Anti-Inflammatory, Antiallergic, and COVID-19 Main Protease (Mpro) Inhibitory Activities of Butenolides from a Marine-Derived Fungus Aspergillus terreus
Abstract
1. Introduction
2. Results and Discussion
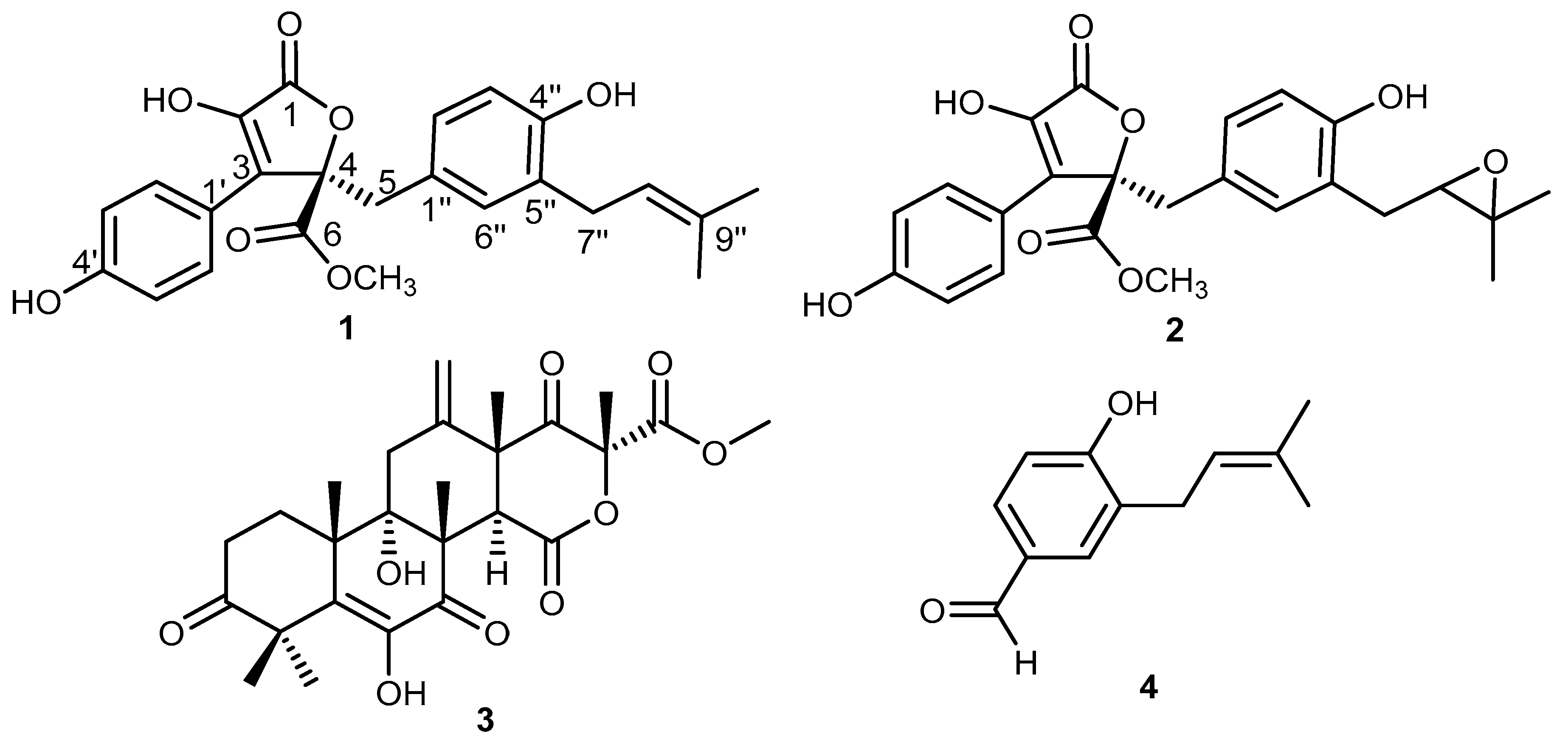
2.1. Isolation and Characterization of Main Secondary Metabolites in the Fungal Extract
2.2. Degranulation Assay in Mast Cells
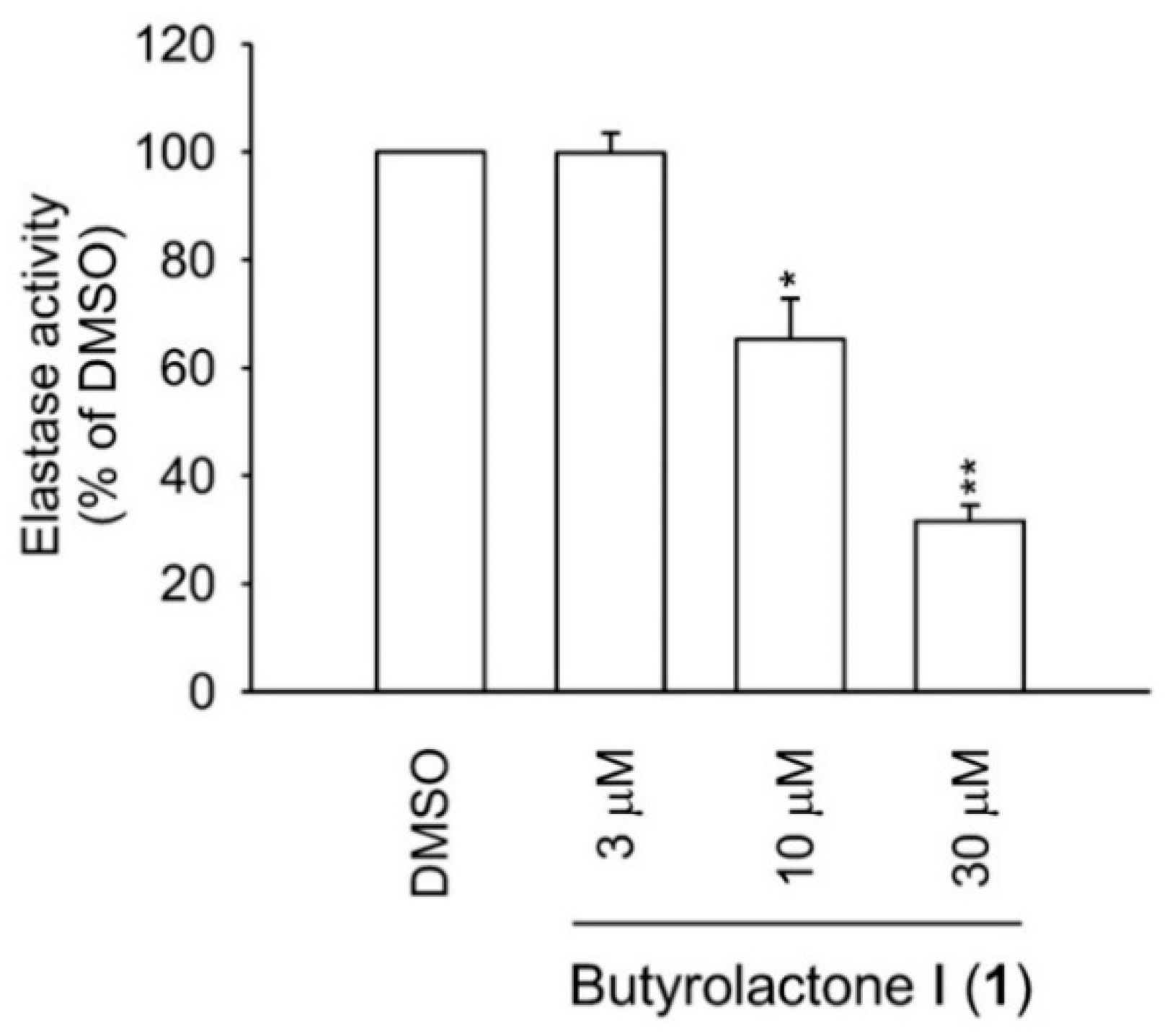
2.3. Human Neutrophil Viability, Elastase Release, and Elastase Enzymatic Assays
