Nanoformulation Development to Improve the Biopharmaceutical Properties of Fisetin Using Design of Experiment Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination and Analytical Method Validation of FST
2.2. Formulation Optimization of FST–NP by CCD
2.2.1. The Effect on Particle Size (Y1)
2.2.2. The Effect on Encapsulation Efficiency (Y2)
2.2.3. The Effect on Zeta Potential (Y3)
2.2.4. The Effect on PDI (Y4)
2.3. Characterization of FST–NP
2.3.1. Morphological Study of FST–NP
2.3.2. Differential Scanning Calorimetry
2.3.3. Powder X-ray Diffraction
2.3.4. Fourier Transform–Infrared Spectroscopy
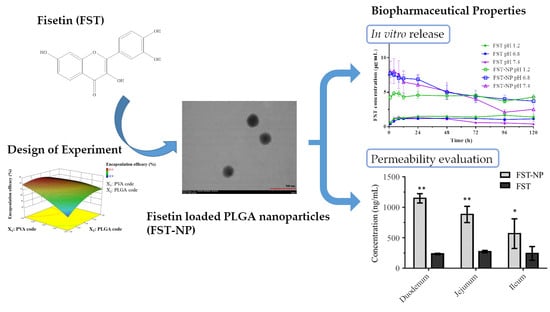
2.4. In Vitro Release
2.5. Permeability Evaluation of FST–NP
2.6. Storage and pH Stabilities of FST–NP
3. Materials and Methods
3.1. Materials
3.2. HPLC–DAD Analysis of Fisetin
3.3. HPLC–Electrochemical Analysis of Fisetin
3.4. Preparation of FST–NP
3.5. Formulation Optimization and Characterization of FST–NP
3.6. Characterization of FST–NP
3.6.1. Particle Size, Polydispersity Index, and Zeta Potential
3.6.2. Encapsulation Efficiency (EE)
3.7. Morphological Study of FST–NP
3.7.1. Differential Scanning Calorimetry
3.7.2. Powder X-ray Diffraction
3.7.3. Fourier Transform–Infrared Spectroscopy
3.8. In Vitro Release of FST–NP
3.9. Permeability Evaluation of FST–NP
3.10. Storage and pH Stabilities of FST–NP
3.11. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kimira, M.; Arai, Y.; Shimoi, K.; Watanabe, S. Japanese Intake of Flavonoids and Isoflavonoids from Foods. J. Epidemiol. 1998, 8, 168–175. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018, 194, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-S.; Li, C.-B.; Li, X.-Y.; Wu, J.; Li, Y.; Fu, X.; Zhang, Y.; Hu, W.-Z. Fisetin Attenuates Metabolic Dysfunction in Mice Challenged with a High-Fructose Diet. J. Agric. Food Chem. 2018, 66, 8291–8298. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Demchuk, O.M. New perspectives for fisetin. Front. Chem. 2019, 7, 697. [Google Scholar] [CrossRef] [PubMed]
- Krasieva, T.B.; Ehren, J.; O’Sullivan, T.; Tromberg, B.J.; Maher, P. Cell and brain tissue imaging of the flavonoid fisetin using label-free two-photon microscopy. Neurochem. Int. 2015, 89, 243–248. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Ding, J.; Li, Q.; He, S.; Xie, J.; Liang, X.; Wu, T.; Li, D. Luteolin-loading of Her-2-poly (lactic-co-glycolic acid) nanoparticles and proliferative inhibition of gastric cancer cells via targeted regulation of forkhead box protein O1. J. Cancer Res. Ther. 2020, 16, 263. [Google Scholar] [CrossRef]
- Zheng, L.T.; Ock, J.; Kwon, B.-M.; Suk, K. Suppressive effects of flavonoid fisetin on lipopolysaccharide-induced microglial activation and neurotoxicity. Int. Immunopharmacol. 2008, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Dargusch, R.; Bodai, L.; Gerard, P.E.; Purcell, J.M.; Marsh, J.L. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington′s disease. Hum. Mol. Genet. 2011, 20, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?term=fisetin&Search=Search (accessed on 12 May 2021).
