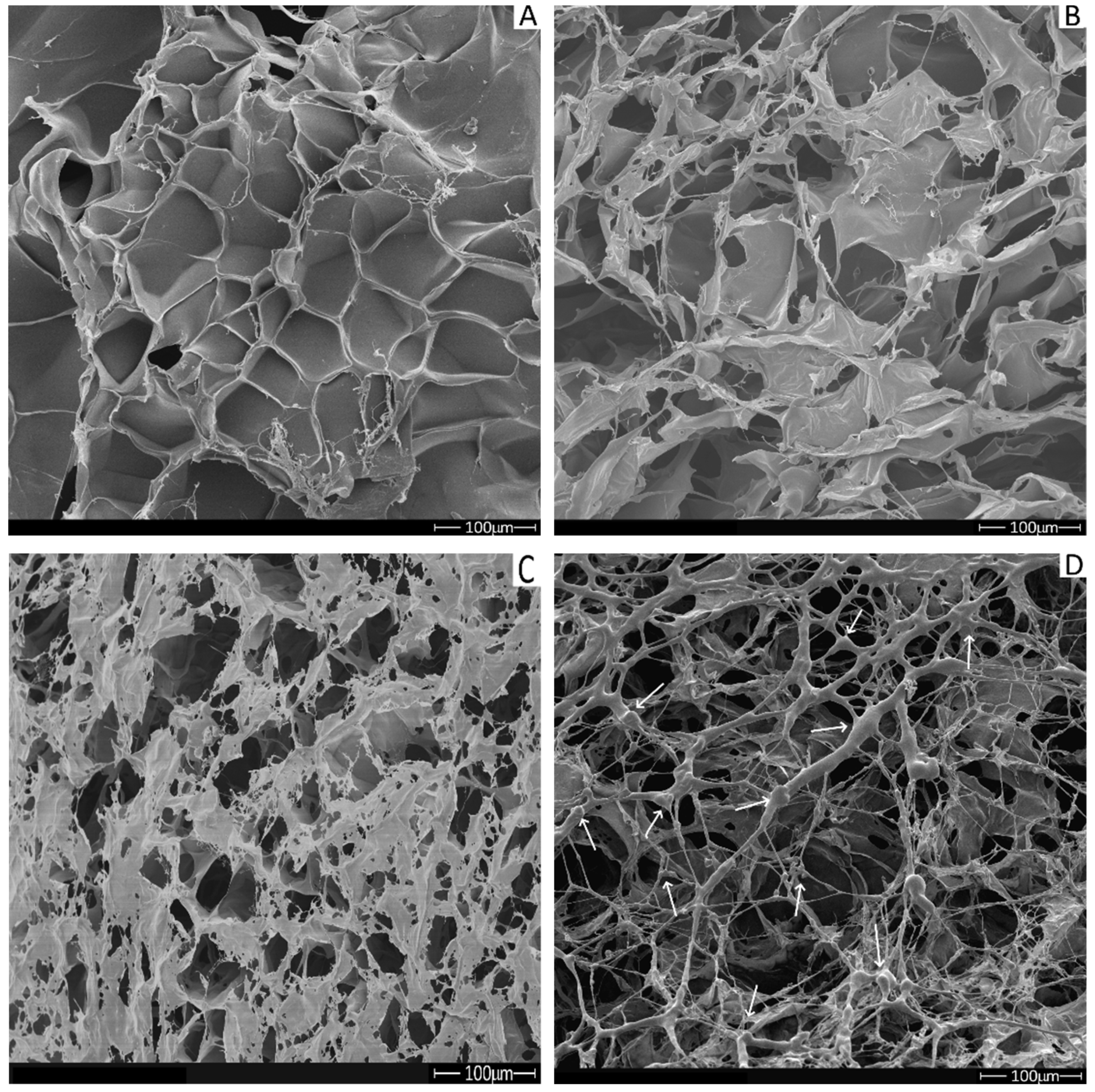
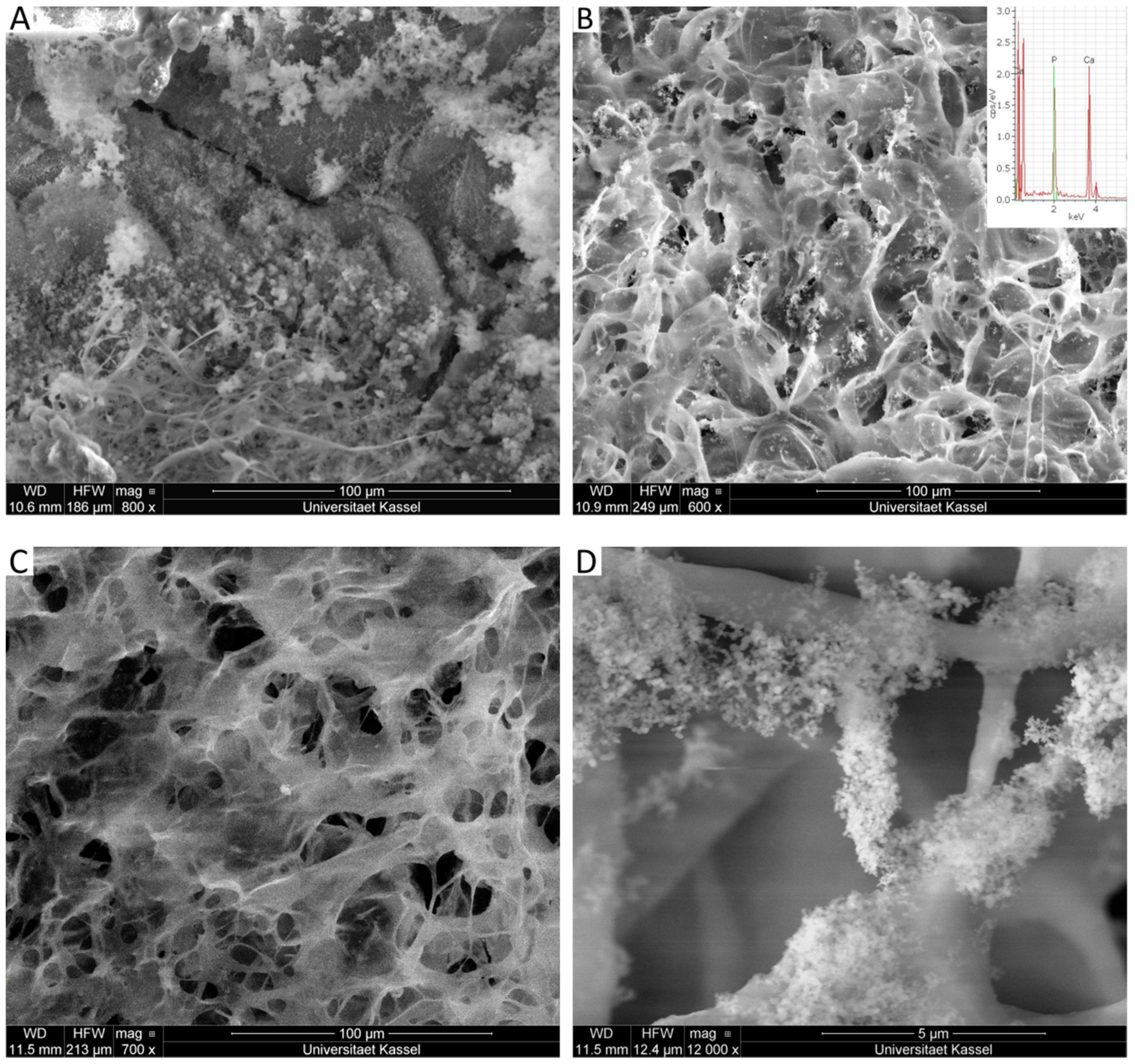
2.6. Scanning Electron Microscopy (SEM)
2.7. Energy Dispersive X-Ray Spectroscopy (EDX)
3. Materials and Methods
3.1. Materials
3.2. Extraction of Collagen
3.3. Mangosteen Extraction
3.4. Phosphorylation of Collagen Scaffold
3.5. Mineralization of Scaffolds
3.6. Characterization
3.6.1. Collagen SDS-PAGE
3.6.2. Phosphate Quantification
3.6.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.6.4. Differential Scanning Calorimetry (DSC)
3.6.5. Thermogravimetric Analysis (TGA)
3.6.6. Scanning Electron Microscopy (SEM)
3.6.7. Energy Dispersive X-Ray Spectroscopy (EDX)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Salgado, C.L.; Sahu, A.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J. Biomed. Mater. Res. A 2013, 101, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.; Ringshia, R.A.; Legeros, R.Z.; Clark, E.; Yost, M.J.; Terracio, L.; Teixeira, C.C. An improved collagen scaffold for skeletal regeneration. J. Biomed. Mater. Res. A. 2010, 94, 371–379. [Google Scholar] [CrossRef]
- León-Lopez, A.; Molares-Peñaloza, A.; Matínez-Juárez, V.M.; Vargas-Torrer, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Potaros, T.; Raksakulthai, N.; Runglerdkreangkrai, J.; Wprawattanamateekul, W. Characteristics of Collagen from Nile Tilapia (Oreochromis niloticus) Skin Isolated by Two Different Methods. Kasetsart. J. Nat. Sci. 2009, 43, 584–593. [Google Scholar]
- Valenzuela-Rojo, R.D.; López-Cervantes, J.; Sánchez-Machado, D.I.; Escárcega-Galaz, A.A.; Del Martínez-Macias, M.R. Antibacterial, mechanical and physical properties of collagen-chitosan sponges from aquatic source. Sustain. Chem. Pharm. 2020, 15, 100218. [Google Scholar] [CrossRef]
