Bertholletia excelsa Seeds Reduce Anxiety-Like Behavior, Lipids, and Overweight in Mice
Abstract
1. Introduction
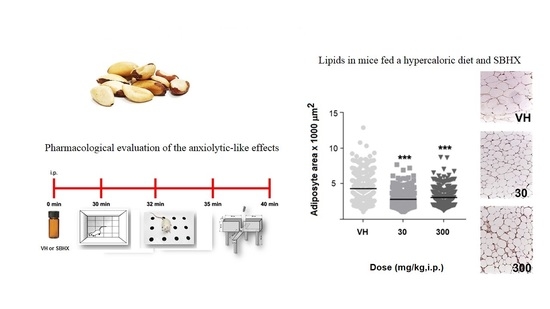
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents and Drugs
4.3. Extract Preparation
Fatty Acid Composition of the Bertholletia Excelsa Oil
4.4. Pharmacological Evaluation
4.4.1. Bertholletia Excelsa Acute Effects on the Central Nervous System
Open-Field Test
Hole-Board Test
Elevated Plus-Maze Test
Sodium Pentobarbital (SPB)-Induced Hypnosis Potentiation
4.4.2. Bertholletia. Excelsa Chronic Effects on the Overweight and Lipids
4.4.3. Histological and Morphometric Analyses
4.4.4. Tissue Lipids
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cardoso, B.R.; Duarte, G.B.S.; Reis, B.Z.; Cozzolino, S.M.F. Brazil nuts: Nutritional composition, health benefits and safety aspects. Food Res. Int. 2017, 100, 9–18. [Google Scholar] [CrossRef]
- Martins, M.; Klusczcovski, A.M.; Scussel, V.M. In vitro activity of the Brazil nut (Bertholletia excelsa H.B.K.) oil in aflatoxigenic strains of Aspergillus parasiticus. Eur. Food Res. Technol. 2014, 239, 687–693. [Google Scholar] [CrossRef]
- Callisaya, J.C.; Alvarado, J.A. Total phenol contents and antioxidant capacity of Bertholletia excelsa, amazonian almonds from Bolivia. Rev. Boliv. Quim. 2016, 33, 34–42. [Google Scholar]
- Stockler-Pinto, M.B.; Carrero, J.J.; Weide, L.D.C.C.; Cozzolino, S.M.F.; Mafra, D. Effect of selenium supplementation via brazil nut (Bertholletia excelsa, Hbk) on thyroid hormones levels in hemodialysis patients: A pilot study. Nutr. Hosp. 2015, 32, 1808–1812. [Google Scholar] [CrossRef]
- López-Uriarte, P.; Nogués, R.; Saez, G.; Bulló, M.; Romeu, M.; Masana, L.; Tormos, C.; Casas-Agustench, P.; Salas-Salvadó, J. Effect of nut consumption on oxidative stress and the endothelial function in metabolic syndrome. Clin. Nutr. 2010, 29, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Brazil nuts and associated health benefits: A review. LWT-Food Sci. Technol. 2009, 42, 1573–1580. [Google Scholar] [CrossRef]
- Anselmo, N.A.; Paskakulis, L.C.; Garcias, R.C.; Botelho, F.F.R.; Toledo, G.Q.; Cury, M.F.R.; Carvalho, N.Z.; Mendes, G.E.F.; Iembo, T.; Bizotto, T.S.G.; et al. Prior intake of Brazil nuts attenuates renal injury induced by ischemia and reperfusion. J. Bras. Nefrol. 2017, 40, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Zhao, G.; Binkoski, A.E.; Coval, S.M.; Etherton, T.D. The effects of nuts on coronary heart disease risk. Nutr. Rev. 2001, 59, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Huguenin, G.V.B.; Oliveira, G.M.M.; Moreira, A.S.B.; Saint’Pierre, T.D.; Gonçalves, R.A.; Pinheiro-Mulder, A.R.; Teodoro, A.J.; Luiz, R.R.; Rosa, G. Improvement of antioxidant status after Brazil nut intake in hypertensive and dyslipidemic subjects. Nutr. J. 2015, 14, 54–59. [Google Scholar] [CrossRef]
- Huguenin, G.V.B.; Moreira, A.S.B.; Siant’Pierre, T.D.; Gonçalves, R.A.; Rosa, G.; Oliveira, G.M.M.; Luiz, R.R.; Tibirica, E. Effects of dietary supplementation with Brazil nuts on microvascular endothelial function in hypertensive and dyslipidemic patients: A randomized crossover placebo-controlled trial. Microcirculation 2015, 22, 687–699. [Google Scholar] [CrossRef]
