Densazalin, a New Cytotoxic Diazatricyclic Alkaloid from the Marine Sponge Haliclona densaspicula
Abstract
:1. Introduction
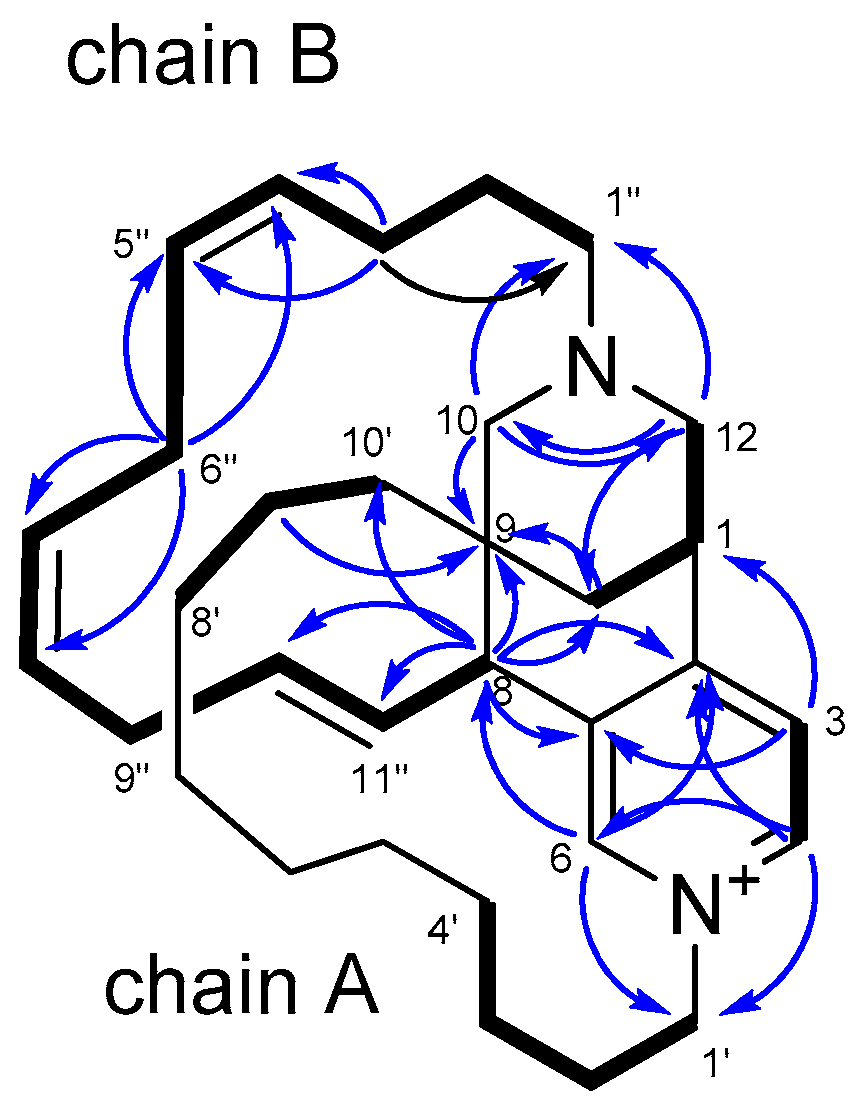
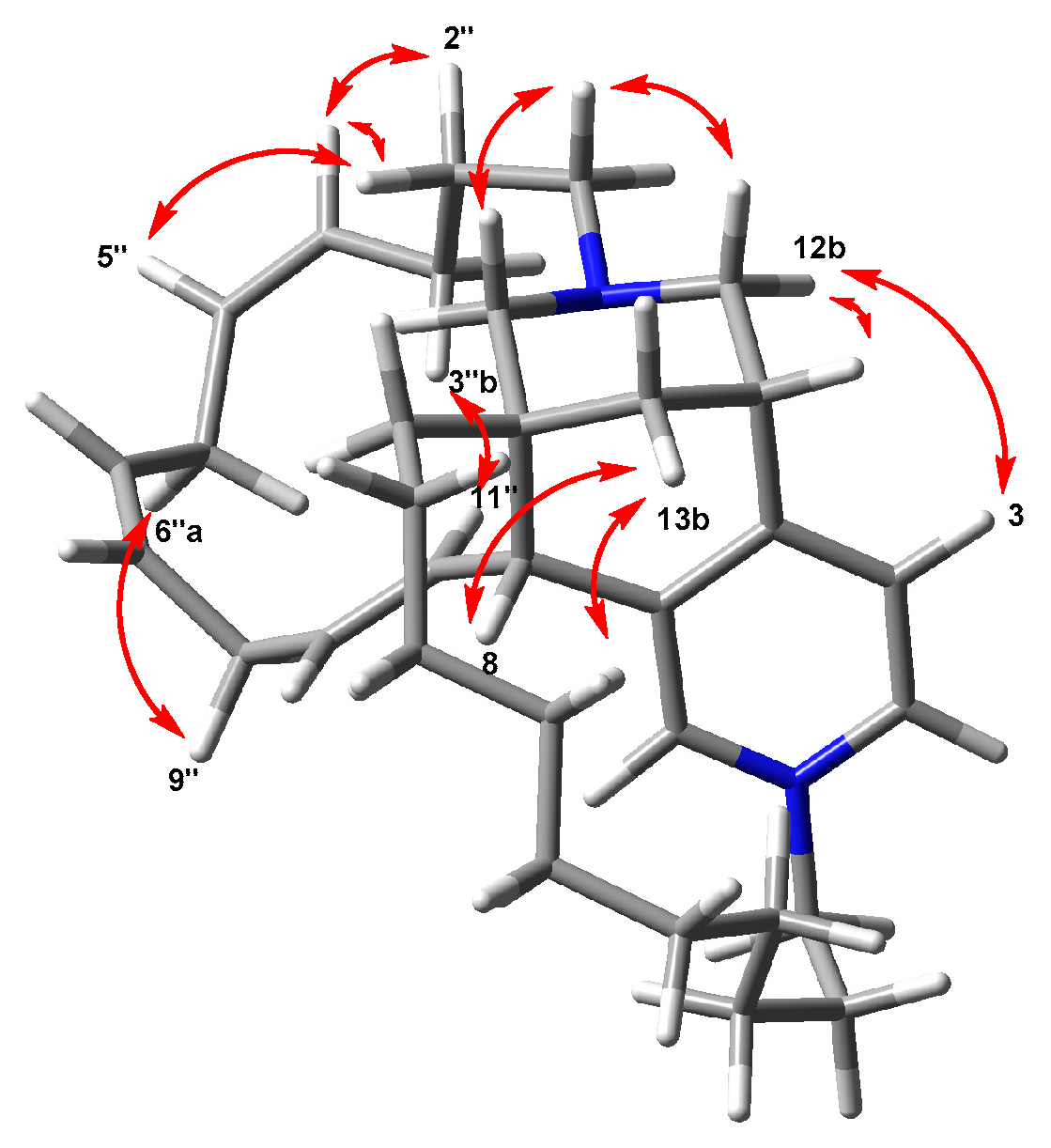
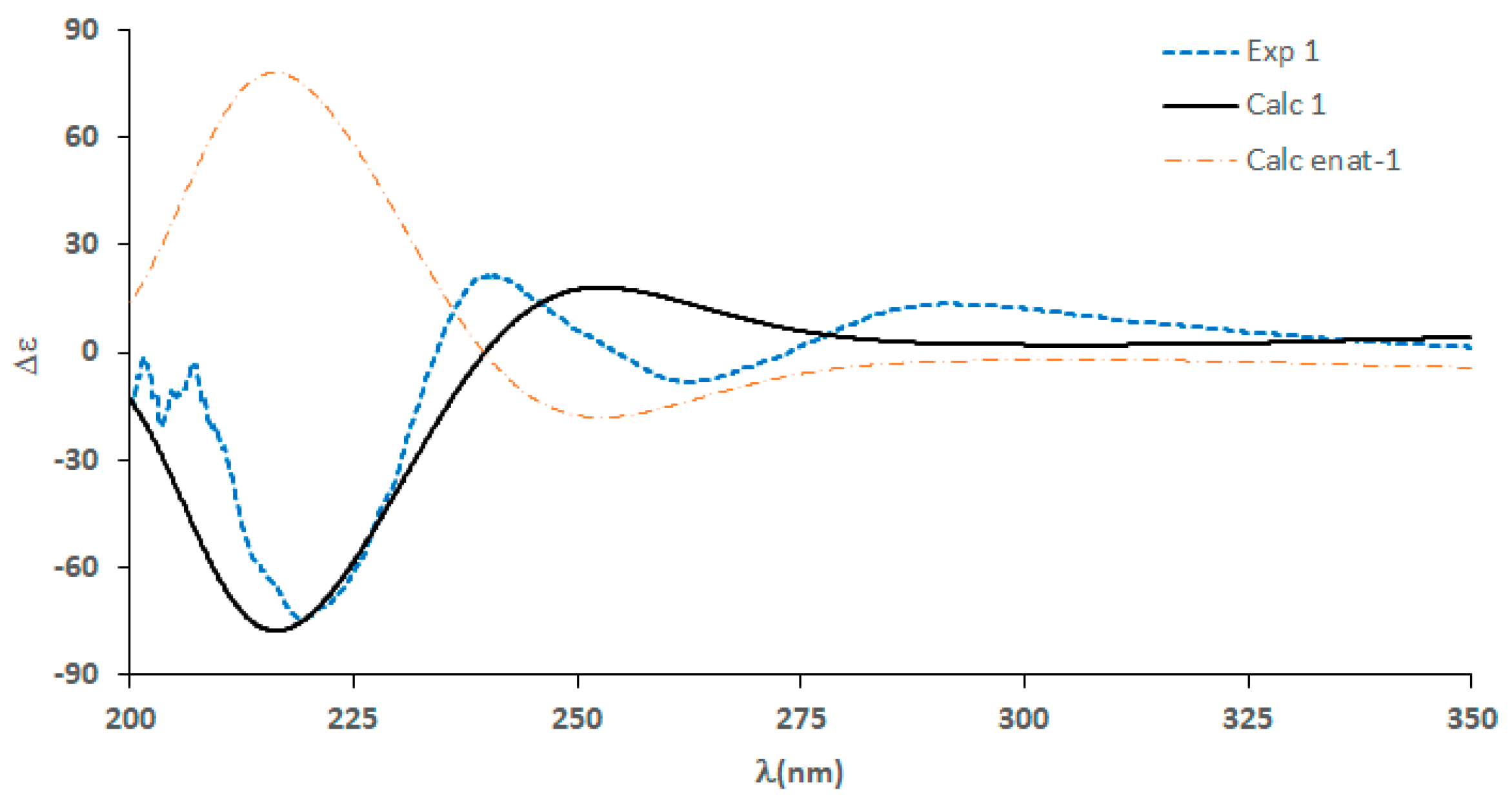
2. Results and Discussion
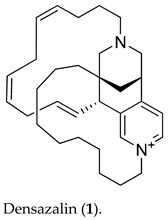
3. Materials and Methods
3.1. Instrumentation
3.2. Material
3.3. Extraction and Isolation
3.4. Cell Culture and Cytotoxicity Assay
3.5. Statistical Analysis
3.6. Calculation of ECD Spectrum of 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Perdicaris, S.; Vlachogianni, T.; Valavanidis, A. Bioactive natural substances from marine sponges: New developments and prospects for future pharmaceuticals. Nat. Prod. Chem. Res. 2013, 1, 1–8. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.B.; Xiao, H.; Fan, C.-Q.; Lu, Y.-N.; Zhang, G.; Yin, S. Bioactive polyhydroxylated sterols from the marine sponge Haliclona crassiloba. Steroids 2013, 78, 1343–1358. [Google Scholar] [CrossRef]
- Sera, Y.; Adachi, K.; Fujii, K.; Shizuri, Y. A new antifouling hexapeptide from a Palauan sponge, Haliclona sp. J. Nat. Prod. 2003, 66, 719–721. [Google Scholar] [CrossRef]
- Erickson, K.L.; Beutler, J.A.; Cardellina, J.H.; Boyd, M.R. Salicylihalamides A and B, Novel cytotoxic macrolides from the marine sponge Haliclona sp. J. Org. Chem. 1997, 62, 8188–8192. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.-E.; Herbette, G.; Chendo, C.; Clerc, P.; Tintillier, F.; de Voogd, N.J.; Papangnou, E.-D.; Trougakos, I.P.; Jerabek, M.; Bignon, J.; et al. Osirisynes GI, New Long-Chain Highly Oxygenated Polyacetylenes from the Mayotte Marine Sponge Haliclona sp. Mar. Drugs 2020, 18, 350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, Y.; Liu, Z.; Wang, H.; Zhang, H. Bioactive nitrogenous secondary metabolites from the marine sponge genus Haliclona. Mar. Drugs 2019, 17, 682. [Google Scholar] [CrossRef] [Green Version]
