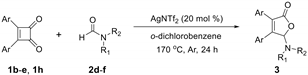
Abstract
As an important moiety in natural products, N,O-acetal has attracted wide attention in the past few years. An efficient method to construct N,O-acetal has been developed. Using silver catalyst, cyclobutenediones were smoothly converted to the corresponding γ-aminobutenolides in the presence of formamides, in which cyclobutenediones likely proceed with a key decarbonylative [3 + 2] cycloaddition process. In this way, a series of products with varied substituents were isolated in moderate yield and fully characterized.
1. Introduction
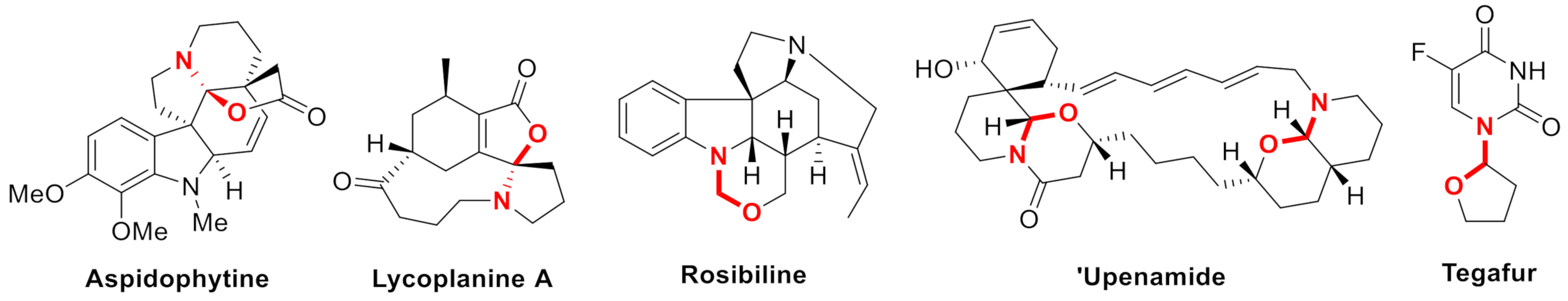
N,O-Acetal/ketal represent an important architecture in bioactive natural and pharmaceutical products [1,2,3]. Typical structures such as aspidosperma alkaloid (-)-aspidophytine [4,5], lycopodium alkaloid lycoplanine A [6], indoline alkaloid rosibiline [7], and macrocyclic marine alkaloid ‘upenamide [8,9] are shown in Figure 1. Besides, Tegafur [10] containing a hemiaminal ether skeleton is used as an anticancer reagent and indolinooxzolidines [11] are effective in molecular switching.
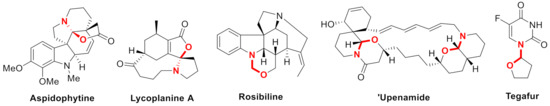
Figure 1.
Selected examples containing N,O-acetal/ketal.
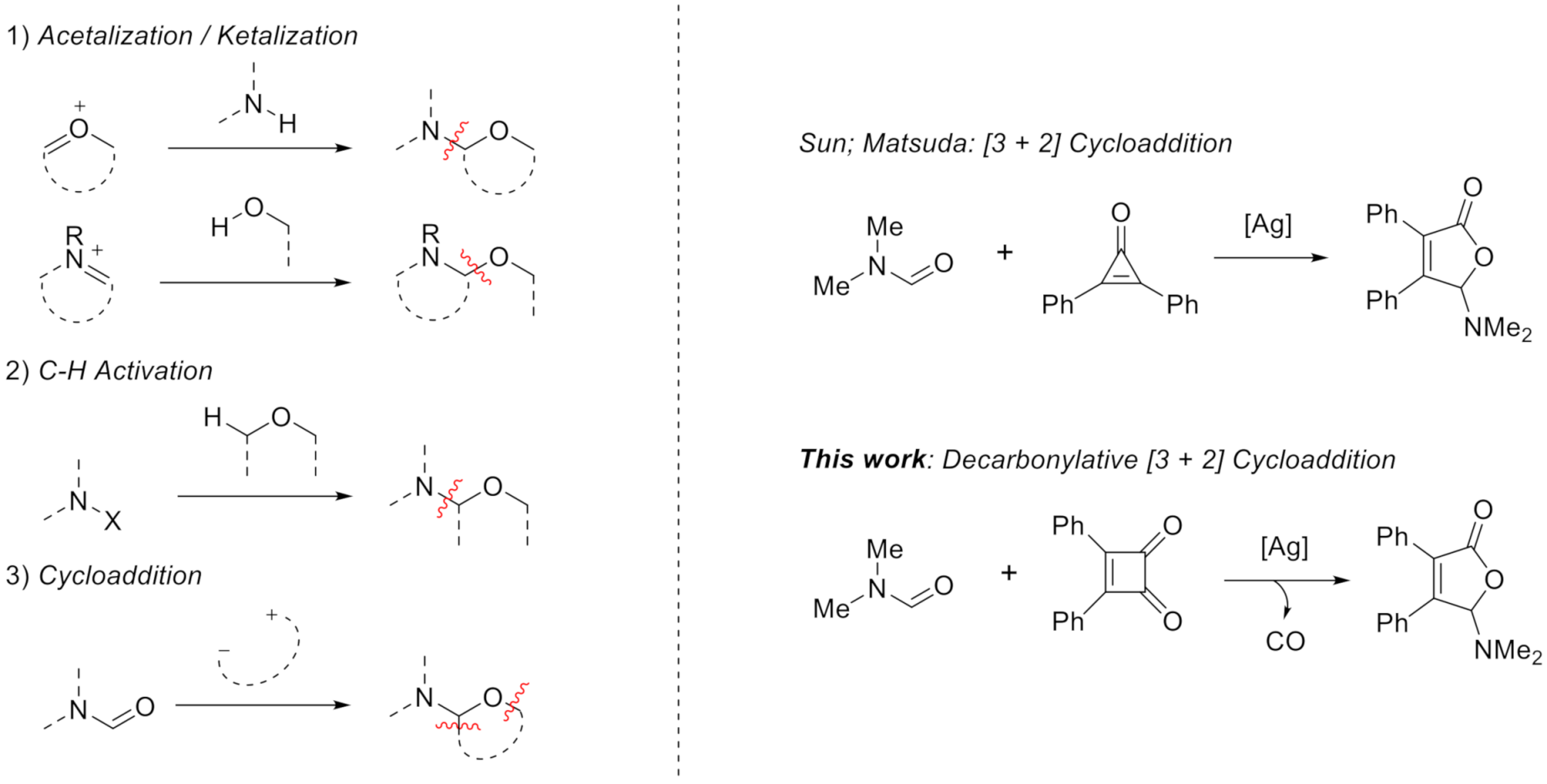
Many efforts [12,13] have been made to achieve the N,O-acetal/ketal moiety including: (1) acetalization/ketalization of oxonium or imine ion (eq. 1, Scheme 1) [14,15], (2) C-H bond activation of ether with various nitrogen reagents (eq. 2) [16,17,18,19,20,21], and (3) cycloaddition of dipole with or derived from amide (eq. 3) [22,23]. In this respect, Sun group reported a type of [3 + 2] cycloaddition to achieve 5-aminofuran-2(5H)-one using cyclopropenone and amide under Ag catalyst [24]. Similar work was reported by Matsuda and co-workers [25].
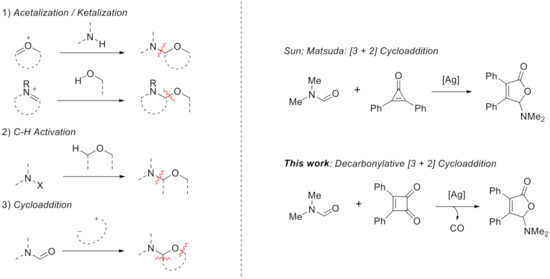
Scheme 1.
Pathways to construct N,O-acetal/ketal.