- Guzzo, M.R.; Uemi, M.; Donate, P.M.; Nikolaou, S.; Machado, A.E.H.; Okano, L.T. Study of the Complexation of Fisetin with Cyclodextrins. J. Phys. Chem. A. 2006, 110, 10545–10551. [Google Scholar] [CrossRef] [PubMed]
- Mignet, N.; Seguin, J.; Ramos Romano, M.; Brullé, L.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a liposomal formulation of the natural flavonoid fisetin. Int. J. Pharm. 2012, 423, 69–76. [Google Scholar] [CrossRef]
- Shia, C.-S.; Tsai, S.-Y.; Kuo, S.-C.; Hou, Y.-C.; Chao, P.-D.L. Metabolism and Pharmacokinetics of 3,3′,4′,7-Tetrahydroxyflavone (Fisetin), 5-Hydroxyflavone, and 7-Hydroxyflavone and Antihemolysis Effects of Fisetin and Its Serum Metabolites. J. Agric. Food Chem. 2009, 57, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-C.; Hsueh, T.Y.; Cheng, Y.-Y.; Lin, L.-C.; Tsai, T.-H. Pharmacokinetics and Biliary Excretion of Fisetin in Rats. J. Agric. Food Chem. 2018, 66, 6300–6307. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int. J. Pharm. 2012, 427, 452–459. [Google Scholar] [CrossRef]
- Kulbacka, J.; Pucek, A.; Kotulska, M.; Dubińska-Magiera, M.; Rossowska, J.; Rols, M.-P.; Wilk, K.A. Electroporation and lipid nanoparticles with cyanine IR-780 and flavonoids as efficient vectors to enhanced drug delivery in colon cancer. Bioelectrochemistry 2016, 110, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int. J. Biol. Macromol. 2019, 125, 700–710. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Khalil, N.M.; Nascimento, T.C.F.D.; Casa, D.M.; Dalmolin, L.F.; Mattos, A.C.D.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloids Surf. B Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Cham, T.-M.; Tsai, T.-R. Development of HPLC with Photo-diode Array Method for the Determination of Ramipril in Tablets Using Factorial Design. J. Chin. Chem. Soc. TAIP 2014, 61, 1388–1394. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C. 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Y.; Hou, S.; Xu, F.; Zhao, R.; He, J.; Cai, Z.; Li, Y.; Chen, Q. Dual agents loaded PLGA nanoparticles: Systematic study of particle size and drug entrapment efficiency. Eur. J. Pharm. Biopharm. 2008, 69, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Feczkó, T.; Tóth, J.; Dósa, G.; Gyenis, J. Optimization of protein encapsulation in PLGA nanoparticles. Chem. Eng. Process. 2011, 50, 757–765. [Google Scholar] [CrossRef]
- Tefas, L.R.; Tomuţă, I.; Achim, M.; Vlase, L. Development and optimization of quercetin-loaded PLGA nanoparticles by experimental design. Clujul. Med. 2015, 88, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Stolnik, S.; Garnett, M.C.; Davies, M.C.; Illum, L.; Bousta, M.; Vert, M.; Davis, S.S. The colloidal properties of surfactant-free biodegradable nanospheres from poly(β-malic acid-co-benzyl malate)s and poly(lactic acid-co-glycolide). Colloids Surf. A. Physicochem. Eng. ASP 1995, 97, 235–245. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Kadari, A.; Gudem, S.; Kulhari, H.; Bhandi, M.M.; Borkar, R.M.; Kolapalli, V.R.M.; Sistla, R. Enhanced oral bioavailability and anticancer efficacy of fisetin by encapsulating as inclusion complex with HPβCD in polymeric nanoparticles. Drug Deliv. 2017, 24, 224–232. [Google Scholar] [CrossRef]