- González, C.A.; Salas-Salvadó, J. The potential of nuts in the prevention of cancer. Br. J. Nutr. 2006, 96, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; McIntosh, G.H.; Le Leu, R.K.; Somashekar, R.; Meng, X.Q.; Gopalsamy, G.; Bambaca, L.; McKinnon, R.A.; Young, G.P. Supplementation with Brazil nuts and green tea extract regulates targeted biomarkers related to colorectal cancer risk in humans. Br. J. Nutr. 2016, 116, 1901–1911. [Google Scholar] [CrossRef]
- Peña-Muñiz, M.A.; Ferreira Dos Santos, M.N.M.; Ferreira Da Costa, C.E.; Morais, L.; Nobre-Lamarão, M.L.; Ribeiro-Costa, R.M.; Carrera Silva-Júnior, J.O. Physicochemical characterization, fatty acid composition, and thermal analysis of Bertholletia excelsa HBK oil. Pharmacogn. Mag. 2015, 11, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Dodds, E.D.; McCoy, M.R.; Rea, L.D.; Kennish, J.M. Gas chromatographic quantification of fatty acid methyl esters: Flame ionization detection vs. electron impact mass spectrometry. Lipids 2005, 40, 419–428. [Google Scholar] [CrossRef]
- Scott, K.M.; McGee, M.A.; Wells, J.E.; Oakley Browne, M.A. Obesity and mental disorders in the adult general population. J. Psychosom. Res. 2008, 64, 97–105. [Google Scholar] [CrossRef]
- Atlantis, E.; Goldney, R.D.; Wittert, G.A. Obesity and depression or anxiety. BMJ 2009, 339, 871–876. [Google Scholar] [CrossRef]
- Baker, K.D.; Loughman, A.; Spencer, S.J.; Reichelt, A.C. The impact of obesity and hypercaloric diet consumption on anxiety and emotional behavior across the lifespan. Neurosci. Biobehav. Rev. 2017, 83, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Pichot, P.; López-Ibor-Aliño, J.J.; Valdés-Miyar, M. DSM-IV. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Masson: Barcelona, Spain, 1995. [Google Scholar]
- Guyenet, S.J.; Carlson, S.E. Increase in adipose tissue linoleic acid of US adults in the last half century. Adv. Nutr. 2015, 6, 660–664. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Sundseth, S.S.; Jones, S.A.; Brown, P.J.; Wisely, G.B.; Koble, C.S.; Devchand, P.; Wahli, W.; Willson, T.M.; Lenhard, J.M.; et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA 1997, 94, 4318–4323. [Google Scholar] [CrossRef]
- Wahli, W.; Devchand, P.R.; Desvergne, B. Fatty acids, eicosanoids, and hypolipidemic agents regulate gene expression through direct binding to peroxisome proliferator-activated receptors. Adv. Exp. Med. Biol. 1999, 447, 199–209. [Google Scholar] [CrossRef]
- Contreras, C.M.; Rodríguez-Landa, J.F.; Gutiérrez-García, A.J.; Mendoza-López, M.R.; García-Ríos, R.I.; Cueto-Escobedo, J. Anxiolytic-like effects of human amniotic fluid and its fatty acids in Wistar rats. Behav. Pharmacol. 2011, 22, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Landa, J.F.; García-Riíos, R.I.; Cueto-Escobedo, J.; Bernal-Morales, B.; Contreras, C.M. Participation of GABAA chloride channels in the anxiolytic-like effects of a fatty acid mixture. BioMed. Res. Int. 2013, 2013, 121794. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Morales, B.; Cueto-Escobedo, J.; Guillén-Ruiz, G.; Rodríguez-Landa, J.F.; Contreras, C.M. A fatty acids mixture reduces anxiety-like behaviors in infant rats mediated by GABAA receptors. BioMed. Res. Int. 2017, 2017, 8798546. [Google Scholar] [CrossRef]