As part of the transformation of small ring compounds, especially squaric acid in our group, we wish to establish an alternative approach for the construction of N,O-acetal from squaric acid or other four-membered cyclic compounds. Although the transformation of squaric acid and other cyclobutenediones to cyclopropenones under photolysis is known [26,27,28], the investigation of their thermal stability is still lacking. Based on this, we anticipated that the strained ring compound cyclobutenediones [29,30,31] could proceed with a decarbonylation process under metal-catalyst to form cyclopropenone intermediate similar to their photochemical nature, and thus achieve N,O-acetal/ketal skeleton.
2. Results
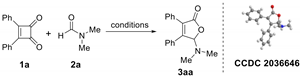
We initiated our studies by probing various reaction conditions for the cycloaddition of diphenylcyclobutenedione [32] (1a) with N,N-dimethylformamide (DMF, 2a), and the results are given in Table 1. Heating the reaction mixture at 170 °C in the absence of catalyst, no reaction occurred (Table 1, entry 1). Considering the wide catalytic applications of transition metals in carbonylation and/or decarbonylation reaction, rhodium [33] and palladium [34] salts were firstly adopted in our decarbonylative [3 + 2] cycloaddition. Fortunately, the reaction occurred under the rhodium catalyst [Cp*RhCl2]2 or Rh(PPh3)3Cl (Entries 2 and 3). Due to the low yield of 3aa under rhodium catalyst, we thus turn our attention to the palladium catalyst such as Pd(OAc)2 or Pd(PPh3)4. It was found that Pd(II) leads to the decomposition of cyclobutenedione, and Pd(0) catalyzes the formation of 3aa (Entries 4 and 5).

Table 1.
Studies on the reaction conditions a.
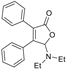
Based on these results, other transition metals without obvious coordination effect with CO were screened. Most of the metals yielded similar results as Cu(OTf)2, and gave no desired product during the reaction (Table 1, entry 6). According to the [3 + 2] cycloaddition of cyclopropenone, various Ag salts were then investigated and proved effective for this transformation. When AgSbF6 was employed as the catalyst, the desired product 3aa was isolated in only 21% yield (Entry 7). Further examination demonstrated that the reaction proceeded most efficiently with AgNTf2 catalyst and the product 3aa was offered in 60% yield (Entries 8–10). It should be mentioned that longer reaction time proved ineffective for this transformation (Entry 11). Moreover, no annulation reaction took place upon lowering the temperature to 160 °C (Entry 12). Other solvents such as chlorobenzene were also investigated for the reaction and the product 3aa was obtained in 52% yield, which was slightly lower than o-dichlorobenzene (Entry 13). Copies of 1H and 13C NMR spectra for 3aa and its crystallographic data are available in the Supplementary Materials.
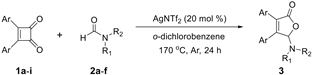
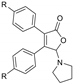
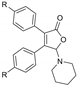
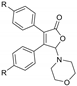
With the optimized [3 + 2] cycloaddition conditions in hand, the scope and generality of the annulation reactions for the formation of products 3 were then investigated (Table 2). We firstly examined the reaction of various formamides (2a–f) with diphenylcyclobutenedione (1a) under standard conditions. The experiment results demonstrated that the steric hinerance of formamides had an obvious effect on the reaction. Compared with the yield of N,N-dimethylformamide (2a), the coupling product (3ab) of N,N-diethylformamide (2b) with 1a was isolated in only 36% yield. In addition, the extension of N-methyl-N-phenylformamide (2c) was unsuccessful, and only a few products (3ac) could be detected. Although the increasingly steric hindrance led to the lower yield, the various cyclic formamides (2d–2f) could be converted smoothly to give the corresponding products (3ad–3af) in moderate yield.

Table 2.
Scope of the [3 + 2] cycloaddition reactions a,b.
Further exploration demonstrated that various aromatic substituted cyclobutenediones were suitable for this reaction (Table 2). As indicated, diarylcyclobutenediones bearing electron-donating groups on the phenyl rings such as 4-methyl, 4-ethyl and 4-methoxy, respectively, provided the corresponding annulation products (3ba, 3ca and 3da) in higher yields, thus broadening the application of current methodology. Meanwhile, this transformation can be extended to the halogen substituted cyclobutenediones, although the isolated yields of products (3ea, 3fa and 3ga) were decreased slightly. Moreover, when the position of the substituent on the phenyl rings changed, the corresponding products (3ha, 3ia) were still isolated in good yields.
To further expand the application of the Ag-catalyzed [3 + 2]-cycloaddition reaction, cross experiments with various substituent groups on formamides or the phenyl rings were screened. Results are given in Table 3; ten new corresponding annulation products were obtained in moderate yield.

Table 3.
Other [3 + 2] cycloaddition products a,b.
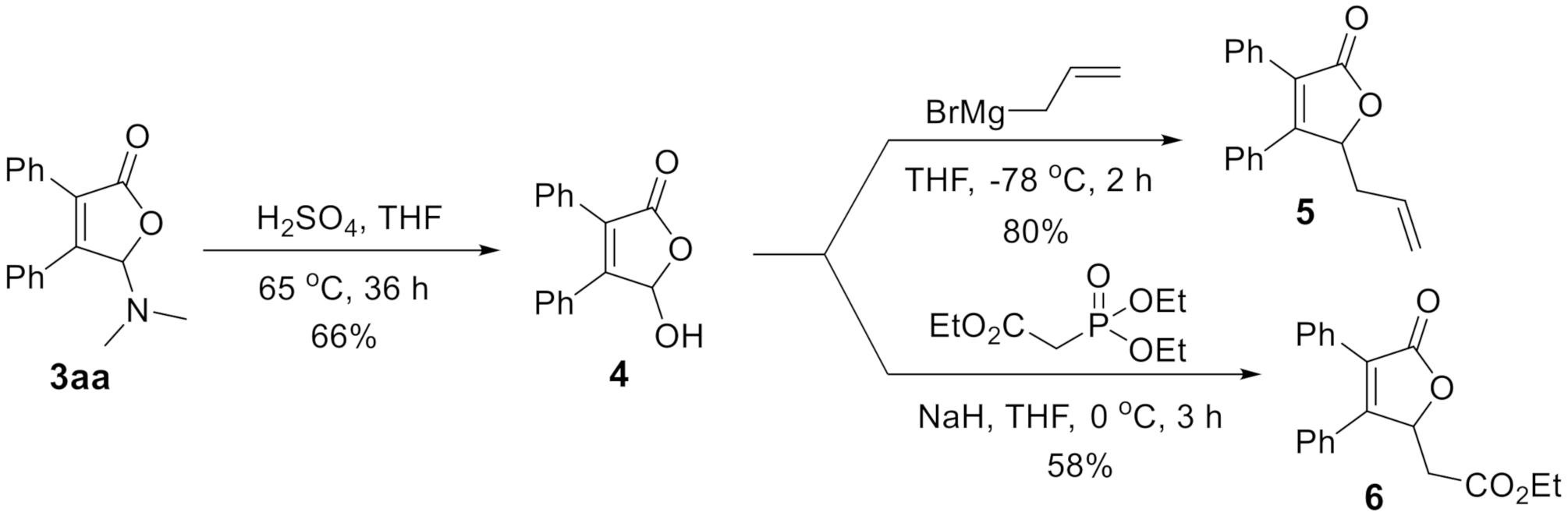
Finally, the derivatization experiments of [3 + 2]-annulation product were investigated. N,O-Acetal was easily converted to hemiacetal under acid condition. Upon heating the product 3aa in THF at 65 °C in the presence of H2SO4, 4 was isolated in 66% yield, which could be further alkylated to 5 or 6 using Grignard reaction or HWE olefination [35], respectively (Scheme 2).
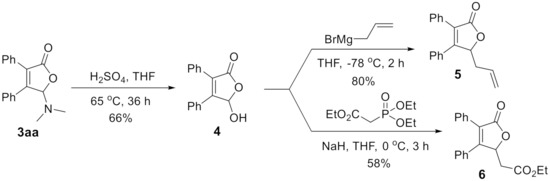
Scheme 2.
Derivatization experiments.