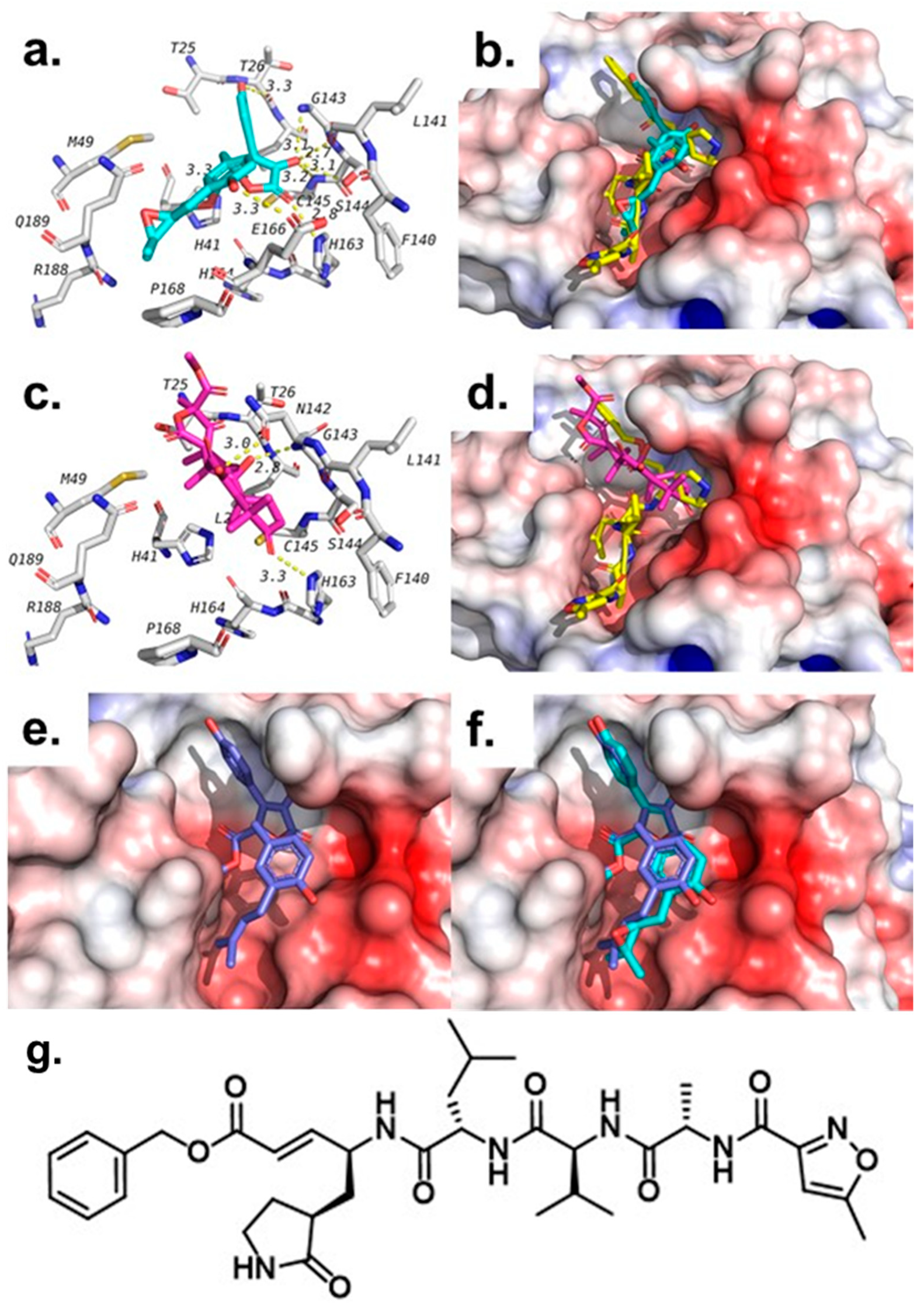
2.4. Molecular Docking Studies
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Sponge and Fungal Strain Material
3.3. Fermentation, Extraction, and Isolation
3.4. Degranulation Assay and MTT Cell Viability Assay in Mast Cells
3.5. Elastase Release Assay and Lactate Dehydrogenase (LDH) Viability Assay by Human Neutrophils
3.6. Determination of Elastase Enzymatic Activity
3.7. Molecular Modeling Studies
3.8. Coronavirus 229E Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 108, 102433. [Google Scholar] [CrossRef]
- da Silva, P.G.; Mesquita, J.R.; Nascimento, M.D.J.; Ferriera, V.A.M. Viral, host and environmental factors that favour anthropozoonotic spillover of coronaviruses: An opinionated review, focusing on SARS-CoV, MERS-CoV and SARS-CoV-2. Sci. Total Environ. 2021, 750, 141483. [Google Scholar] [CrossRef]
- Pfizer-BioNtech COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine (accessed on 4 January 2021).