- Prabu, E.; Rajagopalsamy, C.B.T.; Ahilan, B.; Jeevagan, I.J.M.A.; Renuhadevi, M. Tilapia–An Excellent Candidate Species for World Aquaculture: A Review. ARRB 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Tang, J.; Saito, T. Biocompatibility of Novel Type I Collagen Purified from Tilapia Fish Scale: An In Vitro Comparative Study. Biomed Res. Int. 2015, 2015, 139476. [Google Scholar] [CrossRef]
- Lau, C.S.; Hassanbhai, A.; Wen, F.; Wang, D.; Chanchareonsook, N.; Goh, B.T.; Yu, N.; Teoh, S.H. Evaluation of decellularized tilapia skin as a tissue engineering scaffold. J. Tissue. Eng. Regen. Med. 2019, 10, 1779–1791. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-X.; Hoff, S.E.; Huang, X.-Q.; Liu, J.; Wan, Q.-Q.; Song, Q.; Gu, J.-T.; Heinz, H.; Tay, F.R.; Niu, L.-N. Involvement of prenucleation clusters in calcium phosphate mineralization of collagen. Acta. Biomater. 2021, 120, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Niu, X.; Hou, S.; Li, Z.; Li, P.; Fan, Y. Apatite minerals derived from collagen phosphorylation modification induce the hierarchical intrafibrillar mineralization of collagen fibers. J. Biomed. Mater. Res. A. 2019, 107, 2403–2413. [Google Scholar] [CrossRef]
- Gu, L.; Kim, J.; Kim, Y.K.; Liu, Y.; Dickens, S.H.; Pashley, D.H.; Ling, J.; Tay, F.R. A chemical phosphorylation-inspired design for Type I collagen biomimetic remineralization. Dent. Mater. 2010, 26, 1077–1089. [Google Scholar] [CrossRef]
- Marzaimi, I.N.; Aizat, W.M. Current Review on Mangosteen Usages in Antiinflammation and Other Related Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Academic Press: Cambrigde, MA, USA, 2019; pp. 273–289. ISBN 9780128138205. [Google Scholar]
