Structure–Activity Relationship Assessment of Sophorolipid Ester Derivatives against Model Bacteria Strains
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Antibacterial Efficacy and Structure–Activity Relationship of SL Derivatives
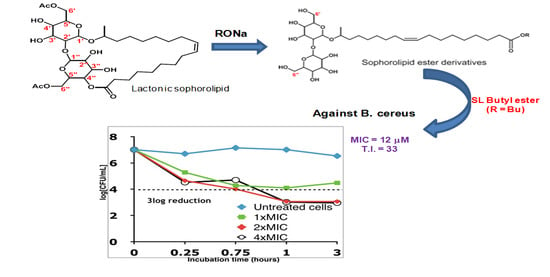
2.2. Time–Kill Studies
2.3. Membrane Depolarization Assay
2.4. In Vitro Cytotoxicity
3. Materials and Methods
3.1. Materials and Reagents
3.2. SL Synthesis
3.3. Bacteria Strain Culture and Antibacterial Testing
3.4. Time–Kill Studies
3.5. Cytotoxicity Study
3.6. Dye Leakage Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Antibiotic Resistance. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 30 July 2020).
- Antibiotic/Antimicrobial Resistance (AR/AMR): Biggest Threats and Data. CDC’s Antibiotic Resistance Threats in The United States (2019 AR Threats Report). Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 19 May 2021).
- European Antibiotic Awareness Day (EAAD). European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/news-events/european-antibiotic-awareness-day-eaad-2020 (accessed on 19 May 2021).
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O’neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Felse, P.A.; Shah, V.; Chan, J.; Rao, K.J.; Gross, R.A. Sophorolipids biosynthesis by candida bombicola from industrial fatty acid residues. Enzyme Microb. Technol. 2007, 40, 316–323. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.; Saerens, K.; De Muynck, C.; Develter, D.; Soetaert, W.; Vandamme, E.J. Microbial production and application of sophorolipids. Appl. Microbiol. Biotechnol. 2007, 76, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gorin, P.A.; Spencer, J.F.T.; Tulloch, A.P. Hydroxy fatty acid glycosides of sophorose from torulopsis magnolia. Can. J. Chem. 1961, 39, 846–855. [Google Scholar] [CrossRef] [Green Version]
- Solaiman, D.K.Y.; Ashby, R.D.; Zerkowski, J.A.; Foglia, T.A. Simplified soy molasses-based medium for reduced-cost production od sophorolipids by Candida bombicola. Biotechnol. Lett. 2007, 29, 1341–1347. [Google Scholar] [CrossRef]
- Zhang, L.; Somasundaran, P.; Singh, S.K.; Felse, A.P.; Gross, R. Synthesis and interfacial properties of sophorolipid derivatives. Colloids Surf. A 2004, 240, 75–82. [Google Scholar] [CrossRef]
- Koh, A.; Linhardt, R.; Gross, R. Effect of Sophorolipid n-alkyl ester chain length on its interfacial properties at the almond oil-water interface. Langmuir 2016, 32, 5562–5572. [Google Scholar] [CrossRef]
- Koh, A.; Wong, A.; Quinteros, A.; Desplat, C.; Gross, R. Influence of sophorolipid structure on interfacial properties of aqueous-arabian light crude and related constituent emulsions. J. Am. Oil Chem. Soc. 2017, 94, 107–119. [Google Scholar] [CrossRef]
- Azim, A.; Shah, V.; Doncel, G.F.; Peterson, N.; Gao, W.; Gross, R. Amino acid conjugated sophorolipids: A new family of biologically active functionalized glycolipids. Bioconjugate Chem. 2006, 17, 1523–1529. [Google Scholar] [CrossRef]
- Shah, V.; Doncel, G.F.; Seyoum, T.; Eaton, K.M.; Zalenskaya, I.; Hagver, R.; Azim, A.; Gross, R. Sophorolipids, microbial glycolipids with anti-human imunodeficiency virus and sperm-immobilizing activities. Antimicrob. Agents Chemother. 2005, 49, 4093–4100. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, I.A.; Faustino, C.; Guerreiro, P.S.; Frade, R.F.; Bronze, M.R.; Castro, M.F.; Ribeiro, M.H. Development of novel sophorolipids with improved cytotoxic activity toward Mda-Mb-231 breast cancer cells. J. Mol. Recognit. 2015, 28, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Song, X.; Ma, X.; Li, H.; Qu, Y. Bioactivities of sophorolipid with different structures against human esophageal cancer cells. J. Surg. Res. 2012, 173, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, P.; Chen, H.; Erndt-Marino, J.D.; Liu, F.; Totsingan, F.; Gross, R.A.; Hahn, M.S. Impact of select sophorolipid derivatives on macrophage polarization and viability. ACS Appl. Bio. Mater. 2019, 2, 601–612. [Google Scholar] [CrossRef]
- Hardin, R.; Pierre, J.; Schulze, R.; Mueller, C.M.; Fu, S.L.; Wallner, S.R.; Stanek, A.; Shah, V.; Gross, R.A.; Weedon, J.; et al. Sophorolipids improve sepsis survival: Effects of dosing and derivatives. J. Surg. Res. 2007, 142, 314–319. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Kosaric, N. Utilization of canola and lactose to produce biosurfactant with Candida Bombicola. J. Am. Oil Chem. Soc. 1995, 72, 67–71. [Google Scholar] [CrossRef]