- COVID-19: Oxford-AstraZeneca Vaccine Approved for Use in UK. Available online: https://www.bbc.com/news/health-55280671 (accessed on 4 January 2021).
- Risk Assessment: Risk related to Spread of New SARS-CoV-2 Variants of Concern in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-risk-assessment-spread-new-sars-cov-2-variants-eueea (accessed on 4 January 2021).
- Henriksen, P.A. The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr. Opin. Hematol. 2014, 21, 23–28. [Google Scholar] [CrossRef]
- Hoenderdos, K.; Condliffe, A. The neutrophil in chronic obstructive pulmonary disease. Am. J. Respir. Cell. Mol. Biol. 2013, 48, 531–539. [Google Scholar] [CrossRef]
- Moraes, T.J.; Chow, C.W.; Downey, G.P. Proteases and lung injury. Crit. Care Med. 2003, 31, S189–S194. [Google Scholar] [CrossRef]
- Chiang, C.-C.; Korinek, M.; Cheng, W.-J.; Hwang, T.-L. Targeting neutrophils to treat acute respiratory distress syndrome in coronavirus disease. Front. Pharmacol. 2020, 11, 572009. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, J.; Jiang, N.; Lu, Y.H.; Wang, L.; Xu, S.H.; Wang, W.; Zhang, G.F.; Xu, Q.; Ge, H.M.; et al. Immunosuppressive polyketides from mantis-associated Daldinia eschscholzii. J. Am. Chem. Soc. 2011, 133, 5931–5940. [Google Scholar] [CrossRef]
- Ebada, S.S.; El-Neketi, M.; Ebrahim, W.; Mándi, A.; Kurtán, T.; Kalscheuer, R.; Müller, W.E.G.; Proksch, P. Cytotoxic secondary metabolites from the endophytic fungus Aspergillus versicolor KU258497. Phytochem. Lett. 2018, 24, 88–93. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.K.; Liu, P.P.; Wang, W.; Zhu, W.M. Three new compounds from Aspergillus terreus PT06-2 grown in a high salt medium. Mar. Drugs 2011, 9, 1368–1378. [Google Scholar] [CrossRef]
- Liao, W.Y.; Shen, C.N.; Lin, L.H.; Yang, Y.L.; Han, H.Y.; Chen, J.W.; Kuo, S.C.; Wu, S.H.; Liaw, C.C. Asperjinone, a Nor-Neolignan, and terrein, a suppressor of ABCG2-expressing breast cancer cells, from thermophilic Aspergillus terreus. J. Nat. Prod. 2012, 75, 630–635. [Google Scholar] [CrossRef]
- Deng, C.M.; Huang, C.H.; Wu, Q.L.; Pang, J.Y.; Lin, Y.C. A new sesquiterpene from the mangrove endophytic fungus Aspergillus terreus (No. GX7-3B). Nat. Prod. Res. 2013, 27, 1882–1887. [Google Scholar] [CrossRef]
- Wijeratne, E.M.K.; Turbyville, T.J.; Zhang, Z.G.; Bigelow, D.; Pierson, L.S.; VanEtten, H.D.; Whitesell, L.; Canfield, L.M.; Gunatilaka, A.A.L. Cytotoxic constituents of Aspergillus terreus from the rhizosphere of Opuntia versicolor of the Sonoran Desert. J. Nat. Prod. 2003, 66, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Ebrahim, W. A new antibacterial quinolone derivative from the endophytic fungus Aspergillus versicolor strain Eich.5.2.2. S. Afr. J. Bot. 2020, 134, 151–155. [Google Scholar] [CrossRef]
- Zhou, M.; Miao, M.M.; Du, G.; Li, X.N.; Shang, S.Z.; Zhao, W.; Liu, Z.H.; Yang, G.Y.; Che, C.T.; Hu, Q.F.; et al. Aspergillines A-E, highly oxygenated hexacyclic indole-tetrahydrofuran-tetramic acid derivatives from Aspergillus versicolor. Org. Lett. 2014, 16, 5016–5019. [Google Scholar] [CrossRef]
- Gao, H.Q.; Zhou, L.N.; