Design of Chitosan Sterilization Agents by a Structure Combination Strategy and Their Potential Application in Crop Protection
Abstract
:1. Introduction
2. Structure Combination Strategy for Antimicrobial Chitosan Derivatives
2.1. Introduction of Active Groups Containing Sulfur and Phosphorus
2.2. Introduction of the Quaternary Ammonium Group
2.3. Introduction of Active Aldehydes or Ketones
2.4. Introduction of Metal Ions
2.5. Introduction of Alkyl
3. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leonhardt, E.E.; Kang, N.; Hamad, M.A.; Wooley, K.L.; Elsabahy, M. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Shu, G.; Jin, F.; Qi, J.; Xu, X.; Du, Y.; Yu, H.; Wang, J.; Sun, M.; You, Y.; et al. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci. Adv. 2020, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Abu Elella, M.H.; Mohamed, R.R.; Abdel-Aziz, M.M.; Sabaa, M.W. Green synthesis of antimicrobial and antitumor N,N,N-trimethyl chitosan chloride/poly (acrylic acid)/silver nanocomposites. Int. J. Biol. Macromol. 2018, 111, 706–716. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, L.; Li, L.; Zhang, T.; Li, M.; Mou, H. Eco-friendly preparation of chitooligosaccharides with different degrees of deacetylation from shrimp shell waste and their effects on the germination of wheat seeds. Marine Life Sci. Technol. 2019, 1, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Kim, J.-H.; Kalia, V.C.; Lee, J.-K. Antimicrobial activity of amino-derivatized cationic polysaccharides. Indian J. Microbiol. 2019, 59, 96–99. [Google Scholar] [CrossRef]
- Govindan, S.; Nivethaa, E.A.K.; Saravanan, R.; Narayanan, V.; Stephen, A. Synthesis and characterization of chitosan-silver nanocomposite. Appl. Nanosci. 2012, 2, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Guo, M.; Qian, R.; Liu, C.; Zong, X.; Li, Y.-Q.; Li, W. Binding efficacy and kinetics of chitosan with DNA duplex: The effects of deacetylation degree and nucleotide sequences. Carbohydr. Polym. 2017, 169, 451–457. [Google Scholar] [CrossRef]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Farahani, M.S.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of curcumin loaded chitosan nanoparticle within poly (epsilon-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Cazon, P.; Velazquez, G.; Ramirez, J.A.; Vazquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Coll. Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization and antifungal efficacy of C-coordinated O-carboxymethyl chitosan Cu(II) complexes. Carbohydr. Polym. 2017, 160, 97–105. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; Delattre, C.; de Baynast, H.; Grediac, M.; Mathias, J.-D.; Ursu, A.V.; Desbrieres, J.; Michaud, P. Alkyl-chitosan-based adhesive: Water resistance improvement. Molecules 2019, 24, 1987. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, S.; Deng, D.; Xiao, Z.; Dong, Z.; Wang, Z.; Lei, Q.; Gao, S.; Huang, G.; Zhang, E.; et al. Fluorinated chitosan to enhance transmucosal delivery of sonosensitizer- conjugated catalase for sonodynamic bladder cancer treatment post-intravesical instillation. ACS Nano 2020, 14, 1586–1599. [Google Scholar] [CrossRef]