- Ghosh, P.; Singha Roy, A.; Chaudhury, S.; Jana, S.K.; Chaudhury, K.; Dasgupta, S. Preparation of albumin based nanoparticles for delivery of fisetin and evaluation of its cytotoxic activity. Int. J. Biol. Macromol. 2016, 86, 408–417. [Google Scholar] [CrossRef]
- Sowa, M.; Ślepokura, K.; Matczak-Jon, E. Improving solubility of fisetin by cocrystallization. CrystEngComm 2014, 16, 10592–10601. [Google Scholar] [CrossRef]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of dietary flavonoid fisetin: Formulation and in vitro antioxidant and α-glucosidase inhibition activities. Mater. Sci. Eng. C 2016, 68, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Q.; Jiang, K.-M.; An, K.; Ren, S.-H.; Xie, X.-G.; Jin, Y.; Lin, J. Novel water-soluble fisetin/cyclodextrins inclusion complexes: Preparation, characterization, molecular docking and bioavailability. Carbohydr. Res. 2015, 418, 20–28. [Google Scholar] [CrossRef]
- Chen, L.-F.; Xu, P.-Y.; Fu, C.-P.; Kankala, R.K.; Chen, A.-Z.; Wang, S.-B. Fabrication of Supercritical Antisolvent (SAS) Process-Assisted Fisetin-Encapsulated Poly (Vinyl Pyrrolidone) (PVP) Nanocomposites for Improved Anticancer Therapy. Nanomaterials 2020, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Zhu, R.; Wang, Y.; Li, B.; Ma, Y.; Yin, Y. Synthesis and characterization of serial random and block-copolymers based on lactide and glycolide. Polym. Sci. Ser. B 2016, 58, 720–729. [Google Scholar] [CrossRef]
- Mistry, P.; Mohapatra, S.; Gopinath, T.; Vogt, F.G.; Suryanarayanan, R. Role of the Strength of Drug–Polymer Interactions on the Molecular Mobility and Crystallization Inhibition in Ketoconazole Solid Dispersions. Mol. Pharm. 2015, 12, 3339–3350. [Google Scholar] [CrossRef]
- SciFinder. Available online: https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf (accessed on 27 April 2021).
- Wang, J.; Zhao, X.-H. Degradation kinetics of fisetin and quercetin in solutions as effected by pH, temperature and coexisted proteins. J. Serbian Chem. Soc. 2016, 81, 243–253. [Google Scholar] [CrossRef]
- Krajčíková, K.; Suváková, M.; Glinská, G.; Ohlasová, J.; Tomečková, V. Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops. Open Chem. 2020, 18, 325–332. [Google Scholar] [CrossRef]
- Faisant, N.; Siepmann, J.; Benoit, J.P. PLGA-based microparticles: Elucidation of mechanisms and a new, simple mathematical model quantifying drug release. Eur. J. Pharm. Sci. 2002, 15, 355–366. [Google Scholar] [CrossRef]
- Song, H.Y.; Moon, T.W.; Choi, S.J. Impact of antioxidant on the stability of β-carotene in model beverage emulsions: Role of emulsion interfacial membrane. Food Chem. 2019, 279, 194–201. [Google Scholar] [CrossRef]
- Cao, S.-J.; Xu, S.; Wang, H.-M.; Ling, Y.; Dong, J.; Xia, R.-D.; Sun, X.-H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef] [PubMed]