- Lister, R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bautista, C.J.; Rodríguez-González, G.L.; Morales, A.; Lomas-Soria, C.; Cruz-Pérez, F.; Reyes-Castro, L.A.; Zambrano, E. Maternal obesity in the rat impairs male offspring aging of the testicular antioxidant defence system. Reprod Fertil. Dev. 2017, 29, 1950–1957. [Google Scholar] [CrossRef]
- Torres, N.; Bautista, C.J.; Tovar, A.R.; Ordáz, G.; Rodríguez-Cruz, M.; Ortiz, V.; Granados, O.; Nathanielsz, P.E.; Larrea, F.; Zambrano, E. Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. Am. J. Physiol. Endocrinol. Metab. 2010, 298, 270–277. [Google Scholar] [CrossRef]
- Ribeiro, M.B.N.; Jerozolimski, A.; De Robert, P.; Salles, N.V.; Kayapó, B.; Pimentel, T.P.; Magnusson, W.E. Anthropogenic landscape in southeastern Amazonia: Contemporary impacts of low-intensity harvesting and dispersal of Brazil nuts by the Kayapó indigenous people. PLoS ONE 2014, 9, e102187. [Google Scholar] [CrossRef]
- Reynolds, K.; Pietrzak, R.H.; El-Gabalawy, R.; Mackenzie, C.S.; Sareen, J. Prevalence of psychiatric disorders in U.S. older adults: Findings from a nationally representative survey. World Psychatry 2015, 14, 74–81. [Google Scholar] [CrossRef]
- Sieghart, W. Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv. Pharmacol. 2015, 72, 53–96. [Google Scholar] [CrossRef]
- Torres-Villalobos, G.; Hamdan-Pérez, N.; Tovar, A.R.; Ordaz-Nava, G.; Martínez-Benítez, B.; Torre-Villalvazo, I.; Morán-Ramos, S.; Díaz-Villaseñor, A.; Noriega, L.G.; Hiriart, M.; et al. Combined high-fat diet and sustained high sucrose consumption promotes NAFLD in a murine model. Ann. Hepatol. 2015, 14, 540–546. [Google Scholar] [CrossRef]
- Cox, R.A.; García-Palmieri, M.R. Chapter 31. Cholesterol, triglycerides, and associated lipoproteins. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, D.W., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; pp. 153–160. ISBN 0-409-90077-X. [Google Scholar]
- Maranhão, P.A.; Kraemer-Aguiar, L.G.; De Oliveira, C.L.; Kuschnir, M.C.; Vieira, Y.R.; Souza, M.G.; Koury, J.C.; Boudkela, E. Brazil nuts intake improves lipid profile, oxidative stress and microvascular function in obese adolescents: A randomized controlled trial. Nutr. Metab. 2011, 8, 1–8. [Google Scholar] [CrossRef]
- Colpo, E.; Dalton, D.A.V.C.; Vilanova, C.; Reetz, L.G.B.; Duarte, M.M.M.F.; Farias, I.L.G.; Meinerz, D.F.; Mariano, D.O.C.; Vendrusculo, R.G.; Boligon, A.A.; et al. Brazilian nut consumption by healthy volunteers improves inflammatory parameters. Nutrition 2014, 30, 459–465. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Huguenin, G.V.B.; Luiz, R.R.; Moreira, A.S.B.; Oliveira, G.M.M.; Rosa, G. Intake of partially defatted Brazil nut flour reduces serum cholesterol in hypercholesterolemic patients—A randomized controlled trial. Nutr. J. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Zambrano, E.; Guzmán, C.; Rodríguez-González, G.L.; Durand-Carbajal, M.; Nathanielsz, P.W. Fetal programming of sexual development and reproductive function. Mol Cell Endocrinol 2014, 382, 538–549. [Google Scholar] [CrossRef]
- Arteaga, A. Overweight and obesity as an universal health problem. Rev. Med. Clin. Condes 2012, 23, 145–153. [Google Scholar] [CrossRef]