- Tong, T.; Li, Y.; Hou, R.; Wang, X.; Wang, S. Decoration of chitosan microspheres with Bronsted heteropolyacids and Lewis ion Ti: Trifunctional catalysts for esterification to biodiesel. RSC Adv. 2017, 7, 42422–42429. [Google Scholar] [CrossRef] [Green Version]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, F.; Wu, Z.; Jin, J.; Li, F.; Wang, Y.; Wang, Z.; Tang, S.; Wu, C.; Wang, Y. O-acylation of chitosan nanofibers by short-chain and long-chain fatty acids. Carbohydr. Polym. 2017, 177, 203–209. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Chen, S.; Yu, H.; Liu, D.; Sun, W. Metal ion-coordinated carboxymethylated chitosan grafted carbon nanotubes with enhanced antibacterial properties. RSC Adv. 2016, 6, 39–43. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Li, Y.; Wei, Q.; Hu, L.; Yan, T.; Feng, R.; Yan, L.; Du, B. Phosphorylated chitosan/CoFe2O4 composite for the efficient removal of Pb(II) and Cd(II) from aqueous solution: Adsorption performance and mechanism studies. J. Mol. Liq. 2019, 277, 181–188. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Attjioui, M.; Ferreira, A.P.G.; Dockal, E.R.; El Gueddari, N.E.; Moerschbacher, B.M.; Cavalheiro, E.T.G. Synthesis, characterization and biological activities of biopolymeric schiff bases prepared with chitosan and salicylaldehydes and their Pd(II) and Pt(II) Complexes. Molecules 2017, 22, 1987. [Google Scholar] [CrossRef] [Green Version]
- Antony, R.; Arun, T.; Manickam, S.T.D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef]
- Rashid, S.; Shen, C.; Yang, J.; Liu, J.; Li, J. Preparation and properties of chitosan-metal complex: Some factors influencing the adsorption capacity for dyes in aqueous solution. J. Environ. Sci. 2018, 66, 301–309. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P. Antimicrobial chitosan conjugates: Current synthetic strategies and potential applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Eweis, M.; Elkholy, S.S.; Elsabee, M.Z. Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugar-beet pathogens. Int. J. Biol. Macromol. 2006, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, S.S.; Salem, H.A.; Eweis, M.; Elsabee, M.Z. Synthesis and characterization of some acyl thiourea derivatives of chitosan and their biocidal activities. Int. J. Biol. Macromol. 2014, 70, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Z.; Xiu, L.; Huang, J.; Zhou, F. Selective antifungal activity of chitosan and sulfonated chitosan against postharvest fungus isolated from blueberry. J. Food Biochem. 