- Ersoz, M.; Erdemir, A.; Derman, S.; Arasoglu, T.; Mansuroglu, B. Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm. Dev. Technol. 2020, 25, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, B.; Li, B.; Li, Z.; Jiang, B.-H.; Chen, Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar] [CrossRef]
- Tariq, M.; Alam, M.A.; Singh, A.T.; Iqbal, Z.; Panda, A.K.; Talegaonkar, S. Biodegradable polymeric nanoparticles for oral delivery of epirubicin: In vitro, ex vivo, and in vivo investigations. Colloids Surf. B Biointerfaces 2015, 128, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Shailender, J.; Ravi, P.R.; Saha, P.; Dalvi, A.; Myneni, S. Tenofovir disoproxil fumarate loaded PLGA nanoparticles for enhanced oral absorption: Effect of experimental variables and in vitro, ex vivo and in vivo evaluation. Colloids Surf. B Biointerfaces 2017, 158, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Singh, S.; Rajalakshmi, S.; Shaikh, K.; Bothiraja, C. Development of fisetin-loaded folate functionalized pluronic micelles for breast cancer targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 347–361. [Google Scholar] [CrossRef]
- Yen, C.-C.; Tung, C.-W.; Chang, C.-W.; Tsai, C.-C.; Hsu, M.-C.; Wu, Y.-T. Potential Risk of Higenamine Misuse in Sports: Evaluation of Lotus Plumule Extract Products and a Human Study. Nutrients 2020, 12, 285. [Google Scholar] [CrossRef]
- Fatma, S.; Talegaonkar, S.; Iqbal, Z.; Panda, A.K.; Negi, L.M.; Goswami, D.G.; Tariq, M. Novel flavonoid-based biodegradable nanoparticles for effective oral delivery of etoposide by P-glycoprotein modulation: An in vitro, ex vivo and in vivo investigations. Drug Deliv. 2016, 23, 500–511. [Google Scholar] [CrossRef]
- Kumar, S.; Dilbaghi, N.; Saharan, R.; Bhanjana, G. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. Bionanoscience 2012, 2, 227–250. [Google Scholar] [CrossRef]
- Yen, C.-C.; Chen, Y.-C.; Wu, M.-T.; Wang, C.-C.; Wu, Y.-T. Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. Int. J. Nanomed. 2018, 13, 669. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-mohizea, A.M. Everted gut sac model as a tool in pharmaceutical research: Limitations and applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef] [PubMed]









| Run Order | Factors and Levels | Responses | ||||
|---|---|---|---|---|---|---|
| X1: PVA (%) | X2: PLGA (mg) | Y1: Particle Size (nm) | Y2: EE 1 (%) | Y3: Zeta Potential (mV) | Y4: PDI 2 | |
| 1 | 0.295 | 100 | 224.0 | 83.6 | −20.62 | 0.152 |
| 2 | 0.5 | 35 | 158.3 | 76.0 | −23.51 | 0.568 |
| 3 | 0.5 | 165 | 211.1 | 71.6 | −22.70 | 0.144 |
| 4 | 1 | 100 | 186.4 | 81.5 | −20.52 | 0.152 |
| 5 | 1 | 191.5 | 244.4 | 65.6 | −14.15 | 0.201 |
| 6 | 1 | 100 | 190.3 | 77.7 | −17.34 | 0.090 |
| 7 | 1 | 100 | 198.1 | 74.2 | −22.65 | 0.053 |
| 8 | 1 | 100 | 205.0 | 77.8 | −21.48 | 0.145 |
| 9 | 1 | 100 | 194.1 | 77.2 | −19.86 | 0.083 |
| 10 | 1 | 8.35 | 102.9 | 60.1 | −7.68 | 0.792 |
| 11 | 1.5 | 35 | 145.5 | 42.8 | −12.32 | 0.144 |
| 12 | 1.5 | 165 | 216.8 | 68.9 | −17.36 | 0.228 |
| 13 | 1.705 | 100 | 193.8 | 57.8 | −25.94 | 0.078 |
| Factor | Optimal Conditions | ||
|---|---|---|---|
| PVA Concentration (%) | 0.5 | ||
| PLGA Amount (mg) | 75.3 | ||