- Yan, W.-J.; Mu, Y.; Yu, N.; Yi, T.-L.; Zhang, Y.; Pang, X.-L.; Cheng, D.; Yang, J. Protective effects of metformin on reproductive function in obese male rats induced by high-fat diet. J. Assist. Reprod Genet 2015, 32, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-F.; Feng, Q.; Ge, Z.-Y.; Guo, Y.; Zhou, F.; Zhang, K.-S.; Wang, X.-W.; Lu, W.-H.; Liang, X.-W.; Gu, Y.-Q. Obesity impairs male fertility through long-term effects on spermatogenesis. BMC urology 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Arisha, S.M.; Sakr, S.A.; Abd-Elhaseeb, F.R. Cinnamomum zeylanicum alleviate testicular damage induced by high fat diet in albino rats; histological and ultrastructural studies. Heliyon 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Seijas Buschiazzo, D.; Feuchtmann, S.C. Obesity: Psychiatric and psychological factors. ARS MEDICA Rev. Ciencias Médicas 2018, 26, 1–6. [Google Scholar] [CrossRef][Green Version]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- OECD. Test No. 423: Acute Oral toxicity—Acute Toxic Class Method. OECD Guidel. Test. Chem. 2002, 1–14. [Google Scholar] [CrossRef]
- González-Stuart, A.; Rivera, J.O. Yellow oleander seed, or “Codo de Fraile” (Thevetia spp.): A review of its potential toxicity as a purported weight-loss supplement. J. Diet. ESuppl. 2018, 15, 352–364. [Google Scholar] [CrossRef]
- Martínez-Enríquez, M.E.; Moreno-Ruiz, L.A.; Luna-Rosas, M.; Magos-Guerrero, G.A.; Aguilar-Contreras, A.; Campos-Sepúlveda, A.E. Acute toxicity of Thevetia peruviana in rodents. Proc West. Pharmacol Soc. 2002, 45, 131–133. [Google Scholar] [PubMed]
- Gonzalez-Trujano, M.E.; Carrera, D.; Ventura-Martinez, R.; Cedillo-Portugal, E.; Navarrete, A. Neuropharmacological profile of an ethanol extract of Ruta chalepensis L. in mice. J. Ethnopharmacol. 2006, 106, 29–35. [Google Scholar] [CrossRef]
- Clark, G.; Koester, A.G.; Pearson, D.W. Exploratory behavior in chronic disulfoton poisoning in mice. Psychopharmacologia 1971, 20, 169–171. [Google Scholar] [CrossRef] [PubMed]
- González-Trujano, M.E.; Navarrete, A.; Reyes, B.; Hong, E. Some pharmacological effects of the ethanol extract of leaves of Annona diversifolia on the central nervous system in mice. Phytother Res. 1998, 12, 600–602. [Google Scholar] [CrossRef]
- van Beek, L.; van Klinken, J.B.; Pronk, A.C.M.; van Dam, A.D.; Dirven, E.; Rensen, P.C.N.; Koning, F.; van Dijk, K.O.; van Harmelen, V. The limited storage capacity of gonadal adipose tissue directs the development of metabolic disorders in male C57Bl/6J mice. Diabetologia 2015, 58, 1601–1609. [Google Scholar] [CrossRef]
- Chusyd, D.E.; Wang, D.; Huffman, D.M.; Nagy, T.R. Relationships between rodent white adipose fat pads and human white adipose fat depots. Front. Nutr. 2016, 3, 1–12. [Google Scholar] [CrossRef]
- Gariepy, G.; Nitka, D.; Schmitz, N. The association between obesity and anxiety disorders in the population: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 407–419. [Google Scholar] [CrossRef]
- Rajan, T.M.; Menon, V. Psychiatric disorders and obesity: A review of association studies. J. Postgrad Med. 2017, 63, 182–190. [Google Scholar] [CrossRef]