2018, 42, e12658. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A. Preparation and antimicrobial activity of some carboxymethyl chitosan acyl thiourea derivatives. Int. J. Biol. Macromol. 2012, 50, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis and antimicrobial activity of some novel terephthaloyl thiourea cross-linked carboxymethyl chitosan hydrogels. Cellulose 2012, 19, 1879–1891. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Fahmy, M.M. Synthesis and antimicrobial activity of some novel cross-linked chitosan hydrogels. Int. J. Mol. Sci. 2012, 13, 11194–11209. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Al-mehbad, N.Y. Novel terephthaloyl thiourea cross-linked chitosan hydrogels as antibacterial and antifungal agents. Int. J. Biol. Macromol. 2013, 57, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Mohamed, R.R.; Seoudi, R.S. Synthesis and characterization of some novel antimicrobial thiosemicarbazone O-carboxymethyl chitosan derivatives. Int. J. Biol. Macromol. 2014, 63, 163–169. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A.; Fahmy, M.M. Novel antimicrobial superporous cross-linked chitosan/pyromellitimide benzoyl thiourea hydrogels. Int. J. Biol. Macromol. 2016, 82, 589–598. [Google Scholar] [CrossRef]
- Zhong, Z.; Xing, R.; Liu, S.; Wang, L.; Cai, S.; Li, P. Synthesis of acyl thiourea derivatives of chitosan and their antimicrobial activities in vitro. Carbohydr. Res. 2008, 343, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Aotegen, B.; Xu, H. The influence of the different inductivity of acetyl phenyl-thiosemicarbazone-chitosan on antimicrobial activities. Int. J. Biol. Macromol. 2011, 48, 713–719. [Google Scholar] [CrossRef]
- Qin, Y.; Xing, R.; Liu, S.; Li, K.; Meng, X.; Li, R.; Cui, J.; Li, B.; Li, P. Novel thiosemicarbazone chitosan derivatives: Preparation, characterization, and antifungal activity. Carbohydr. Polym. 2012, 87, 2664–2670. [Google Scholar] [CrossRef]
- Qin, Y.; Xing, R.; Liu, S.; Li, K.; Hu, L.; Yu, H.; Chen, X.; Li, P. Synthesis of chitosan derivative with diethyldithiocarbamate and its antifungal activity. Int. J. Biol. Macromol. 2014, 65, 369–374. [Google Scholar] [CrossRef]
- Zhong, Z.; Aotegen, B.; Xu, H.; Zhao, S. The influence of chemical structure on the antimicrobial activities of thiosemicarbazone-chitosan. Cellulose 2014, 21, 105–114. [Google Scholar] [CrossRef]
- Zhong, Z.; Aotegen, B.; Xu, H.; Zhao, S. Structure and antimicrobial activities of benzoyl phenyl-thiosemicarbazone-chitosans. Int. J. Biol. Macromol. 2012, 50, 1169–1174. [Google Scholar] [CrossRef]