- Charan, R.D.; Garson, M.J.; Brereton, I.M.; Willis, A.C.; Hooper, J.N.A. Haliclonacyclamines A and B, cytotoxic alkaloids from the tropical marine sponge Haliclona sp. Tetrahedron 1996, 52, 9111–9120. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Hollenbeak, K.H.; Campbell, D.C. Marine natural products: Halitoxin, toxic complex of several marine sponges of the genus Haliclona. J. Org. Chem. 1978, 43, 3916–3922. [Google Scholar] [CrossRef]
- Sakai, R.; Higa, T.J. Manzamine A, a novel antitumor alkaloid from a sponge. Am. Chem. Soc. 1986, 108, 6404–6405. [Google Scholar] [CrossRef]
- Sakai, R.; Kohmoto, S.; Higa, T.; Jefford, C.W.; Bernardinelli, G. Manzamine B and C, two novel alkaloids from the sponge Haliclona sp. Tetrahedron Lett. 1987, 28, 5493–5496. [Google Scholar] [CrossRef]
- Fusetani, N.; Yasumuro, K.; Matsunaga, S.; Hirota, H. Haliclamines A and B, cytotoxic macrocyclic alkaloids from a sponge of the genus Haliclona. Tetrahedron Lett. 1989, 30, 6891–6894. [Google Scholar] [CrossRef]
- Crews, P.; Cheng, X.C.; Adamczeski, M.; Rodríguez, J.; Jaspars, M.; Schmitz, F.J.; Pordesimo, E.O. 1, 2, 3, 4-tetrahydro-8-hydroxymanzamines, alkaloids from two different haplosclerid sponges. Tetrahedron 1994, 50, 13567–13574. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Whitehead, R.C. On the biosynthesis of manzamines. Tetraheadron Lett. 1992, 33, 2059–2062. [Google Scholar] [CrossRef]