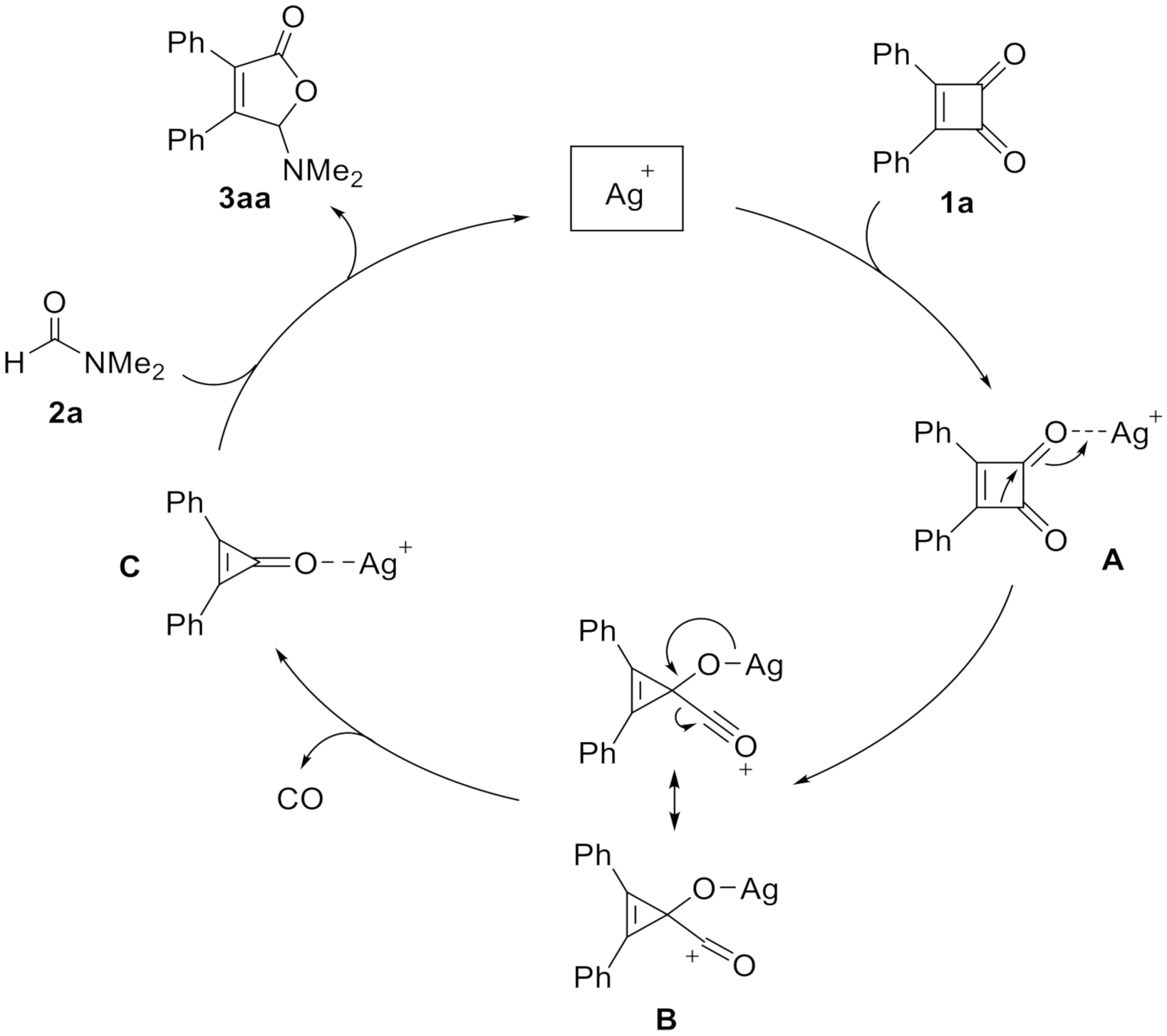
A possible mechanism for the decarbonylative [3 + 2] cycloaddition of cyclobutenedione 1a with formamide 2a is tentatively proposed. As shown in Scheme 3, following the initial chelation of Ag+ with the carbonyl group to generate A, the ring-reducing process is occurred and lead to the formation of intermediate B [36]. After the extrusion of CO, the cyclopropenone intermediate C is formed. The next steps involving ring-opening, nucleophilic addition of formamide, and regeneration of silver catalyst are the same with the literatures [24,25].
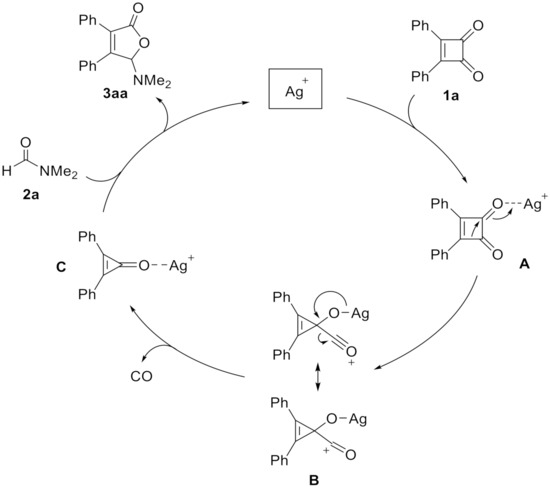
Scheme 3.
Proposed mechanism.
3. Materials and Methods
3.1. General Methods
Unless otherwise stated, all reactions were carried out under argon atmosphere. All commercial available reagents (Energy Chemical, Shanghai, China) were used without further purification. Anhydrous solvents including chlorobenzene and o-dichlorobenzene were commercially available. Tetrahydrofuran (THF) was distilled from sodium. Column chromatography was performed on silica gel (200–300 mesh). 1H NMR spectra (Bruker, Fällanden, Switzerland) were recorded on a 500 MHz NMR spectrometer and 13C NMR spectra were recorded on a 125 MHz NMR spectrometer. HRMS data were recorded on Thermo Scientific LTQ Orbitrap XL. Melting points were uncorrected.
3.2. Experiment Procedures
General procedure for the synthesis of cyclobutenedione. THF (150 mL) was added to a mixture of Fe2(CO)9 (5.457 g, 15.00 mmol) and t-BuOK (2.245 g, 20.00 mmol) at room temperature under argon. The resulting mixture was stirred for 0.5 h at room temperature and another 15 min at 65 °C. Diphenylacetylene (0.892 g, 5.00 mmol) was added and then further stirred for 12 h at 75 °C. The mixture was cooled to room temperature, and CuCl2·2H2O (12.786 g, 75.00 mmol) in acetone (50 mL) was added. After filtration, the filtrate was concentrated under reduced pressure. The residue was purified by flash chromatography (petrol ether/EtOAc = 20:1) to give products. The NMR data are consistent with previous reports in the literature (1a [32,37], 1b, 1d, 1f–1h [37], 1e [38]).
General procedure for [3 + 2] cycloaddition of cyclobutenediones with formamides. A pressure tube was charged with AgNTf2 (0.012 g, 0.03 mmol) and 3,4-diphenylcyclobut-3-ene-1,2-dione 1a (0.035 g, 0.15 mmol), and then was evacuated and backfilled with argon. DMF 2a (0.25 mL, 3.00 mmol) and o-dichlorobenzene (1.5 mL) were added via a syringe. The mixture was heated at 170 °C for 24 h. After cooling to room temperature, the mixture was subjected to flash chromatography (petrol ether/EtOAc = 10:1) to give products. The NMR data are consistent with previous reports in the literature (3aa, 3ab, 3af, 3ba, 3da [24,25], 3ad, 3da [25], 3ae [39], 3ea–3ga [24]). Copies of 1H and 13C NMR spectra for all products and crystallographic data for 3aa are available in the Supplementary Materials.
Procedure for the synthesis of product 4. To a solution of 3aa (0.144 g, 0.51 mmol) in THF (3 mL) was added 0.5 M H2SO4 (3mL). The reaction mixture was heated at 65 °C for 36 h. After cooled to room temperature, the mixture was quenched with saturated NaHCO3 solution. The separated layer was extracted with ethyl acetate twice. The combined organic layers were dried over anhydrous Na2SO4, and then concentrated under reduced pressure. The crude product was purified by flash chromatography (petrol ether/EtOAc = 3:1) to give product 4. The NMR data are consistent with previous reports in the literature. [40]
Procedure for the synthesis of product 5. To a solution of 4 (0.032 g, 0.13 mmol) in THF (3 mL) at −78 °C under argon was added a solution of allylmagnesium bromide in diethyl ether (0.39 mL, 1.0 M, 0.39 mmol) via syringe. The reaction was stirred at room temperature for 1.5 h, and then quenched with saturated NH4Cl solution. The separated aqueous layer was extracted with ethyl acetate twice. The combined organic layers were dried over anhydrous Na2SO4, and then concentrated. The crude product was purified by flash chromatography (petrol ether/EtOAc = 10:1) to give product 5.