- Ansori, A.N.M.; Fadholly, A.; Hayaza, S.; Susilo, R.J.K.; Inayatillah, B.; Winarni, D.; Husen, S.A. A Review on Medicinal Properties of Mangosteen (Garcinia mangostana L.). Rese. J. Pharm. Technol. 2020, 13, 974. [Google Scholar] [CrossRef]
- Kresnoadi, U.; Ariani, M.D.; Djulaeha, E.; Hendrijantini, N. The potential of mangosteen (Garcinia mangostana) peel extract, combined with demineralized freeze-dried bovine bone xenograft, to reduce ridge resorption and alveolar bone regeneration in preserving the tooth extraction socket. J. Indian Prosthodont. Soc. 2017, 17, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.R.; Covington, A.D.; Hancock, R.A. Structure-activity relationships in the hydrophobic interactions of polyphenols with cellulose and collagen. Biopolymers 2003, 70, 403–413. [Google Scholar] [CrossRef]
- Li, P.-H.; Lu, W.-C.; Chan, Y.-J.; Ko, W.-C.; Jung, C.-C.; Le Huynh, D.T.; Ji, Y.-X. Extraction and characterization of collagen from sea cucumber (Holothuria cinerascens) and its potential application in moisturizing cosmetics. Aquaculture 2020, 515, 734590. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef]
- Yu, F.; Zong, C.; Jin, S.; Zheng, J.; Chen, N.; Huang, J.; Chen, Y.; Huang, F.; Yang, Z.; Tang, Y.; et al. Optimization of Extraction Conditions and Characterization of Pepsin-Solubilised Collagen from Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 29. [Google Scholar] [CrossRef]