- Jaspars, M.; Pasupathy, V.; Crews, P.J. A tetracyclic diamine alkaloid, halicyclamine A, from the marine sponge Haliclona sp. Org. Chem. 1994, 59, 3253–3255. [Google Scholar] [CrossRef]
- Sisko, J.; Weinreb, S.M. Construction of the tricyclic core of the marine alkaloid sarain A. J. Org. Chem. 1991, 56, 3210–3211. [Google Scholar] [CrossRef]
- Hwang, B.S.; Oh, J.S.; Jeong, E.J.; Sim, C.J.; Rho, J.-R. Densanins A and B, new macrocyclic pyrrole alkaloids isolated from the marine sponge Haliclona densaspicula. Org. Lett. 2012, 14, 6154–6157. [Google Scholar] [CrossRef]
- Fahy, E.; Molinski, T.F.; Harper, M.K.; Sullivan, B.W.; Faulkner, D.J.; Parkanyi, L.; Clardy, J. Haliclonadiamine, an antimicrobial alkaloid from the sponge Haliclona sp. Tetrahedron Lett. 1988, 29, 3427–3428. [Google Scholar] [CrossRef]
- Kara, A.U.; Higa, T.; Holmes, M. Antimalarial Activity of β-carboline Alkaloids. PCT Int. Appl. US 6143756A, 7 November 2000. [Google Scholar]
- Uli, H.; Noor, A.; Mandey, F.W.; Sapar, A.; Kemerdekaan, J.P. Isolation, Identification, and Bioactivity Test of Non Polar Compounds on n-Hexana Extract of Haliclona (Reniera) fascigera from Samalona Island-Spermonde Archipelago. Int. J. Mar. Chim. Acta 2016, 17, 32–34. [Google Scholar]
- Wattanadilok, R.; Sawangwong, P.; Rodrigues, C.; Cidade, H.; Pinto, M.; Pinto, E. Antifungal activity evaluation of the constituents of Haliclona baeri and Haliclona cymaeformis, collected from the Gulf of Thailand. Mar. Drugs 2007, 5, 40–51. [Google Scholar] [CrossRef]
- Amraoui, B.E.; Wahidi, M.E.; Fassouane, A. In vitro screening of antifungal activity of marine sponge extracts against five phytopathogenic fungi. SpringerPlus 2014, 3, 629–633. [Google Scholar] [CrossRef] [Green Version]