- Amiri, S.; Behnezhad, S. Obesity and anxiety symptoms: A systematic review and meta-analysis. Neuropsychiatr 2019, 33, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Loeber, S.; Herpertz, S. Personality disorders and obesity: A systematic review. Obes. Rev. 2016, 17, 691–723. [Google Scholar] [CrossRef] [PubMed]
- Geiker, N.R.W.; Astrup, A.; Hjorth, M.F.; Sjödin, A.-; Pijls, L.; Markus, C.R. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and viceversa? Obes. Rev. 2018, 19, 81–97. [Google Scholar] [CrossRef]
- Barry, D.; Pietrzak, R.H.; Petry, N.M. Gender differences in associations between body mass index and DSM-IV mood and anxiety disorders: Results from the National Epidemiologic Survey on alcohol and related conditions. Ann. Epidemiol. 2008, 18, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Jaremka, L.M.; Pacanowski, C.R. Social anxiety symptoms moderate the link between obesity and metabolic function. Psychoneuroendocrinology 2019, 110, 1–7. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Ruiz-Padilla, A.J.; Ramírez-Morales, M.A.; Alcocer-García, S.G.; Ruiz-Noa, Y.; Ibarra-Reynoso, L.D.R.; Solorio-Alvarado, C.R.; Zapata-Morales, J.R.; Mendoza-Macias, C.L.; Deveze-Álvarez, M.A.; et al. Self-treatment with herbal products for weight-loss among overweight and obese subjects from central Mexico. J. Ethnopharmacol. 2019, 234, 21–26. [Google Scholar] [CrossRef]
- Mori, S.A.; Prance, G.T. Taxonomy, ecology, and economic botany of the Brazil nut (Bertholletia excelsa Humb. & Bonpl.: Lecythidaceae). Adv. Econ. Bot 1990, 8, 130–150. [Google Scholar]






| Treatment | Dose (mg/kg) | Latency (min) | Duration of the Hypnosis (min) | |
|---|---|---|---|---|
| Sedation | Hypnosis | |||
| SP + VH | - | 1.22 ± 0.04 | 3.19 ± 0.21 | 17.92 ± 04.19 |
| SP + SBHX | 0.1 | 1.10 ± 0.08 * | 1.98 ± 0.12 | 40.80 ± 03.27 * |
| SP + SBHX | 3 | 1.23 ± 0.14 | 3.32 ± 0.25 | 64.32 ± 06.47 * |
| SP + SBHX | 10 | 1.05 ± 0.09 | 2.65 ± 0.22 | 74.04 ± 17.91 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frausto-González, O.; Bautista, C.J.; Narváez-González, F.; Hernandez-Leon, A.; Estrada-Camarena, E.; Rivero-Cruz, F.; González-Trujano, M.E. Bertholletia excelsa Seeds Reduce Anxiety-Like Behavior, Lipids, and Overweight in Mice. Molecules 2021, 26, 3212. https://doi.org/10.3390/molecules26113212
Frausto-González O, Bautista CJ, Narváez-González F, Hernandez-Leon A, Estrada-Camarena E, Rivero-Cruz F, González-Trujano ME. Bertholletia excelsa Seeds Reduce Anxiety-Like Behavior, Lipids, and Overweight in Mice. Molecules. 2021; 26(11):3212. https://doi.org/10.3390/molecules26113212
Chicago/Turabian StyleFrausto-González, Oswaldo, Claudia J. Bautista, Fernando Narváez-González, Alberto Hernandez-Leon, Erika Estrada-Camarena, Fausto Rivero-Cruz, and María Eva González-Trujano. 2021. "Bertholletia excelsa Seeds Reduce Anxiety-Like Behavior, Lipids, and Overweight in Mice" Molecules 26, no. 11: 3212. https://doi.org/10.3390/molecules26113212
APA StyleFrausto-González, O., Bautista, C. J., Narváez-González, F., Hernandez-Leon, A., Estrada-Camarena, E., Rivero-Cruz, F., & González-Trujano, M. E. (2021). Bertholletia excelsa Seeds Reduce Anxiety-Like Behavior, Lipids, and Overweight in Mice. Molecules, 26(11), 3212. https://doi.org/10.3390/molecules26113212