- Arvand, M.; Mirroshandel, A.A. Highly-sensitive aptasensor based on fluorescence resonance energy transfer between L-cysteine capped ZnS quantum dots and graphene oxide sheets for the determination of edifenphos fungicide. Biosens. Bioelectron. 2017, 96, 324–331. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, T.; Xu, D.; Wang, S.; Yuan, Y.; He, S.; Cao, Y. The removal of pesticide residues from pakchoi (Brassica rape L. ssp chinensis) by ultrasonic treatment. Food Control 2019, 95, 176–180. [Google Scholar] [CrossRef]
- Devarayan, K.; Sathishkumar, Y.; Lee, Y.S.; Kim, B.-S. Effect of microgravity on fungistatic activity of an alpha-aminophosphonate chitosan derivative against Aspergillus niger. PLoS ONE 2015, 10, e0139303. [Google Scholar] [CrossRef]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization, and antifungal evaluation of diethoxyphosphoryl polyaminoethyl chitosan derivatives. Carbohydr. Polym. 2018, 190, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Li, P.; Xing, R.; Chen, X.; Liu, S. Preparation, characterization and antifungal properties of 2-(alpha-arylamino phosphonate)-chitosan. Int. J. Biol. Macromol. 2009, 45, 255–259. [Google Scholar] [CrossRef]
- Qin, Y.; Xing, R.; Liu, S.; Yu, H.; Li, K.; Hu, L.; Li, P. Synthesis and antifungal properties of (4-tolyloxy)-pyrimidyl-alpha-aminophosphonates chitosan derivatives. Int. J. Biol. Macromol. 2014, 63, 83–91. [Google Scholar] [CrossRef]
- Curti, E.; de Britto, D.; Campana, S.P. Methylation of chitosan with iodomethane: Effect of reaction conditions on chemoselectivity and degree of substitution. Macromol. Biosci. 2003, 3, 571–576. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Synthesis and characterization of N,N,N-trimethyl-O-(ureidopyridinium)acetyl chitosan derivatives with antioxidant and antifungal activities. Marine Drugs 2020, 18, 163. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization, and anti-phytopathogen evaluation of 6-oxychitosan derivatives containing N-quaternized moieties in its backbone. Int. J. Polym. Sci. 2018, 2018. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. Synthesis and antifungal property of N-(aryl) and quaternary N-(aryl) chitosan derivatives against Botrytis cinerea. Cellulose 2014, 21, 3121–3137. [Google Scholar] [CrossRef]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization and antifungal efficacy of chitosan derivatives with triple quaternary ammonium groups. Int. J. Biol. Macromol. 2018, 114, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wu, H.; Li, S.; Tian, H.; Li, Y.; Wang, J. Homogeneous synthesis of cationic chitosan via new avenue. Molecules 2018, 23, 1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Han, Q.; Zhang, F.; Meng, X.; Liu, B. Preparation, characterization and antibacterial properties of 6-deoxy-6-arginine modified chitosan. Carbohydr. Polym. 2020, 230, 115635. [Google Scholar] [CrossRef]