- Hutagalung, R.A.; Victor Karjadidjaja, M.; Prasasty, V.D.; Mulyono, N. Extraction and characterization of bioactive compounds from cultured and natural sponge, Haliclona molitba and Stylotella aurantium origin of Indonesia. Int. J. Biosci. Biochem. Bioinform. 2014, 4, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Ivana, P.; Zoran, K.; Nikola, D.; Dusan, S.; Zorica, J.; Miroslave, G. A novel lectin from the sponge Haliclona cratera: Isolation, characterization and biological activity. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 213–221. [Google Scholar]
- Hirsch, S.; Kashman, Y. New glycosphingolipids from marine organisms. Tetrahedron 1989, 45, 3897–3906. [Google Scholar] [CrossRef]
- Boyd, M.R.; Mckee, T.C.; Pannell, L. Macrocyclic Lactones, Compositions, and Methods of Use. PCT Int. Appl. WO 9905136, 4 February 1999. [Google Scholar]
- Valentin, B.B.; Beulah, M.C.; Vinod, V. Efficacy of bioactive compounds extracted from marine sponge Haliclona exigua. Asian J. Pharm. Clin. Res. 2013, 6, 347–349. [Google Scholar]
- Rivera, P.; Uy, M.M. In vitro antioxidant and cytotoxic activities of some marine sponges collected off Misamis Oriental Coast, Philippines. E-J. Chem. 2012, 9, 354–358. [Google Scholar] [CrossRef]
- Randazzo, A.; Bifulco, G.; Giannini, C.; Bucci, M.; Debitus, C.; Cirino, G.J. Halipeptins A and B: Two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J. Am. Chem. Soc. 2001, 123, 10870–10876. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Demers, S.; Stevenson, H.; Candler, J.; Bashore, C.G.; Arnold, E.P.; O’Neill, B.T.; Coe, J.W. Pyridine analogs of (−)-cytisine and varenicline: Cholinergic receptor probes. Tetrahedron Lett. 2008, 49, 3368–3371. [Google Scholar] [CrossRef]