- Chen, C.; Mao, C.; Sun, J.; Chen, Y.; Wang, W.; Pan, H.; Tang, R.; Gu, X. Glutaraldehyde-induced remineralization improves the mechanical properties and biostability of dentin collagen. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Mao, C.; Gu, T.; Pan, H.; Shao, C.; Sun, J.; Chen, C.; Tang, R.; Gu, X. Phosphorylated chitosan to promote biomimetic mineralization of type I collagen as a strategy for dentin repair and bone tissue engineering. New J. Chem. 2019, 43, 2002–2010. [Google Scholar] [CrossRef]
- Zhang, J.; Jeevithan, E.; Bao, B.; Wang, S.; Gao, K.; Zhang, C.; Wu, W. Structural characterization, in-vivo acute systemic toxicity assessment and in-vitro intestinal absorption properties of tilapia (Oreochromis niloticus) skin acid and pepsin solublilized type I collagen. Process. Biochem. 2016, 51, 2017–2025. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef]
- Abifarin, J.K.; Obada, D.O.; Dauda, E.T.; Dodoo-Arhin, D. Experimental data on the characterization of hydroxyapatite synthesized from biowastes. Data Brief. 2019, 26, 104485. [Google Scholar] [CrossRef] [PubMed]
- Silva Júnior, Z.S.; Botta, S.B.; Ana, P.A.; França, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Deana, A.; Bussadori, S.K. Effect of papain-based gel on type I collagen-spectroscopy applied for microstructural analysis. Sci. Rep. 2015, 5, 11448. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.M.; Da Fontoura, A.M.; Vidal, A.R.; Dornelles, R.C.P.; Kubota, E.H.; Mello, R.D.O.; Cansian, R.L.; Demiate, I.M.; de Oliveira, C.S. Characterization of hydrolysates of collagen from mechanically separated chicken meat residue. Food Sci. Technol. 2020, 40, 355–362. [Google Scholar] [CrossRef]