- Rabea, E.I.; El Badawy, M.; Rogge, T.M.; Stevens, C.V.; Hofte, M.; Steurbaut, W.; Smagghe, G. Insecticidal and fungicidal activity of new synthesized chitosan derivatives. Pest Manag. Sci. 2005, 61, 951–960. [Google Scholar] [CrossRef]
- Rabea, E.I.; El Badawy, M.; Rogge, T.M.; Stevens, C.V.; Steurbaut, W.; Hofte, M.; Smagghe, G. Enhancement of fungicidal and insecticidal activity by reductive alkylation of chitosan. Pest Manag. Sci. 2006, 62, 890–897. [Google Scholar] [CrossRef]
- Jagadish, R.S.; Divyashree, K.N.; Viswanath, P.; Srinivas, P.; Raj, B. Preparation of N-vanillyl chitosan and 4-hydroxybenzyl chitosan and their physico-mechanical, optical, barrier, and antimicrobial properties. Carbohydr. Polym. 2012, 87, 110–116. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. Synthesis and structure-activity relationship of N-(cinnamyl) chitosan analogs as antimicrobial agents. Int. J. Biol. Macromol. 2013, 57, 185–192. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Chen, R.; Xing, R.E.; Liu, S.; Yu, H.H.; Wang, P.B.; Li, C.P.; Li, P.C. Novel derivatives of chitosan and their antifungal activities in vitro. Carbohydr. Res. 2006, 341, 351–354. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A. Novel aminohydrazide cross-linked chitosan filled with multi-walled carbon nanotubes as antimicrobial agents. Int. J. Biol. Macromol. 2018, 115, 651–662. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Elzanaty, A.M.; Abdel-Gawad, O.F.; Arafa, E.G. Synthesis, characterization and antimicrobial activity of Schiff bases modified chitosan-graft-poly(acrylonitrile). Int. J. Biol. Macromol. 2018, 109, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dutta, J.; Dutta, P.K. Preparation and characterization of N-heterocyclic chitosan derivative based gels for biomedical applications. Int. J. Biol. Macromol. 2009, 45, 330–337. [Google Scholar] [CrossRef]
- Wei, L.; Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Synthesis, characterization, and antifungal activity of Schiff bases of inulin bearing pyridine ring. Polymers 2019, 11, 371. [Google Scholar] [CrossRef] [Green Version]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual crosslinked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Abdel-Monem, R.A.; Darwesh, O.M.; Hashim, A.I.; Nada, A.A.; Rabie, S.T. Synthesis, characterization, and evaluation of antimicrobial activities of chitosan and carboxymethyl chitosan Schiff-base/silver nanoparticles. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Hui, L.; Ying, H. Chitosan-metal complexes as antimicrobial agent: Synthesis, characterization and structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Mekahlia, S.; Bouzid, B. Chitosan-Copper (II) complex as antibacterial agent: Synthesis, characterization and coordinating bond- activity correlation study. Phys. Proc. 2009, 2, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Du, Y.M.; Liu, H. Preparation, characterization and antimicrobial activity of chitosan-Zn complex. Carbohydr. Polym. 2004, 56, 21–26. [Google Scholar] [CrossRef]