- Rodríguez, J.; Peters, B.M.; Kurz, L.; Schatzman, R.C.; McCarley, D.; Lou, L.; Crews, P. An alkaloid protein kinase C inhibitor, xestocyclamine A, from the marine sponge Xestospongia sp. J. Am. Chem. Soc. 1993, 115, 10436–10437. [Google Scholar] [CrossRef]
- Oh, K.-B.; Mar, W.; Kim, S.; Kim, J.-Y.; Lee, T.-H.; Kim, J.-G.; Shin, D.; Sim, C.J.; Shin, J. Antimicrobial activity and cytotoxicity of bis (indole) alkaloids from the sponge Spongosorites sp. Biol. Pharm. Bull. 2006, 29, 570–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urda, C.; Mèrez, M.; Rodríguez, J.; Fernández, R.; Jiménez, C.; Cuevas, C. Njaoamine I, a cytotoxic polycyclic alkaloid from the Haplosclerida sponge Haliclona (Reniera) sp. Tetrahedron Lett. 2018, 59, 2577–2580. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.P.; Boswell, J.L.; Boyd, M.R. Haligramides A and B, two new cytotoxic hexapeptides from the marine sponge Haliclona nigra. J. Nat. Prod. 2000, 63, 956–959. [Google Scholar] [CrossRef] [PubMed]


| no. | δC | δH, mult (J Hz) |
|---|---|---|
| 1 | 38.4, CH | 3.35, m |
| 2 | 163.6, C | |
| 3 | 128.6, CH | 7.92, d(6.1) |
| 4 | 141.5, CH | 8.65, d(6.1) |
| 6 | 145.1, CH | 8.59, s |
| 7 | 144.5, C | |
| 8 | 46.4, CH | 3.77, d(8.8) |
| 9 | 38.2, C | |
| 10 | 66.0, CH2 | a 1.59, d(11.3); b 2.87, brd(11.3) |
| 12 | 60.3, CH2 | a 2.39, dd(11.6, 3.2); b 3.06, d(11.6) |
| 13 | 35.6, CH2 | a 1.58, dd(13.2, 2.7); b 2.02, brd(13.2) |
| 1′ | 62.8, CH2 | a 4.54, td(12.5, 3.2); b 4.72, dt(12.5, 3.9) |
| 2′ | 30.9, CH2 | a 1.72, m; b 2.15, m |
| 3′ | 26.2, CH2 | a 1.24, m; b 1.44, m |
| 4′ | 29.5, CH2 | a 0.07, m; b 1.07, m |
| 5′ | 29.1, CH2 | a 1.17, m; b 1.25, m |
| 6′ | 30.3, CH2 | 1.12, m |
| 7′ | 31.0, CH2 | 1.19, m |
| 8′ | 30.5, CH2 | 1.24, m |
| 9′ | 23.1, CH2 | a 1.36, m; b 1.67, m |
| 10′ | 35.9, CH2 | a 1.23, m; b 1.57, m |
| 1″ | 59.2, CH2 | a 2.19, m; b 2.28, dd(7.6, 3.7) |
| 2″ | 27.9, CH2 | 1.41, dd(7.6, 3.4) |
| 3″ | 27.4, CH2 | a 1.67, m; b 2.17, m |
| 4″ | 132.4, CH | 5.29, td(10.9, 5.1) |
| 5″ | 128.9, CH | 5.48, m |
| 6″ | 26.3, CH2 | a 2.65, m; b 2.96, m |
| 7″ | 130.9, CH | 5.41, dt(11.3, 6.1) |
| 8″ | 128.0, CH | 5.54, m |
| 9″ | 30.6, CH2 | 2.92, m |
| 10″ | 134.9, CH | 6.10, dt(15.9, 6.1) |
| 11″ | 131.8, CH | 5.94, dd(15.9, 9.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, B.S.; Jeong, Y.T.; Lee, S.; Jeong, E.J.; Rho, J.-R. Densazalin, a New Cytotoxic Diazatricyclic Alkaloid from the Marine Sponge Haliclona densaspicula. Molecules 2021, 26, 3164. https://doi.org/10.3390/molecules26113164
Hwang BS, Jeong YT, Lee S, Jeong EJ, Rho J-R. Densazalin, a New Cytotoxic Diazatricyclic Alkaloid from the Marine Sponge Haliclona densaspicula. Molecules. 2021; 26(11):3164. https://doi.org/10.3390/molecules26113164
Chicago/Turabian StyleHwang, Buyng Su, Yong Tae Jeong, Sangbum Lee, Eun Ju Jeong, and Jung-Rae Rho. 2021. "Densazalin, a New Cytotoxic Diazatricyclic Alkaloid from the Marine Sponge Haliclona densaspicula" Molecules 26, no. 11: 3164. https://doi.org/10.3390/molecules26113164
APA StyleHwang, B. S., Jeong, Y. T., Lee, S., Jeong, E. J., & Rho, J.-R. (2021). Densazalin, a New Cytotoxic Diazatricyclic Alkaloid from the Marine Sponge Haliclona densaspicula. Molecules, 26(11), 3164. https://doi.org/10.3390/molecules26113164