Procedure for the synthesis of product 6. To a solution of Triethyl phosphonoacetate (0.088 g, 0.36 mmol) in THF (2 mL) at 0 °C was added NaH (0.015 g, 0.36 mmol). After 30 min, a solution of 4 (0.030 g, 0.12 mmol) in THF (1 mL) was added dropwise. The resulting mixture was stirred for 3 h, and then quenched with saturated NH4Cl solution. The separated aqueous layer was extracted with ethyl acetate twice. The combined organic layers were dried over anhydrous Na2SO4, and then concentrated. The crude product was purified by flash chromatography (petrol ether/EtOAc = 5:1) to give product 6.
3.3. Characterization of the Products
3,4-Diphenylcyclobut-3-ene-1,2-dione (1a). Yellow solid, mp 89.8–91.2 °C; 0.878 g, yield 75%; 1H NMR (500 MHz, CDCl3) δ: 8.10–8.04 (m, 4H), 7.64–7.51(m, 6H); 13C NMR (125 MHz, CDCl3) δ: 196.07, 187.40, 133.35, 129.26, 128.15, 128.08; HRMS (ESI) m/z calcd for C16H10O2Na+ [M+Na]+: 257.05730, found: 257.05728.
3,4-Di-p-tolylcyclobut-3-ene-1,2-dione (1b). Yellow solid, mp 169.4–172.3 °C; 0.630 g, yield 48%; 1H NMR (500 MHz, CDCl3) δ: 7.99 (d, J = 8.0 Hz, 4H), 7.35 (d, J = 8.0 Hz, 4H), 2.46 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 196.37, 186.54, 144.39, 129.97, 128.25, 125.64, 21.97; HRMS (ESI) m/z calcd for C18H14O2Na+ [M+Na]+: 285.08860, found: 285.08859.
3,4-Bis(4-ethylphenyl)cyclobut-3-ene-1,2-dione (1c). Yellow solid, mp 93.2–95.6 °C; 0.653 g, yield 45%; 1H NMR (500 MHz, CDCl3) δ: 8.03 (d, J = 8.0 Hz, 4H), 7.37 (d, J = 8.0 Hz, 4H), 2.75 (q, J = 7.5 Hz, 4H), 1.30 (t, J = 7.5 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 196.40, 186.54, 150.55, 128.78, 128.36, 125.83, 29.20, 15.08; HRMS (ESI) m/z calcd for C20H18O2Na+ [M+Na]+: 313.11990, found: 313.11984.
3,4-Bis(4-methoxyphenyl)cyclobut-3-ene-1,2-dione (1d). Yellow solid, mp 173.6–176.4 °C; 0.912 g, yield 62%; 1H NMR (500 MHz, CDCl3) δ: 8.11 (d, J = 8.8 Hz, 4H), 7.04 (d, J = 8.8 Hz, 4H), 3.91 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 196.27, 184.38, 163.47, 130.40, 121.23, 114.71, 55.58; HRMS (ESI) m/z calcd for C18H14O4Na+ [M+Na]+: 317.07843, found: 317.07846.
3,4-Bis(4-fluorophenyl)cyclobut-3-ene-1,2-dione (1e). Yellow solid, mp 172.4–174.0 °C; 0.851 g, yield 63%; 1H NMR (500 MHz, CDCl3) δ: 8.15–8.07 (m, 4H), 7.30–7.23 (m, 4H); 13C NMR (125 MHz, CDCl3) δ: 195.56, 185.52, 165.53 (d, J = 256.2 Hz), 130.75 (d, J = 8.7 Hz), 124.40 (d, J = 3.8 Hz), 116.93 (d, J = 22.5 Hz); HRMS (ESI) m/z calcd for C16H8F2O2Na+ [M+Na]+: 293.03846, found: 293.03851.
3,4-Bis(4-chlorophenyl)cyclobut-3-ene-1,2-dione (1f). Yellow solid, mp 138.2–141.3 °C; 0.712 g, yield 47%; 1H NMR (500 MHz, CDCl3) δ: 8.00 (d, J = 8.0 Hz, 4H), 7.55 (d, J = 8.0 Hz, 4H); 13C NMR (125 MHz, CDCl3) δ: 195.32, 185.91, 139.92, 129.90, 129.42, 126.28; HRMS (ESI) m/z calcd for C16H8Cl2O2Na+ [M+Na]+: 324.97936, found: 324.97946.
3,4-Bis(4-bromophenyl)cyclobut-3-ene-1,2-dione (1g). Yellow solid, mp 163.1–165.3 °C; 1.000 g, yield 51%; 1H NMR (500 MHz, CDCl3) δ: 7.91 (d, J = 8.5 Hz, 4H), 7.71 (d, J = 8.5 Hz, 4H); 13C NMR (125 MHz, CDCl3) δ: 195.24, 186.08, 132.89, 129.44, 128.59, 126.68; HRMS (ESI) m/z calcd for C16H8Br2O2Na+ [M+Na]+: 412.87833, found: 412.87857.
3,4-Di-m-tolylcyclobut-3-ene-1,2-dione (1h). Yellow solid, mp 113.4–115.7 °C; 0.708 g, yield 54%; 1H NMR (500 MHz, CDCl3) δ: 7.92 (s, 2H), 7.85 (d, J = 6.8 Hz, 2H), 7.46–7.39 (m, 4H), 2.43 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 196.34, 187.65, 139.21, 134.16, 129.12, 128.76, 128.17, 125.24, 21.34; HRMS (ESI) m/z calcd for C18H14O2Na+ [M+Na]+: 285.08860, found: 285.08878.
3,4-Bis(3-methoxyphenyl)cyclobut-3-ene-1,2-dione (1i). Yellow solid, mp 118.0–119.5 °C; 0.706 g, yield 48%; 1H NMR (500 MHz, CDCl3) δ: 7.66 (d, J = 7.5 Hz, 2H), 7.63–7.59 (m, 2H), 7.45 (t, J = 8.0 Hz, 2H), 7.17–7.11 (m, 2H), 3.84 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 196.03, 187.45, 159.94, 130.32, 129.15, 120.69, 119.70, 112.79, 55.48; HRMS (ESI) m/z calcd for C18H14O4Na+ [M+Na]+: 317.07843, found: 317.07849.
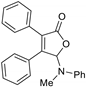
5-Dimethylamino-3,4-diphenylfuran-2(5H)-one (3aa). Yellow solid, mp 145.1–146.9 °C; 0.025 g, yield 60%; 1H NMR (500 MHz, CDCl3) δ: 7.43–7.36 (m, 4H), 7.34–7.24 (m, 6H), 6.04 (s, 1H), 2.43 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.09, 154.09, 130.76, 129.91, 129.85, 129.40, 129.22, 128.61, 128.50, 128.34, 128.32, 97.81, 38.57; HRMS (ESI) m/z calcd for C18H17NO2Na+ [M+Na]+: 302.11515, found: 302.11511.
5-Diethylamino-3,4-diphenylfuran-2(5H)-one (3ab). Yellow solid, mp 117.4–118.7 °C; 0.017 g, yield 36%; 1H NMR (500 MHz, CDCl3) δ: 7.48–7.25 (m, 10H), 6.25 (s, 1H), 2.88–2.74 (m, 4H), 1.02 (t, J = 7.2 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.49, 154.42, 131.17, 130.29, 129.86, 129.82, 129.46, 128.81, 128.74, 128.52, 128.34, 96.88, 42.35, 13.26; HRMS (ESI) m/z calcd for C20H21NO2Na+ [M+Na]+: 330.14645, found: 330.14633.
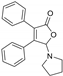
5-(Pyrrolidin-1-yl)-3,4-diphenylfuran-2(5H)-one (3ad). Yellow solid, mp 142.9–144.5 °C; 0.024 g, yield 52%; 1H NMR (500 MHz, CDCl3) δ: 7.44–7.26 (m, 10H), 6.29 (s, 1H), 2.96–2.82 (m, 4H), 1.81–1.73 (m, 4H); 13C NMR (125 MHz, CDCl3) δ: 171.72, 154.87, 131.16, 130.25, 129.93, 129.44, 129.11, 128.73, 128.64, 128.50, 128.44, 94.59, 46.32, 24.48; HRMS (ESI) m/z calcd for C20H19NO2Na+ [M+Na]+: 328.13080, found: 328.13098.
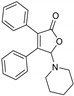
5-(Piperidin-1-yl)-3,4-diphehylfuran-2(5H)-one (3ae). White solid, mp 118.4–119.7 °C; 0.026 g, yield 55%; 1H NMR (500 MHz, CDCl3) δ: 7.48–7.38 (m, 4H), 7.36–7.26 (m, 6H), 5.97 (s, 1H), 2.87–2.74 (m, 4H), 1.60–1.41 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.39, 153.67, 131.06, 130.25, 129.90, 129.62, 129.41, 128.82, 128.73, 128.50, 128.34, 98.50, 48.06, 25.76, 24.05; HRMS (ESI) m/z calcd for C21H21NO2Na+ [M+Na]+: 342.14645, found: 342.14667.
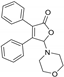
5-Morpholino-3,4-diphenylfuran-2(5H)-one (3af). White solid, mp 166.7–169.3 °C; 0.021 g, yield 43%; 1H NMR (500 MHz, CDCl3) δ: 7.48–7.29 (m, 10H), 5.98 (s, 1H), 3.71–3.55 (m, 4H), 2.90–2.77 (m, 4H); 13C NMR (125 MHz, CDCl3) δ: 170.99, 153.07, 130.79, 130.20, 130.08, 129.96, 129.41, 128.98, 128.72, 128.60, 128.54, 96.96, 66.71, 47.19; HRMS (ESI) m/z calcd for C20H19NO3Na+ [M+Na]+: 344.12571, found: 344.12549.
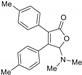
5-Dimethylamino-3,4-di-(4-methylphenyl)furan-2(5H)-one (3ba). Yellow solid, mp 43.8–45.6 °C; 0.027 g, yield 59%; 1H NMR (500 MHz, CDCl3) δ: 7.31 (d, J = 8.0 Hz, 4H), 7.15 (d, J = 8.0 Hz, 2H), 7.11 (d, J = 8.0 Hz, 2H), 6.01 (s, 1H), 2.43 (s, 6H), 2.35 (s, 3H), 2.34 (s, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.65, 153.62, 140.30, 138.65, 129.23, 129.22, 129.20, 128.75, 128.55, 128.18, 127.25, 97.86, 38.73, 21.40, 21.31; HRMS (ESI) m/z calcd for C20H21NO2Na+ [M+Na]+: 330.14645, found: 330.14655.
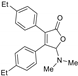
5-Dimethylamino-3,4-di-(4-ethylphenyl)furan-2(5H)-one (3ca). Colorless oil; 0.026 g, yield 52%; 1H NMR (500 MHz, CDCl3) δ: 7.38–7.31 (m, 4H), 7.18 (d, J = 8.0 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 6.01 (s, 1H), 2.69–2.61 (m, 4H), 2.44 (s, 6H), 1.26–1.21 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.74, 153.54, 146.45, 144.89, 129.29, 128.74, 128.64, 128.35, 128.01, 127.99, 127.51, 97.85, 38.74, 28.66, 15.24, 14.92; HRMS (ESI) m/z calcd for C22H25NO2Na+ [M+Na]+: 358.17775, found: 358.17795.
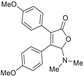
5-Dimethylamino-3,4-di-(4-methoxyphenyl)furan-2(5H)-one (3da). Yellow solid, mp 46.2–48.7 °C; 0.028 g, yield 55%; 1H NMR (500 MHz, CDCl3) δ: 7.42 (d, J = 8.5 Hz, 2H), 7.38 (d, J = 9.0 Hz, 2H), 6.89 (d, J = 8.5 Hz, 2H), 6.82 (d, J = 9.0 Hz, 2H), 5.97 (s, 1H), 3.82 (s, 3H), 3.81 (s, 3H), 2.44 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.97, 160.81, 159.81, 152.52, 130.74, 130.32, 127.21, 123.42, 122.68, 114.04, 113.93, 97.75, 55.21, 38.70; HRMS (ESI) m/z calcd for C20H21NO4Na+ [M+Na]+: 362.13628, found: 362.13632.
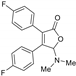
5-Dimethylamino-3,4-di-(4-fluorophenyl)furan-2(5H)-one (3ea). Yellow solid, mp 159.4–161.7 °C; 0.021 g, yield 45%; 1H NMR (500 MHz, CDCl3) δ: 7.45–7.36 (m, 4H), 7.07–6.97 (m, 4H), 5.97 (s, 1H), 2.45 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.90, 163.65 (d, J = 250.0 Hz), 163.11 (d, J = 248.0 Hz), 152.87, 131.43 (d, J = 8.7 Hz), 130.86 (d, J = 7.5 Hz), 128.75, 127.07 (d, J = 3.5 Hz), 126.06 (d, J = 3.7 Hz), 115.92 (d, J = 21.6 Hz), 115.83 (d, J = 21.6 Hz), 97.98, 38.74; HRMS (ESI) m/z calcd for C18H15F2NO2Na+ [M+Na]+: 338.09631, found: 338.09647.
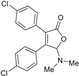
5-Dimethylamino-3,4-di-(4-chlorophenyl)furan-2(5H)-one (3fa). Yellow solid, mp 52.3–53.9 °C; 0.021 g, yield 40%; 1H NMR (500 MHz, CDCl3) δ: 7.37–7.29 (m, 8H), 6.00 (s, 1H), 2.44 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.72, 153.20, 136.49, 135.24, 130.77, 130.00, 129.11, 129.05, 129.03, 128.93, 128.14, 97.98, 38.80; HRMS (ESI) m/z calcd for C18H15Cl2NO2Na+ [M+Na]+: 370.03721, found: 370.03760.
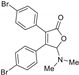
5-Dimethylamino-3,4-di-(4-bromophenyl)furan-2(5H)-one (3ga). Yellow solid, mp 54.6–56.3 °C; 0.023 g, yield 34%; 1H NMR (500 MHz, CDCl3) δ: 7.52–7.45 (m, 4H), 7.31–7.25 (m, 4H), 5.99 (s, 1H), 2.44 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.61, 153.28, 132.09, 131.99, 130.99, 130.16, 129.49, 129.04, 128.58, 124.93, 123.57, 97.94, 38.82; HRMS (ESI) m/z calcd for C18H15Br2NO2Na+ [M+Na]+: 457.93618, found: 457.93671.
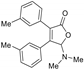
5-Dimethylamino-3,4-di-(3-methylphenyl)furan-2(5H)-one (3ha). Yellow solid, mp 84.9–86.1 °C; 0.025 g, yield 54%; 1H NMR (500 MHz, CDCl3) δ: 7.23–7.10 (m, 8H), 6.03 (s, 1H), 2.45 (s, 6H), 2.32 (s, 3H), 2.28 (s, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.51, 154.34, 138.11, 130.94, 130.80, 130.00, 129.87, 129.62, 129.53, 129.08, 128.35, 128.28, 126.42, 125.83, 97.94, 38.78, 21.37, 21.34; HRMS (ESI) m/z calcd for C20H21NO2Na+ [M+Na]+: 330.14645, found: 330.14642.
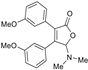
5-Dimethylamino-3,4-di-(3-methoxyphenyl)furan-2(5H)-one (3ia). Yellow solid, mp 113.5–115.2 °C; 0.026 g, yield 51%; 1H NMR (500 MHz, CDCl3) δ: 7.29–7.20 (m, 2H), 7.02–6.93 (m, 4H), 6.93–6.87 (m, 2H), 6.02 (s, 1H), 3.74 (s, 3H), 3.65 (s, 3H), 2.46 (s, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.21, 159.61, 159.38, 154.16, 132.08, 131.36, 129.83, 129.63, 121.84, 121.18, 115.84, 114.91, 114.65, 114.12, 98.01, 55.24, 55.08, 38.86; HRMS (ESI) m/z calcd for C20H21NO4Na+ [M+Na]+: 362.13628, found: 362.13654.
5-(Pyrrolidin-1-yl)-3,4-di-(4-methylphenyl)furan-2(5H)-one (3bd). Yellow solid, mp 38.5–40.2 °C; 0.023 g, yield 45%; 1H NMR (500 MHz, CDCl3) δ: 7.33 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 7.15 (d, J = 7.5 Hz, 2H), 7.09 (d, J = 8.0 Hz, 2H), 6.24 (s, 1H), 2.96–2.80 (m, 4H), 2.35 (s, 3H), 2.34 (s, 3H), 1.80–1.73 (m, 4H); 13C NMR (125 MHz, CDCl3) δ: 172.08, 154.29, 140.18, 138.58, 129.29, 129.23, 129.15, 128.55, 128.42, 128.26, 127.51, 94.48, 46.29, 24.49, 21.43, 21.35; HRMS (ESI) m/z calcd for C22H23NO2Na+ [M+Na]+: 356.16210, found: 356.16229.
5-(Piperidin-1-yl)-3,4-di-(4-methylphenyl)furan-2(5H)-one (3be). White solid, mp 56.7–58.9 °C; 0.028 g, yield 54%; 1H NMR (500 MHz, CDCl3) δ: 7.38 (d, J = 8.5 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 7.15 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 8.5 Hz, 2H), 5.93 (s, 1H), 2.87–2.71 (m, 4H), 2.35 (s, 3H), 2.34 (s, 3H), 1.58–1.42 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.74, 153.03, 140.13, 138.55, 129.23, 129.20, 129.02, 128.73, 128.26, 127.52, 98.38, 47.99, 25.78, 24.07, 21.39, 21.31; HRMS (ESI) m/z calcd for C23H25NO2Na+ [M+Na]+: 370.17775, found: 370.17786.
5-Morpholino-3,4-di-(4-methylphenyl)furan-2(5H)-one (3bf). White solid, mp 78.9–81.4 °C; 0.026 g, yield 49%; 1H NMR (500 MHz, CDCl3) δ: 7.35 (d, J = 8.5 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 8.5 Hz, 2H), 7.11 (d, J = 8.0 Hz, 2H), 5.95 (s, 1H), 3.68–3.56 (m, 4H), 2.87–2.77 (m, 4H), 2.36 (s, 3H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.37, 152.44, 140.50, 138.84, 129.28, 129.20, 129.19, 129.09, 128.61, 127.92, 127.17, 96.83, 66.71, 47.08, 21.43, 21.34; HRMS (ESI) m/z calcd for C22H23NO3Na+ [M+Na]+: 372.15701, found: 372.15720.
5-(Piperidin-1-yl)-3,4-di-(4-ethylphenyl)furan-2(5H)-one (3ce). Yellow oil; 0.035 g, yield 62%; 1H NMR (500 MHz, CDCl3) δ: 7.44 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 7.11 (d, J = 8.5 Hz, 2H), 5.92 (s, 1H), 2.88–2.72 (m, 4H), 2.69–2.60 (m, 4H), 1.59–1.42 (m, 6H), 1.28–1.20 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.84, 152.92, 146.35, 144.81, 129.32, 128.84, 128.75, 128.48, 128.02, 127.78, 98.40, 48.01, 28.66, 25.81, 24.10, 15.25, 14.97; HRMS (ESI) m/z calcd for C25H29NO2Na+ [M+Na]+: 398.20905, found: 398.20908.
5-Morpholino-3,4-di-(4-ethylphenyl)furan-2(5H)-one (3cf). White solid; mp 51.4–53.7 °C; 0.030 g, yield 52%; 1H NMR (500 MHz, CDCl3) δ: 7.41 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 8.5 Hz, 2H), 7.19 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 8.5 Hz, 2H), 5.95 (s, 1H), 3.71–3.57 (m, 4H), 2.89–2.78 (m, 4H), 2.70–2.61 (m, 4H), 1.26–1.22 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.41, 152.32, 146.68, 145.06, 129.25, 129.07, 128.69, 128.08, 127.94, 127.39, 96.76, 66.67, 47.07, 28.66, 15.21, 14.93; HRMS (ESI) m/z calcd for C24H27NO3Na+ [M+Na]+: 400.18831, found: 400.18829.
5-(Pyrrolidin-1-yl)-3,4-di-(4-methoxyphenyl)furan-2(5H)-one (3dd). Yellow solid, mp 46.7–48.8 °C; 0.024 g, yield 43%; 1H NMR (500 MHz, CDCl3) δ: 7.44 (d, J = 9.0 Hz, 2H), 7.37 (d, J = 8.5 Hz, 2H), 6.89 (d, J = 9.0 Hz, 2H), 6.81 (d, J = 9.0 Hz, 2H), 6.21 (s, 1H), 3.82 (s, 3H), 3.81 (s, 3H), 2.95–2.81 (m, 4H), 1.82–1.73 (m, 4H); 13C NMR (125 MHz, CDCl3) δ: 172.35, 160.76, 159.77, 153.22, 130.79, 130.28, 126.69, 123.67, 122.92, 114.05, 113.84, 94.30, 55.22, 46.23, 24.47; HRMS (ESI) m/z calcd for C22H23NO4Na+ [M+Na]+: 388.15193, found: 388.15204.
5-(Piperidin-1-yl)-3,4-di-(4-methoxyphenyl)furan-2(5H)-one (3de). Yellow solid, mp 54.6–56.8 °C; 0.026 g, yield 46%; 1H NMR (500 MHz, CDCl3) δ: 7.54 (d, J = 9.0 Hz, 2H), 7.41 (d, J = 9.0 Hz, 2H), 6.93 (d, J = 9.0 Hz, 2H), 6.86 (d, J = 9.0 Hz, 2H), 5.94 (s, 1H), 3.82 (s, 3H), 3.80 (s, 3H), 2.90–2.76 (m, 4H), 1.64–1.46 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 172.01, 160.72, 159.76, 151.96, 130.76, 130.50, 127.20, 123.59, 122.95, 114.04, 113.72, 98.25, 55.20, 47.97, 25.85, 24.11; HRMS (ESI) m/z calcd for C23H25NO4Na+ [M+Na]+: 402.16758, found: 402.16757.
5-Morpholino-3,4-di-(4-methoxyphenyl)furan-2(5H)-one (3df). White solid, mp 65.4–67.2 °C; 0.032 g, yield 55%; 1H NMR (500 MHz, CDCl3) δ: 7.47 (d, J = 9.0 Hz, 2H), 7.37 (d, J = 9.0 Hz, 2H), 6.90 (d, J = 9.0 Hz, 2H), 6.83 (d, J = 9.0 Hz, 2H), 5.91 (s, 1H), 3.83 (s, 3H), 3.82 (s, 3H), 3.70–3.58 (m, 4H), 2.87–2.77 (m, 4H); 13C NMR (125 MHz, CDCl3) δ: 171.64, 160.92, 159.92, 151.33, 130.74, 130.38, 127.52, 123.21, 122.57, 114.11, 113.89, 96.70, 66.76, 55.25, 55.23, 47.07; HRMS (ESI) m/z calcd for C22H23NO5Na+ [M+Na]+: 404.14684, found: 404.14703.
5-(Piperidin-1-yl)-3,4-di-(4-fluorophenyl)furan-2(5H)-one (3ee). White solid, mp 165.6–167.9 °C; 0.019 g, yield 36%; 1H NMR (500 MHz, CDCl3) δ: 7.51–7.43 (m, 2H), 7.42–7.36 (m, 2H), 7.09–6.98 (m, 4H), 5.93 (s, 1H), 2.85–2.73 (m, 4H), 1.59–1.44 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.14, 163.47 (d, J = 250.7 Hz), 162.94 (d, J = 248.0 Hz), 152.46, 131.38 (d, J = 8.7 Hz), 130.97 (d, J = 8.7 Hz), 129.33 (d, J = 8.7 Hz), 128.48, 126.97 (d, J = 3.7 Hz), 126.04 (d, J = 3.7 Hz), 116.24 (d, J = 22.5 Hz), 115.82 (d, J = 21.2 Hz), 115.73 (d, J = 21.2 Hz), 98.42, 48.07, 25.77, 24.00; HRMS (ESI) m/z calcd for C21H19F2NO2Na+ [M+Na]+: 378.12761, found: 378.12781.
5-Morpholino-3,4-di-(3-methylphenyl)furan-2(5H)-one (3hf). Colorless oil; 0.022 g, yield 42%; 1H NMR (500 MHz, CDCl3) δ: 7.27–7.11 (m, 8H), 5.95 (s, 1H), 3.69–3.57 (m, 4H), 2.88–2.79 (m, 4H), 2.32 (s, 3H), 2.28 (s, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.26, 153.15, 138.29, 138.12, 130.99, 130.79, 130.09, 130.00, 129.92, 129.76, 129.27, 128.45, 128.39, 126.47, 125.94, 96.99, 66.81, 47.21, 21.44, 21.42; HRMS (ESI) m/z calcd for C22H23NO3Na+ [M+Na]+: 372.15701, found: 372.15707.
5-Hydroxy-3,4-diphenylfuran-2(5H)-one (4). White solid, mp 146.2–147.8 °C; 0.084 g, yield 66%; 1H NMR (500 MHz, CD3OD) δ: 7.45–7.43 (m, 2H), 7.38–7.35 (m, 6H), 7.33–7.28 (m, 2H), 6.55 (s, 1H); 13C NMR (125 MHz, CD3OD) δ: 172.83, 157.84, 132.01, 131.39, 131.25, 130.45, 129.95, 129.93, 129.61, 128.97, 99.00; HRMS (ESI) m/z calcd for C16H12O3Na+ [M+Na]+: 275.06787, found: 275.06790.
5-Allyl-3,4-diphenylfuran-2(5H)-one (5). White solid, mp 101.3–102.7 °C; 0.028 g, yield 80%; 1H NMR (500 MHz, CDCl3) δ: 7.40–7.31 (m, 8H), 7.25–7.21 (m, 2H), 5.77–5.66 (m, 1H), 5.54–5.49 (m, 1H), 5.13–5.09 (m, 1H), 5.06–5.00 (m, 1H), 2.72–2.64 (m, 1H), 2.34–2.25 (m, 1H); 13C NMR (125 MHz, CDCl3) δ: 172.26, 159.58, 131.16, 130.63, 130.06, 129.75, 129.25, 128.99, 128.64, 128.45, 128.08, 127.25, 119.55, 80.62, 36.51; HRMS (ESI) m/z calcd for C19H17O2+ [M+H]+: 277.12231, found: 277.12292.
Ethyl 2-(5-oxo-3,4-diphenyl-2,5-dihydrofuran-2-yl)acetate (6). White solid, mp 77.2–78.6 °C; 0.022 g, yield 58%; 1H NMR (500 MHz, CDCl3) δ: 7.41–7.23 (m, 10H), 5.87 (dd, J = 9.0, 3.5 Hz, 1H), 4.19–4.10 (m, 2H), 2.76 (dd, J = 16.0, 3.5 Hz, 1H), 2.51 (dd, J = 16.0, 9.0 Hz, 1H), 1.24 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.75, 169.21, 158.98, 130.62, 130.28, 129.52, 129.22, 129.14, 128.82, 128.49, 128.07, 127.13, 77.84, 61.23, 38.22, 14.09.; HRMS (ESI) m/z calcd for C20H18O4Na+ [M+Na]+: 345.10973, found: 345.10986.
4. Conclusions
We have successfully presented a silver catalyzed ring-opening [3 + 2] cycloaddition of cyclobutenediones with formamides to prepare γ-aminobutenolide, in which cyclobutenediones likely proceed with a key decarbonylative process. In addition, harsh reaction conditions showed the thermochemical stability of cyclobutenedione and made us pay more attention to its mechanism. Further studies including DFT calculations about the decarbonylative process and the applications to address complex synthetic issues are in progress.
Supplementary Materials
Copies of 1H and 13C spectra for all products and crystallographic data for 3aa.
Author Contributions
P.W. and R.Y. performed the experiments and analyzed the data. S.A. participated in the chemical synthesis. Z.L. wrote the manuscript and corrected it. Z.W. and J.-M.G. designed the research and supervised part of the synthesis. H.Z. supervised and coordinated all studies and corrected the manuscript. All authors have read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21772156, 21502151, 21702168, 21702167), the Fundamental Research Funds for the Central Universities (2452017180), the Natural Science Foundation of Shaanxi Province (S2016YFJQ0080, S2019JCQN1597). And the APC was funded by the National Natural Science Foundation of China (21772156).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Crystallographic data for 3aa (CCDC 2036646) has been deposited in the Cambridge Crystallographic Data Centre. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (21772156 and 21502151 to H.Z., 21702168 to Z.W., and 21702167 to Z.L.), the Fundamental Research Funds for the Central Universities (2452017180 to H.Z.), the Natural Science Foundation of Shaanxi Province (S2016YFJQ0080 to Z.L., S2019JCQN1597 to Z.W.), and a Start-Up Grant from Northwest A&F University (Z111021405 to H.Z., Z109021711 to Z.W.).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Ricci, A. Amino Group Chemistry, From Synthesis to the Life Sciences; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Zhang, P.; Wei, Q.; Yuan, X.; Xu, K. Newly reported alkaloids produced by marine-derived penicillium species (covering 2014–2018). Bioorg. Chem. 2020, 99, 103840. [Google Scholar] [CrossRef] [PubMed]
- Maiti, M.; Kumar, G.S. Biophysical aspects and biological implications of the interaction of benzophenanthridine alkaloids with DNA. Biophys. Rev. 2009, 1, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.F.; Snyder, H.R.; Fischer, R.F. Plant insecticides. II. the alkaloids of Haplophyton cimicidum. J. Am. Chem. Soc. 1952, 74, 1987–1989. [Google Scholar] [CrossRef]
- Rae, I.D.; Rosenberger, M.; Szabo, A.G.; Willis, C.R.; Yates, P.; Zacharias, D.E.; Jeffrey, G.A.; Douglas, B.; Kirkpatrick, J.L.; Weisbach, J.A. Haplophytine. J. Am. Chem. Soc. 1967, 89, 3061–3062. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Nian, Y.; Zhu, Q.-F.; Li, X.-N.; Su, J.; Wu, X.-D.; Yang, J.; Zhao, Q.-S. Lycoplanine A, a C16N Lycopodium alkaloid with a 6/9/5 tricyclic skeleton from Lycopodium complanatum. Org. Lett. 2017, 19, 4668–4671. [Google Scholar] [CrossRef]
- Tits, M.; Angenot, L. 12’-Hydroxystrychnobiline, nouvel alcaloïde bisindolinique du Strychnos variabilis. J. Nat. Prod. 1983, 46, 638–645. [Google Scholar] [CrossRef]
- Jiménez, J.I.; Goetz, G.; Mau, C.M.S.; Yoshida, W.Y.; Scheuer, P.J.; Williamson, R.T.; Kelly, M. ‘Upenamide: An unprecedented macrocyclic alkaloid from the indonesian sponge Echinochalina sp. J. Org. Chem. 2000, 65, 8465–8469. [Google Scholar] [CrossRef]
- Unsworth, W.P.; Gallagher, K.A.; Jean, M.; Schmidt, J.P.; Diorazio, L.J.; Taylor, R.J.K. Direct imine acylation: Synthesis of the proposed structures of ‘upenamide. Org. Lett. 2013, 15, 262–265. [Google Scholar] [CrossRef]
- Engel, D.; Nudelman, A.; Tarasenko, N.; Levovich, I.; Makarovsky, I.; Sochotnikov, S.; Tarasenko, I.; Rephaeli, A. Novel prodrugs of tegafur that display improved anticancer activity and antiangiogenic properties. J. Med. Chem. 2008, 51, 314–323. [Google Scholar] [CrossRef]
- Sazlóki, G.; Alévêque, Q.; Pozzo, J.-L.; Hadji, R.; Levillain, E.; Sanguinet, L. Indolinooxazolidine: A versatile switchable unit. J. Phys. Chem. B 2015, 119, 307–315. [Google Scholar] [CrossRef]