- Khan, A.; Mehmood, S.; Shafiq, M.; Yasin, T.; Akhter, Z.; Ahmad, S. Structural and antimicrobial properties of irradiated chitosan and its complexes with zinc. Radiat. Phys. Chem. 2013, 91, 138–142. [Google Scholar] [CrossRef]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. C-coordinated O-carboxymethyl chitosan metal complexes: Synthesis, characterization and antifungal efficacy. Int. J. Biol. Macromol. 2018, 106, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis of C-coordinated O-carboxymethyl chitosan metal complexes and evaluation of their antifungal activity. Sci. Rep. 2018, 8, 4845. [Google Scholar] [CrossRef] [PubMed]
- Quiterio-Gutierrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernandez-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juarez-Maldonado, A. The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubina, M.S.; Vasil’kov, A.Y.; Naumkin, A.V.; Shtykova, E.V.; Abramchuk, S.S.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Synthesis and characterization of chitosan-copper nanocomposites and their fungicidal activity against two sclerotia-forming plant pathogenic fungi. J. Nanostruct. Chem. 2017, 7, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Vanti, G.L.; Masaphy, S.; Kurjogi, M.; Chakrasali, S.; Nargund, V.B. Synthesis and application of chitosan-copper nanoparticles on damping off causing plant pathogenic fungi. Int. J. Biol. Macromol. 2020, 156, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.F.; Youssef, K.; Abd-Elsalam, K.A. Ecofriendly nanomaterials for controlling gray mold of table grapes and maintaining postharvest quality. Eur. J. Plant. Pathol. 2019, 154, 377–388. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Vasil’kov, A.Y.; Said-Galiev, E.E.; Rubina, M.S.; Khokhlov, A.R.; Naumkin, A.V.; Shtykova, E.V.; Alghuthaymi, M.A. Bimetallic blends and chitosan nanocomposites: Novel antifungal agents against cotton seedling damping-off. Eur. J. Plant. Pathol. 2018, 151, 57–72. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Pei, L.; Cai, Z.; Shang, S.; Song, Z. Preparation and application of chitosan derivatives PEI. Chem. Reagents 2013, 35, 897–903. [Google Scholar]
- Li, Y.; Liu, S.; Qin, Y.; Xing, R.; Chen, X.; Li, K.; Li, P. Synthesis of novel pyrimethanil grafted chitosan derivatives with Enhanced antifungal activity. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
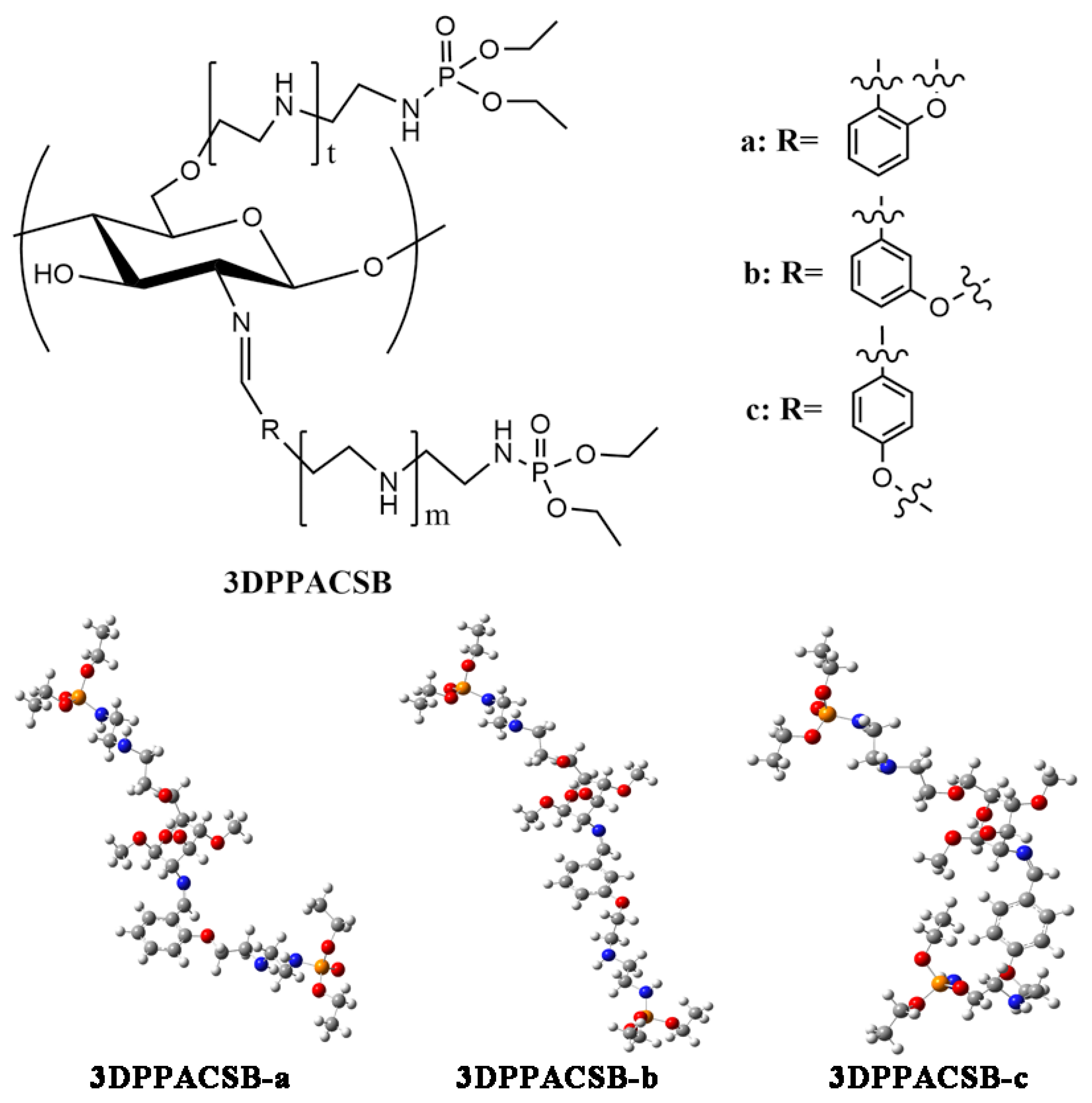
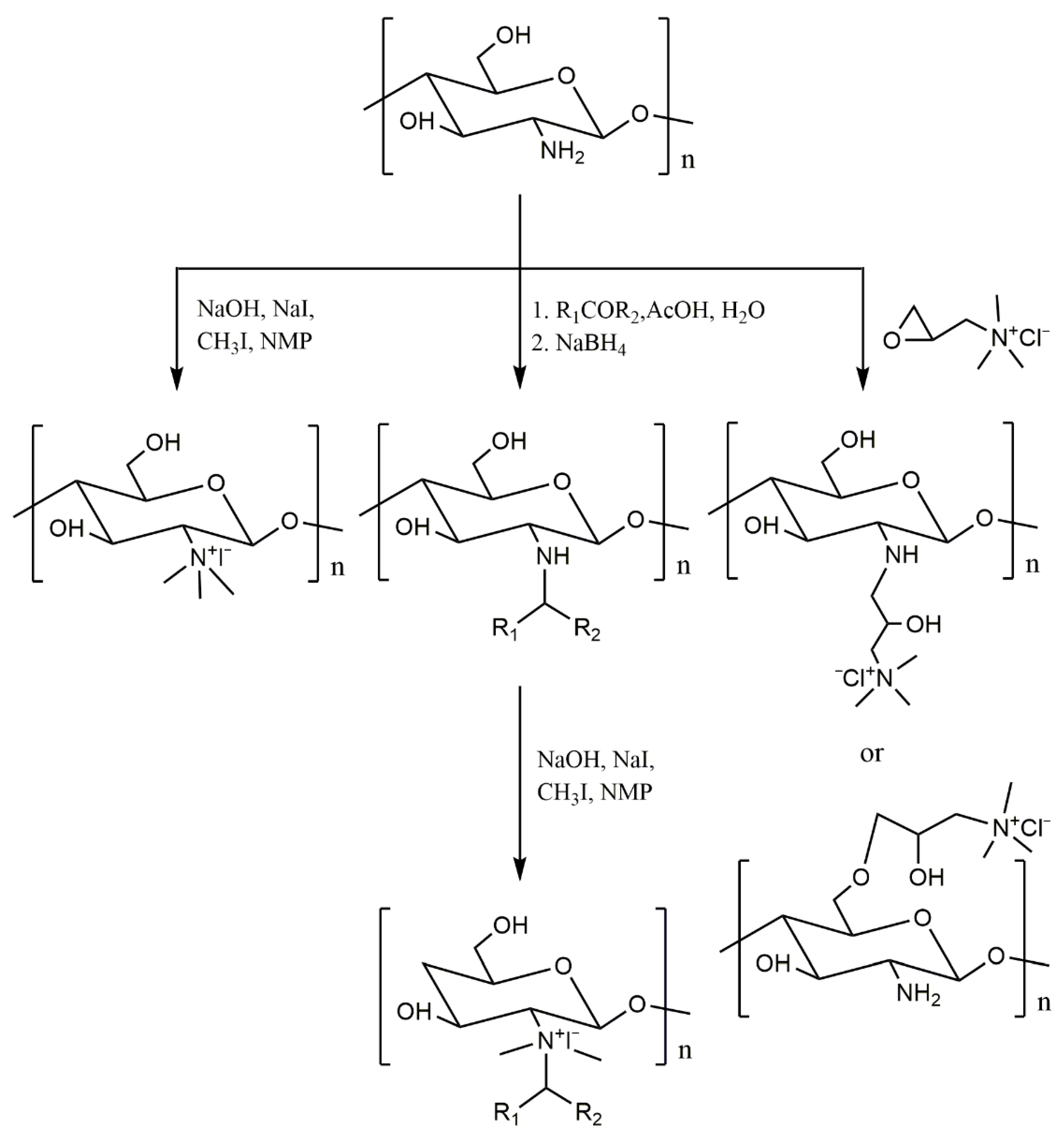
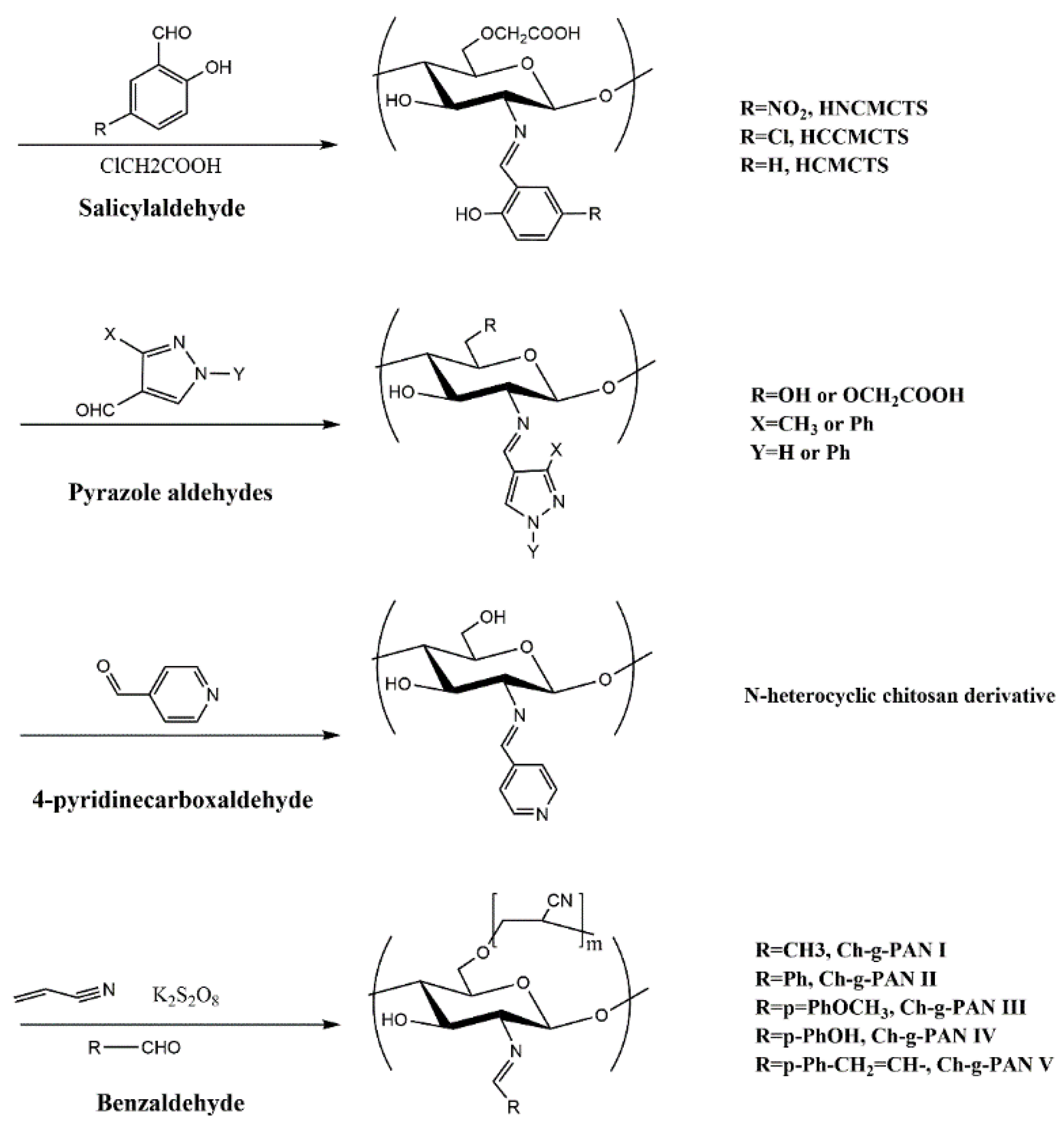
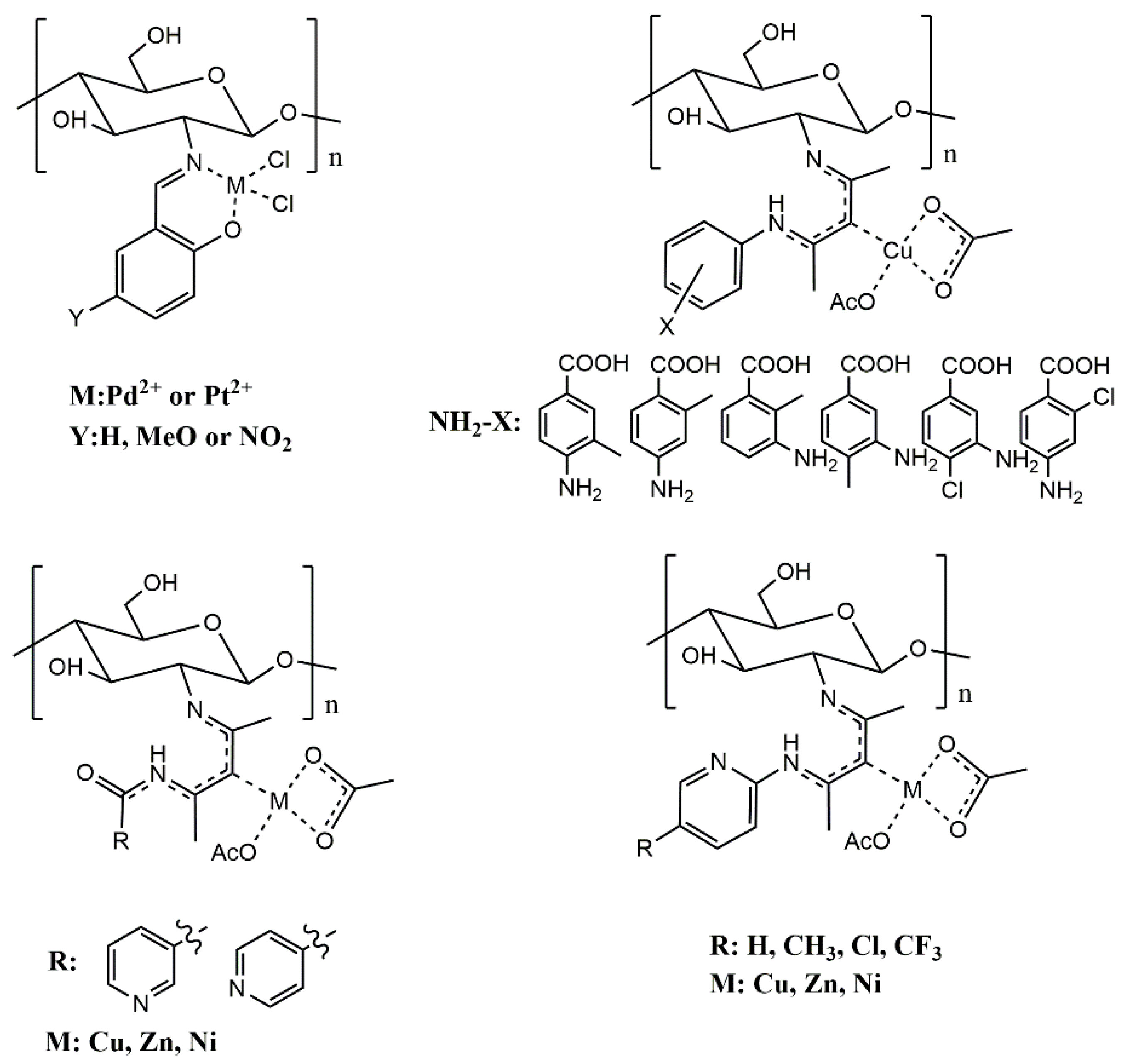

| Sample | Molecular Weight | Sulfur Content | Grafting Degree | Antifungal Activity a | References |
|---|---|---|---|---|---|
| CATUCS | 50 kDa | 9.83% | 91.5% | Colletotrichum gloeosporioides (Penz.) Saec (67%) at 500 μg/mL in vitro | [39] |
| A(p-T)TSCZCS | 200 kDa | 7.44% | 35.3% | Aiternaria solani (93%) at 500 μg/mL in vitro | [40] |
| CATUCMCS | 200 kDa | 7.56% | 88.88% | Aspergillus fumigate (MIC: 7.8 μg/mL) in vitro | [33] |
| o-HPHTCNCS | 230 kDa | 0.81% | 8.62% | Rhizoctonia solani Kühn (100%) at 500 μg/mL in vitro | [41] |
| CA(o-MP)TSCZCS | 200 kDa | 6.73% | 33.7% | Alternaria Solani (82.35%) at 500 μg/mL in vitro | [43] |
| PIBTU-CS-4 | 200 kDa | 7.00% | 88% | Geotricum candidum (MIC: 3.9 μg/mL) in vitro | [38] |
| Sample | Method | Target Fungi | Antifungal Activity a | References |
|---|---|---|---|---|
| N-(p-dimethylaminobenzyl)-dimethyl-α-aminophosphonate chitosan | Radial hyphal growth bioassay in vitro | Aspergillus niger | 76% at 500 μg/mL | [47] |
| Diethoxyphosphoryl polyaminoethyl chitosan Schiff bases | Plate growth rate method in vitro | Phytophthora capsici Leonian | 89% at 0.8 mg/mL | [48] |
| 2-(α-arylamino phosphonate)-chitosan | Plate growth rate method in vitro | Fusarium oxysporum f. sp. vasinfectum | 95.24% at 500 μg/mL | [49] |
| 4-(pyrimidyl-2-yloxy)benzaldehyde and (4-tolyloxy)-pyrimidyl-α-aminophosphonates chitosan | Mycelium growth rate test in vitro | Fusarium oxysporum, Phomopsis asparagi (Sacc.) | 100% at 250 μg/mL | [50] |
| Sample | Method | Target Fungi | Antifungal Activity a | References |
|---|---|---|---|---|
| Chitosan derivatives with triple quaternary ammonium groups | Mycelium growth rate test in vitro | Phytophthora capsici, Rhizoctonia solani | 91.94% against P. capsici and 87.18% against R. solani at 0.8 mg/mL | [55] |
| N,N,N-(diethyl-p-dimethylaminobenzyl) chitosan | Detached leaf method in vivo | Botrytis cinerea | 100% at 1 mg/mL | [54] |
| Quaternized 6-oxychitosan derivatives | Plate growth rate method in vitro | Verticillium albo-atrum | 89.1% at 0.4 μg/mL | [53] |
| N,N,N-trimethyl-O-(ureidopyridinium)acetyl chitosan derivatives | Mycelium growth rate test in vitro | Phomopsis asparagus | 80.65% at 1 mg/mL | [52] |
| Sample | Method | Target Fungi | Antifungal Activity a | References |
|---|---|---|---|---|
| 2-(2-hydroxybenzylideneamino)-6-carboxymethyl-chitosan | Mycelium growth rate test in vitro | Valsa mali | 83% at 500 μg/mL | [62] |
| Chitosan-graft-poly(acrylonitrile) Schiff bases | Filter paper diffusion method in vitro | Geotricum candidum | MIC: 1.95 μg/mL | [64] |
| O-carboxymethyl chitosan Schiff base | Plate growth rate method in vitro | Phytophthora capsici | 49.8% at 0.2 mg/mL | [16] |
| Sample | Method | Target Fungi | Antifungal Activity a | References |
|---|---|---|---|---|
| Chitosan-zinc complexes | Mycelium growth rate test in vitro | Aspergallious fumigatus, Fusarium solani | No growth after two weeks | [72] |
| Chitosan ortho-hydroxyaryl Schiff base palladium complex | Colorimetric method in vitro | Pseudomonas syringae pv. tomato | MIC: 25 μg/mL IC50: 7 μg/mL | [25] |
| O-carboxymethyl aminobenzoic acid chitosan Schiff base Cu complex | Plate growth rate method in vitro | Phytophthora capsici | 100% at 0.2 mg/mL | [16] |
| O-carboxymethyl nicotinamide chitosan Schiff base metal complexes | Plate growth rate method in vitro | Phytophthora capsici, Botrytis cinerea | 100% at 0.2 mg/mL | [73] |
| O-carboxymethyl aminopyridine chitosan Schiff base metal complexes | Pot experiment in vivo | Phytophthora capsici | 85.56% for protective efficacy and 74.41% for curative efficacy at 0.8 mg/mL | [74] |
| Copper-carrying chitosan nanocomposites | Agar medium assay in vitro | Sclerotium rolfsii | 100% at 0.1 mg/mL | [76] |
| Chitosan-coated copper nanoparticles | Mycelium growth rate test in vitro | Rhizoctonia solani, Pythium aphanidermatum | 94.3% against R. solani and 98.3% against P. aphanidermatum at 0.1% | [77] |
| Copper-silica-chitosan nanoparticles | Plate growth rate method in vitro | Botrytis cinerea | 43% at 4 mg/mL | [78] |
| Cu-chitosan nanocomposites | Mycelium growth rate test in vitro | Curvularia lunata | 52.7% at 0.16% | [80] |
| Sample | Method | Target Fungi | Antifungal Activity a | References |
|---|---|---|---|---|
| N-(2,6-dichlorobenzyl)chitosan | Plate growth rate method in vitro | Botrytis cinerea Pers | EC50: 0.52 g/L | [58] |
| N-(2,2-diphenylethyl)chitosan | Plate growth rate method in vitro | Botrytis cinerea Pers | EC50: 0.031 g/L | [59] |
| N-vanillyl chitosan | Direct exposure method in vitro | Aspergillus flavus | 98.9% | [60] |
| N-(cinnamyl) chitosan analogs | Mycelial radial growth technique in vitro | Phytophthora infestans | EC50: 636 mg/L | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Qin, Y.; Li, P. Design of Chitosan Sterilization Agents by a Structure Combination Strategy and Their Potential Application in Crop Protection. Molecules 2021, 26, 3250. https://doi.org/10.3390/molecules26113250
Liu W, Qin Y, Li P. Design of Chitosan Sterilization Agents by a Structure Combination Strategy and Their Potential Application in Crop Protection. Molecules. 2021; 26(11):3250. https://doi.org/10.3390/molecules26113250
Chicago/Turabian StyleLiu, Weixiang, Yukun Qin, and Pengcheng Li. 2021. "Design of Chitosan Sterilization Agents by a Structure Combination Strategy and Their Potential Application in Crop Protection" Molecules 26, no. 11: 3250. https://doi.org/10.3390/molecules26113250
APA StyleLiu, W., Qin, Y., & Li, P. (2021). Design of Chitosan Sterilization Agents by a Structure Combination Strategy and Their Potential Application in Crop Protection. Molecules, 26(11), 3250. https://doi.org/10.3390/molecules26113250