| Responses | Predicted Values | Experimental Values | Error (%) |
| Particle Size (nm) | 180.9 | 187.9 ± 6.1 | 3.9 |
| EE (%) 1 | 83.1 | 79.3 ± 2.7 | −4.6 |
| Zeta Potential (mV) | −20.3 | −29.2 ± 1.6 | −43.8 |
| PDI 2 | 0.289 | 0.121 ± 0.01 | −58.1 |
| Nanomaterial Type | Particle Size (nm) | PDI | Zeta-Potential (mV) | EE (%) 1 | Reference |
|---|---|---|---|---|---|
| HSA 2 | 220 | - | - | 84 | [31] |
| PLA 3 | 226.85 | 0.12 | −15.63 | 90.35 | [19] |
| HPβ-CD/PLGA 4 | 87.27 | 0.25 | −8.71 | 78.8 | [30] |
| PLGA 5 | 187.9 | 0.121 | −29.2 | 79.3 | This study |
| Day | 5 ± 3 °C | 25 ± 2 °C | ||||
|---|---|---|---|---|---|---|
| FST Content (%) | Particle Size (nm) | PDI | FST Content (%) | Particle Size (nm) | PDI | |
| 0 | 100.0 ± 1.1 | 179.3 ± 17.1 | 0.116 ± 0.016 | 100.0 ± 1.1 | 179.3 ± 17.1 | 0.116 ± 0.016 |
| 3 | 99.1 ± 2.9 | 178.2 ± 15.4 | 0.103 ± 0.030 | 102.1 ± 4.4 | 176.8 ± 14.2 | 0.109 ± 0.054 |
| 7 | 96.7 ± 2.9 | 179.7 ± 15.7 | 0.085 ± 0.037 | 100.2 ± 5.0 | 179.1 ± 12.9 | 0.093 ± 0.027 |
| 14 | 101.9 ± 0.9 | 179.3 ± 15.7 | 0.129 ± 0.037 | 95.4 ± 3.7 | 181.7 ± 9.0 | 0.094 ± 0.013 |
| 21 | 101.6 ± 3.4 | 179.5 ± 15.9 | 0.097 ± 0.010 | 98.2 ± 2.1 | 180.5 ± 11.6 | 0.117 ± 0.049 |
| 30 | 99.7 ± 1.0 | 188.3 ± 22.3 | 0.108 ± 0.032 | 103.4 ± 1.7 | 185.4 ± 7.1 | 0.138 ± 0.019 |
| 60 | 100.1 ± 3.3 | 174.9 ± 16.0 | 0.126 ± 0.043 | 103.9 ± 2.7 | 180.7 ± 7.1 | 0.112 ± 0.021 |
| C72 h 1 (μg/mL) | K 2 (h−1) | t1/2 3 of FST (h) | t1/2 of FST–NP (h) | |
|---|---|---|---|---|
| pH 1.2 | 4.73 | 0.001 | 471.8 | N.A. 4 |
| pH 6.8 | 1.72 | 0.015 | 46.6 | 96 |
| pH 7.4 | N.D. 5 | 0.245 | 2.8 | 72 |
| Independent Variables | Level | ||||
|---|---|---|---|---|---|
| −1.414 | −1 | 0 | 1 | 1.414 | |
| X1: PVA Concentration (%) | 0.295 | 0.5 | 1 | 1.5 | 1.705 |
| X2: PLGA Amount (mg) | 8.35 | 35 | 100 | 165 | 191.5 |
| Dependent Variables | Goal | ||||
| Y1: Particle Size (nm) | Minimize | ||||
| Y2: Encapsulation Efficiency (%) | Maximize | ||||
| Y3: Zeta Potential (mV) | <−20 | ||||
| Y4: Polydispersity Index | Minimize | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-Y.; Lin, C.-C.; Hsieh, Y.-S.; Wu, Y.-T. Nanoformulation Development to Improve the Biopharmaceutical Properties of Fisetin Using Design of Experiment Approach. Molecules 2021, 26, 3031. https://doi.org/10.3390/molecules26103031
Liu W-Y, Lin C-C, Hsieh Y-S, Wu Y-T. Nanoformulation Development to Improve the Biopharmaceutical Properties of Fisetin Using Design of Experiment Approach. Molecules. 2021; 26(10):3031. https://doi.org/10.3390/molecules26103031
Chicago/Turabian StyleLiu, Wan-Yi, Chia-Chen Lin, Yun-Shan Hsieh, and Yu-Tse Wu. 2021. "Nanoformulation Development to Improve the Biopharmaceutical Properties of Fisetin Using Design of Experiment Approach" Molecules 26, no. 10: 3031. https://doi.org/10.3390/molecules26103031
APA StyleLiu, W.-Y., Lin, C.-C., Hsieh, Y.-S., & Wu, Y.-T. (2021). Nanoformulation Development to Improve the Biopharmaceutical Properties of Fisetin Using Design of Experiment Approach. Molecules, 26(10), 3031. https://doi.org/10.3390/molecules26103031