- Guo, M.; Wang, X.; Lu, X.; Wang, H.; Brodelius, P.E. α-Mangostin Extraction from the Native Mangosteen (Garcinia mangostana L.) and the Binding Mechanisms of α-Mangostin to HSA or TRF. PLoS ONE 2016, 11, e0161566. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.M.; Martins, V.C.A.; Fernandes, Y.O.M.; Vulcani, V.A.S.; Plepis, A.M.G. Development and characterization of collagen/gelatin films and gels incorporated with pequi oil. J. Appl. Polym. Sci. 2018, 135, 46627. [Google Scholar] [CrossRef]
- Valenzuela-Rojo, D.R.; López-Cervantes, J.; Sánchez-Machado, D.I. Tilapia (Oreochromis aureus) Collagen for Medical Biomaterials. In Seaweed Biomaterials; Maiti, S., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78984-846-5. [Google Scholar]
- Trębacz, H.; Szczęsna, A.; Arczewska, M. Thermal stability of collagen in naturally ageing and in vitro glycated rabbit tissues. J. Therm. Anal. Calorim. 2018, 134, 1903–1911. [Google Scholar] [CrossRef]
- Tziveleka, L.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V.; Rahman, A.; Solva, T.H. Collagen from the Marine Sponges Axinella cannabina and Siberites carnosus: Isolation and Morphological, Biochemical, and Biophysical Characterization. Mar. Drugs. 2017, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Prieur, B.; Meub, M.; Wittemann, M.; Klein, R.; Bellayer, S.; Fontaine, G.; Bourbigot, S. Phosphorylation of lignin: Characterization and investigation of the thermal decomposition. RSC Adv. 2017, 7, 16866–16877. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Ding, S.; Liu, C.; Ai, J. Effect of porous structure and pore size on mechanical strength of 3D-printed comby scaffolds. Mater. Lett. 2018, 223, 21–24. [Google Scholar] [CrossRef]
- Dorozhkin, S. Nanodimensional and Nanocrystalline Calcium Orthophosphates. Am. J. Biomed. Eng. 2012, 2, 48–97. [Google Scholar] [CrossRef]