- Sears, J.E.; Boger, D.L. Tandem intramolecular Diels-Alder/1,3-Dipolar cycloaddition cascade of 1,3,4-Oxadiazoles: Initial scope and applications. Acc. Chem. Res. 2016, 49, 241–251. [Google Scholar] [CrossRef]
- Dian, L.; Xing, Q.; Zhang-Negrerie, D.; Du, Y. Direct functionalization of alkyl ethers to construct Hemiaminal Ether Skeletons (HESs). Org. Biomol. Chem. 2018, 16, 4384–4389. [Google Scholar] [CrossRef]
- Dian, L.; Wang, S.; Zhang-Negrerie, D.; Du, Y.; Zhang, K. Organocatalytic amination of alkyl ethers via n-Bu4NI/t-BuOOH-Mediated intermolecular oxidative C(sp3)-N bond formation: Novel synthesis of hemiaminal ethers. Chem. Commun. 2014, 50, 11738–11741. [Google Scholar] [CrossRef]
- Yang, Q.; Choy, P.Y.; Fu, W.C.; Fan, B.; Kwong, F.Y. Copper-catalyzed oxidative C-H amination of tetrahydrofuran with indole/carbazole derivatives. J. Org. Chem. 2015, 80, 11193–11199. [Google Scholar] [CrossRef]
- Viuf, C.; Bols, M. Radical azidonation of benzylic positions with iodonium azide. Angew. Chem. Int. Ed. 2001, 40, 623–625. [Google Scholar] [CrossRef]
- Albone, D.P.; Challenger, S.; Derrick, A.M.; Fillery, S.M.; Irwin, J.L.; Parsons, C.M.; Takada, H.; Taylor, P.C.; Wilson, D.J. Amination of ethers using chloramine-t hydrate and a copper(I) catalyst. Org. Biomol. Chem. 2005, 3, 107–111. [Google Scholar] [CrossRef]
- Fructos, M.R.; Trofimenko, S.; Díaz-Requejo, M.M.; Pérez, P.J. Facile amine formation by intermolecular catalytic amidation of carbon-hydrogen bonds. J. Am. Chem. Soc. 2006, 128, 11784–11791. [Google Scholar] [CrossRef]
- He, L.; Yu, J.; Zhang, J.; Yu, X.-Q. α-Amidation of cyclic ethers catalyzed by simple copper salt and a mild and efficient preparation method for α, ϖ-amino alcohols. Org. Lett. 2007, 9, 2277–2280. [Google Scholar] [CrossRef]
- Ochiai, M.; Yamane, S.; Hoque, M.M.; Saito, M.; Miyamoto, K. Metal-Free α-CH amination of ethers with hypervalent sulfonylimino-λ3-bromane that acts as an active nitrenoid. Chem. Commun. 2012, 48, 5280–5282. [Google Scholar] [CrossRef]
- Campos, J.; Goforth, S.K.; Crabtree, R.H.; Gunnoe, T.B. Metal-free amidation of ether sp3 c-h bonds with sulfonamides using PhI(OAc)2. RSC Adv. 2014, 4, 47951–47957. [Google Scholar] [CrossRef]
- Mazzocchi, P.H.; Somich, C.; Edwards, M.; Morgan, T.; Ammon, H.L. Electron transfer photochemistry of aromatic imides and phenylcyclopropane. Radical anion-radical cation cycloaddition. J. Am. Chem. Soc. 1986, 108, 6828–6829. [Google Scholar] [CrossRef]
- Padwa, A.; Hertzog, D.L. Bimolecular cycloaddition reactions of isomünchnones derived from the rhodium (II) catalyzed cyclization of diazo pyrrolidinones. Tetrahedron 1993, 49, 2589–2600. [Google Scholar] [CrossRef]
- Ren, J.-T.; Wang, J.-X.; Tian, H.; Xu, J.-L.; Hu, H.; Aslam, M.; Sun, M. Ag(Ⅰ)-Catalyzed [3 + 2]-Annulation of cyclopropenones and formamides via C-C bond cleavage. Org. Lett. 2018, 20, 6636–6639. [Google Scholar] [CrossRef]
- Matsuda, T.; Tabata, Y.; Suzuki, H. Silver-catalyzed ring-opening [3 + 2] annulation of cyclopropenones with amides. New J. Chem. 2018, 42, 19178–19182. [Google Scholar] [CrossRef]
- Dehmlow, E.V.; Neuhaus, R.; Schell, H.G. 2-Alkoxy-3-alkylcyclopropenone. Chem. Ber. 1988, 121, 569–571. [Google Scholar] [CrossRef]
- Simon, J.G.G.; Schweig, A. 1H and 13C NMR spectra of benzocyclopropenone in liquid solutions at 193 K. Chem. Phys. Lett. 1993, 201, 377–382. [Google Scholar] [CrossRef]
- Fu, N.; Allen, A.D.; Kobayashi, S.; Tidwell, T.T.; Vukovic, S. Structural effects on interconversion of oxygen-substituted bisketenes and cyclobutenediones. J. Org. Chem. 2008, 73, 1768–1773. [Google Scholar] [CrossRef]
- Piech, K.; Bally, T. The bisketene radical cation and its formation by oxidative ring-opening of cyclobutenedione. J. Org. Chem. 2013, 78, 2908–2913. [Google Scholar] [CrossRef]
- Liu, F.; Liebeskind, L.S. tert-Butyl substituent as a regiodirecting and novel C-H protecting group in cyclobutenedione-based benzannulation chemistry. J. Org. Chem. 1998, 63, 2835–2844. [Google Scholar] [CrossRef]
- Nguyen, T.V. Convenient access to hydroquinone and quinone derivatives from cyclobutenedione units. Aust. J. Chem. 2010, 63, 1309–1310. [Google Scholar] [CrossRef]
- Beesu, M.; Periasamy, M. Reactive iron carbonyl reagents via reaction of metal alkoxides with Fe(CO)5 or Fe2(CO)9: synthesis of cyclobutenediones via double carbonylation of alkynes. J. Org. Chem. 2011, 76, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yu, T.-Y.; Xu, P.-F.; Wei, H. Selective decarbonylation via transition-metal-catalyzed carbon-carbon bond cleavage. Chem. Rev. 2021, 121, 365–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Nishihara, Y. Nickel or palladium-catalyzed decarbonylative transformations of carboxylic acid derivatives. Chem. Asian J. 2020, 15, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Jette, C.I.; Tong, Z.J.; Hadt, R.G.; Stoltz, B.M. Copper-catalyzed enantioselective allylic alkylation with a γ-butyrolactone- derived silyl ketene acetal. Angew. Chem. Int. Ed. 2020, 59, 2033–2038. [Google Scholar] [CrossRef]
- Jayamani, M.; Pillai, C.N. Reactions of benzoin and benzil over alumina: Decarbonylation of α-diketones. J. Catal. 1985, 92, 422–425. [Google Scholar] [CrossRef]
- Aguilar-Aguilar, A.; Liebeskind, L.S.; Peña-Cabrera, E. Pd-Catalyzed, Cu(I)-Mediated coss-couplings of bisarylthiocyclobutenediones with boronic acids and organostannanes. J. Org. Chem. 2007, 72, 8539–8542. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Suzzarini, L. Novel reactive cyclobutenedione in poly (arylene ether) synthesis. Macromolecules 1996, 29, 1073–1075. [Google Scholar] [CrossRef]
- Bouancheau, C.; Rudler, M.; Chelain, E.; Rudler, H.; Vaissermann, J.; Daran, J.-C. Reaction of aminocarbene complexes of chromium with alkynes IV. New transformations of the nitrogen yield complexes derived thereform. J. Organomet. Chem. 1995, 496, 127–135. [Google Scholar] [CrossRef]
- Zhang, J.; Blazecka, P.G.; Belmont, D.; Davidson, J.G. Reinvestigation of mucohalic acid, versatile and useful building blocks for highly functionalized α,β-unsaturated γ-butyrolactones. Org. Lett. 2002, 4, 4559–4561. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).