- Davila, A.M.; Marchal, R.; Vandecasteele, J.P. Kinetics and balance of fermentation free from product inhibition: Sophorose lipid production by Candida Bombicola. Appl. Microbiol. Biotechnol. 1992, 38, 6–11. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.A.; Sabirova, J.; Develter, D.; Soetaert, W.; Vandamme, E.J. Knocking out the MFE-2 Gene of Candida Bombicola leads to improved medium-chain sophorolipid production. FEMS Yeast Res. 2009, 9, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Bisht, K.S.; Gross, R.A.; Kaplan, D.L. Enzyme-mediated regioselective acylations of sophorolipids. J. Org. Chem. 1999, 64, 780–789. [Google Scholar] [CrossRef]
- Koh, A.; Todd, K.; Sherbourne, E.; Gross, R.A. Fundamental characterization of the micellar self-assembly of sophorolipid esters. Langmuir 2017, 33, 5760–5768. [Google Scholar] [CrossRef]
- Singh, S.K.; Felse, A.P.; Nunez, A.; Foglia, T.A.; Gross, R.A. Regioselective enzyme-catalyzed synthesis of sophorolipid esters, amides, and multifunctional monomers. J. Org. Chem. 2003, 68, 5466–5477. [Google Scholar] [CrossRef]
- Peng, Y.; Totsingan, F.; Meier, M.A.; Steinmann, M.; Wurm, F.; Koh, A.; Gross, R.A. Sophorolipids: Expanding structural diversity by ring-opening cross-metathesis. Eur. J. Lipid Sci. Technol. 2015, 117, 217–228. [Google Scholar] [CrossRef]
- Le Moual, H.; Gruenheid, S. Resistance to antimicrobial peptides in gram-negative bacteria. FEMS Microbiol. Lett. 2012, 330, 81–89. [Google Scholar]
- Kim, K.; Yoo, D.; Kim, Y.; Lee, B.; Shin, D.; Kim, E.K. Characteristics of sophorolipid as an antimicrobial agent. J. Microbiol. Biotechnol. 2002, 12, 235–241. [Google Scholar]
- Lang, S.; Katsiwela, E.; Wagner, F. Antimicrobial effect of biosurfactants. Eur. J. Lipid Sci. Technol. 1989, 91, 363–366. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, J.; Hu, X.; Li, Z.; Fa, K.; Liu, H.; Waigh, T.A.; McBain, A.; Lu, J.R. Hydrophobic control of the bioactivity and cytotoxicity of de novo-designed antimicrobial peptides. ACS Appl. Mater. Interfaces 2019, 11, 34609–34620. [Google Scholar] [CrossRef]
- Stone, T.A.; Cole, G.B.; Ravamehr-Lake, D.; Nguyen, H.Q.; Khan, F.; Sharpe, S.; Deber, C.M. Positive charge patterning and hydrophobicity of membrane-active antimicrobial peptides as determinants of activity, toxicity, and pharmacokinetic stability. J. Med. Chem. 2019, 62, 6276–6286. [Google Scholar] [CrossRef]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal amphotericin B (AmBisome®): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef] [Green Version]



| SLs | MIC (µM) | TI | ||||
|---|---|---|---|---|---|---|
| B. cereus1 | B. subtilis1 | S. aureus1 | L. innocua2 | E. coli1 | ||
| Lactonic SL 3 | 25 | 12 | 25 | 50 | >200 | 2 |
| SL-methyl ester | 50 | 50 | 200 | 100 | >200 | >8 |
| SL-ethyl ester | 50 | 50 | 200 | 50 | >200 | 6 |
| SL-propyl ester | 25 | 25 | 100 | 25 | >200 | 16 |
| SL-butyl ester | 12 | 12 | 100 | 12 | >200 | 33 |
| SL-pentyl ester | 12 | >200 | >200 | 12 | >200 | 17 |
| SL-hexyl ester | >200 | >200 | >200 | >200 | >200 | - |
| SL-octyl ester | >200 | >200 | >200 | >200 | >200 | - |
| Streptomycin | 12 | 12 | 3 | 24 | 12 | - |
| SLs | MIC (µM) | |||
|---|---|---|---|---|
| B. Cereus1 | B. Subtilis1 | L. innocua2 | E. coli1 | |
| ESL-(6′OH, 6″OH) 3 | 50 | 50 | 50 | >200 |
| ESL-(6′Ac, 6″OH) | 25 | 25 | 25 | >200 |
| ESL-(6′OH, 6″Ac) | 25 | 25 | 25 | >200 |
| ESL-(6′Ac, 6″Ac) | 12 | >200 | >200 | >200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totsingan, F.; Liu, F.; Gross, R.A. Structure–Activity Relationship Assessment of Sophorolipid Ester Derivatives against Model Bacteria Strains. Molecules 2021, 26, 3021. https://doi.org/10.3390/molecules26103021
Totsingan F, Liu F, Gross RA. Structure–Activity Relationship Assessment of Sophorolipid Ester Derivatives against Model Bacteria Strains. Molecules. 2021; 26(10):3021. https://doi.org/10.3390/molecules26103021
Chicago/Turabian StyleTotsingan, Filbert, Fei Liu, and Richard A. Gross. 2021. "Structure–Activity Relationship Assessment of Sophorolipid Ester Derivatives against Model Bacteria Strains" Molecules 26, no. 10: 3021. https://doi.org/10.3390/molecules26103021