- Ghomi, H.; Fathi, M.H.; Edris, H. Preparation of nanostructure hydroxyapatite scaffold for tissue engineering applications. J. Sol-Gel Sci. Technol. 2011, 58, 642–650. [Google Scholar] [CrossRef]
- Elhendawi, H.; Felfel, R.M.; Abd El-Hady, B.M.; Reicha, F.M. Effect of Synthesis Temperature on the Crystallization and Growth of In Situ Prepared Nanohydroxyapatite in Chitosan Matrix. ISRN. Biomaterials 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Daskalova, A.; Nathala, C.S.; Bliznakova, I.; Stoyanova, E.; Zhelyazkova, A.; Ganz, T.; Lueftenegger, S.; Husinsky, W. Controlling the porosity of collagen, gelatin and elastin biomaterials by ultrashort laser pulses. Appl. Surf. Sci. 2014, 292, 367–377. [Google Scholar] [CrossRef]
- Kumaran, A.; Sukumaran, P. Antiurolithiatic Evaluation of α- Mangostin Fraction Isolated from Garcinia mangostana Pericarp through Computational, in vitro and in vivo Approach. JPRI 2021, 32, 63–78. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Saeedi, P.; Izadifar, Z.; Bekhit, A.A.; Khademhosseini, A. Polyphenol uses in biomaterials engineering. Biomaterials 2018, 167, 91–106. [Google Scholar] [CrossRef]
- Zarena, A.S.; Sankar, K.U. A Study of Antioxidant Properties from Garcinia Mangostana L. Pericarp Extract. Acta Sci. Pol. Technol. Aliment. 2009, 8, 23–24. [Google Scholar]
- Zhou, Y.; Liu, Q.; Zhao, Z.; Wang, W.; Zheng, L.; Li, Z. Preparation and Characterization of Phosphorylated Collagen and Hydroxyapatite Composite as a Potential Bone Substitute. Chem. Lett. 2013, 42, 83–85. [Google Scholar] [CrossRef]
- Li, X.; Chang, J. Preparation of bone-like apatite-collagen nanocomposites by a biomimetic process with phosphorylated collagen. J. Biomed. Mater. Res. A. 2008, 85, 293–300. [Google Scholar] [CrossRef]
- Horn, M.M.; Martins, V.C.A.; Plepis, A.M.G. In Vitro Mineralization Study of Chitosan/Carbon Nanotubes Scaffolds: Effect of Mineralization Cycles. Macromol. Symp. 2018, 387, 1600148. [Google Scholar] [CrossRef]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.P.B. Extraction, optimization and characterization of collagen from sole fish skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Vaeth, E.; Sladek, P.; Kenar, K. Ionen-Chromatographie von Polyphosphaten und Phosphonaten. Z. Anal. Chem. 1987, 329, 584–589. [Google Scholar] [CrossRef]

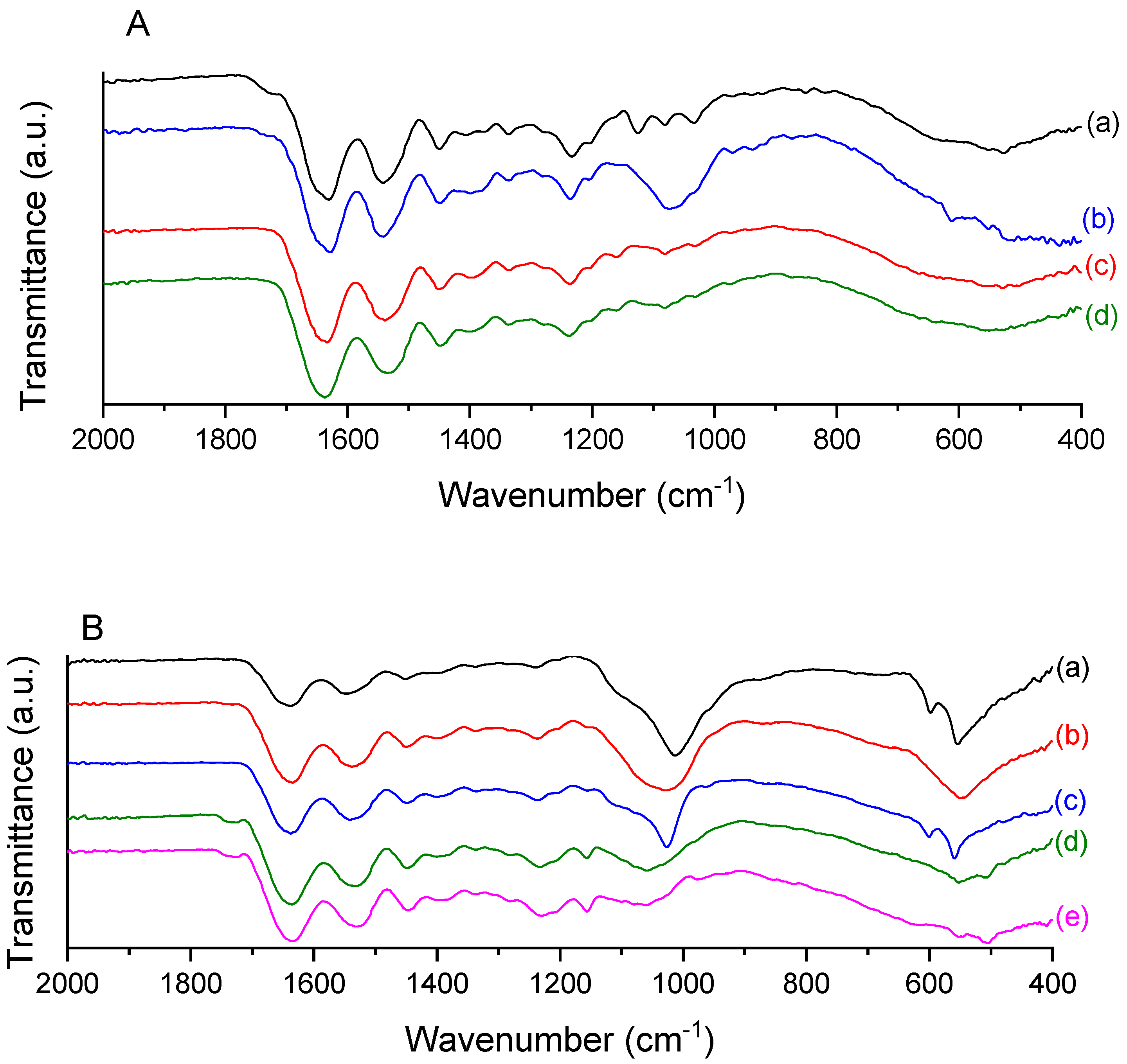
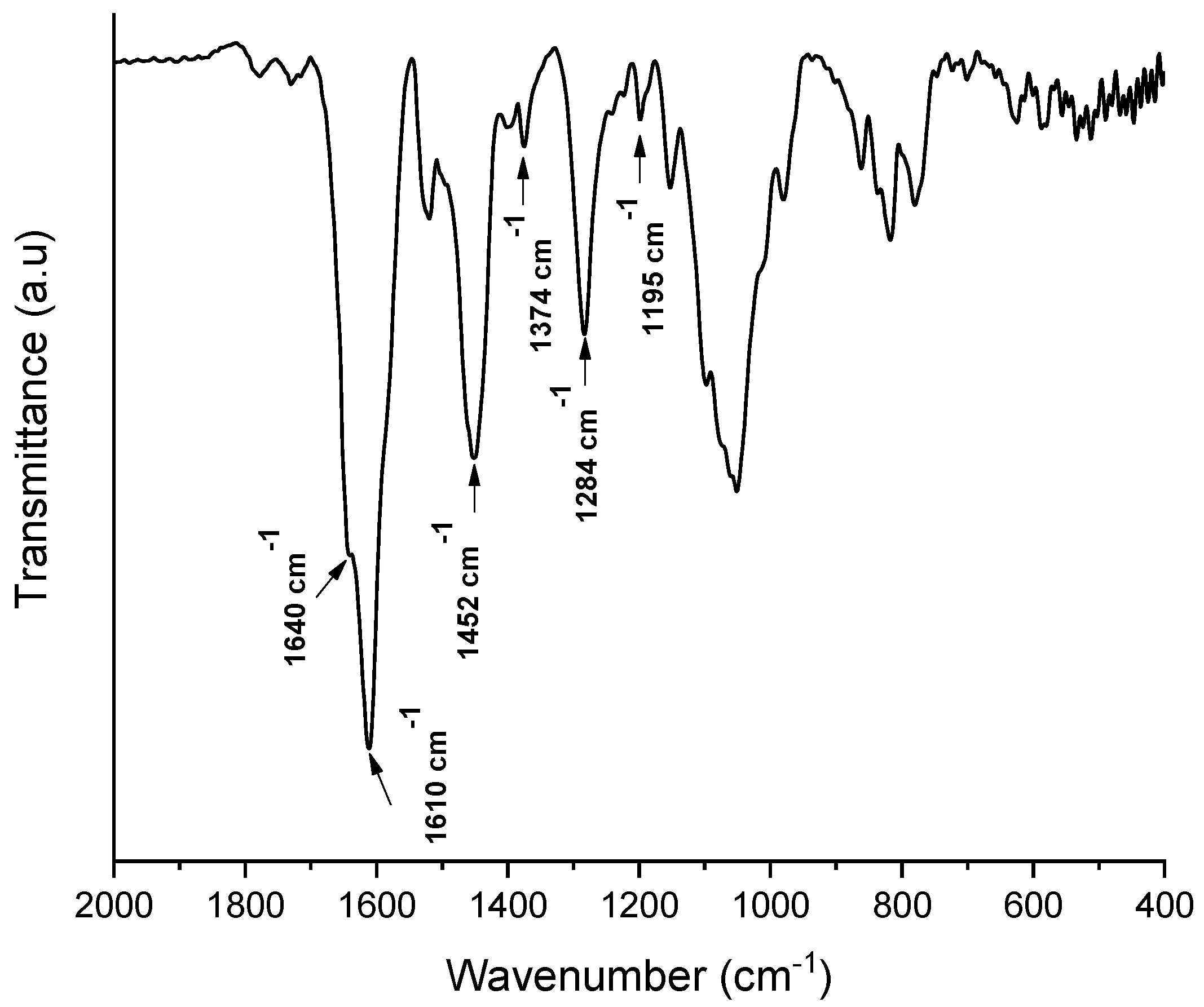
| Scheme 1240. | 1240/1450 cm−1 Ratio |
|---|---|
| C | 0.96 |
| CP | 1.01 |
| C10P | 1.02 |
| C30P | 1.03 |
| CP25 | 1.04 |
| C10P25 | 1.04 |
| C30P25 | 1.00 |
| C10P37 | 1.01 |
| C30P37 | 0.98 |
| Scaffold | Denaturation Temperature (°C) |
|---|---|
| C | 33.5 |
| CP | 40.4 |
| C10P | 41.2 |
| C30P | 41.1 |
| Scaffold | % Weight Loss | %Residue (700 °C) | Tonset (°C) | ||
|---|---|---|---|---|---|
| 30–200 °C | 200–500 °C | 500–700 °C | |||
| C | 8.34 | 63.16 | 27.54 | 1.04 | 228.8 |
| CP | 5.16 | 61.10 | 30.78 | 2.97 | 240.4 |
| C10P | 6.20 | 74.73 | 17.69 | 1.38 | 245.0 |
| C30P | 7.81 | 72.60 | 16.93 | 3.70 | 250.4 |
| CP25 | 9.60 | 43.25 | 11.63 | 35.52 | 247.8 |
| C10P25 | 7.91 | 54.32 | 8.58 | 29.19 | 249.8 |
| C30P25 | 7.88 | 59.97 | 7.48 | 24.68 | 255.2 |
| C10P37 | 8.96 | 56.15 | 8.04 | 26.85 | 262.9 |
| C30P37 | 5.72 | 61.30 | 8.47 | 24.50 | 259.9 |
| Scaffold | Average Pore Size (μM) ± SD |
|---|---|
| C | 85.0 ± 7.4 a |
| CP | 54.3 ± 8.4 b |
| C10P | 54.9 ± 8.6 b |
| C30P | 52.0 ± 7.7 b |
| CP25 | 5.9 ± 1.0 d |
| C10P25 | 24.5 ± 6.6 c |
| C30P25 | 21.1 ± 3.1 c |
| C10P37 | 24.6 ± 3.3 c |
| C30P37 | 21.5 ± 3.1 c |
| Scaffold | Ca/P Ratio ± SD |
|---|---|
| CP25 | 1.53 ± 0.10 c |
| C10P25 | 1.73 ± 0.07 b |
| C30P25 | 1.74 ± 0.08 b |
| C10P37 | 1.91 ± 0.04 a |
| C30P37 | 1.73 ± 0.09 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milan, E.P.; Rodrigues, M.Á.V.; Martins, V.C.A.; Plepis, A.M.G.; Fuhrmann-Lieker, T.; Horn, M.M. Mineralization of Phosphorylated Fish Skin Collagen/Mangosteen Scaffolds as Potential Materials for Bone Tissue Regeneration. Molecules 2021, 26, 2899. https://doi.org/10.3390/molecules26102899
Milan EP, Rodrigues MÁV, Martins VCA, Plepis AMG, Fuhrmann-Lieker T, Horn MM. Mineralization of Phosphorylated Fish Skin Collagen/Mangosteen Scaffolds as Potential Materials for Bone Tissue Regeneration. Molecules. 2021; 26(10):2899. https://doi.org/10.3390/molecules26102899
Chicago/Turabian StyleMilan, Eduardo P., Murilo Á. V. Rodrigues, Virginia C. A. Martins, Ana M. G. Plepis, Thomas Fuhrmann-Lieker, and Marilia M. Horn. 2021. "Mineralization of Phosphorylated Fish Skin Collagen/Mangosteen Scaffolds as Potential Materials for Bone Tissue Regeneration" Molecules 26, no. 10: 2899. https://doi.org/10.3390/molecules26102899
APA StyleMilan, E. P., Rodrigues, M. Á. V., Martins, V. C. A., Plepis, A. M. G., Fuhrmann-Lieker, T., & Horn, M. M. (2021). Mineralization of Phosphorylated Fish Skin Collagen/Mangosteen Scaffolds as Potential Materials for Bone Tissue Regeneration. Molecules, 26(10), 2899. https://doi.org/10.3390/molecules26102899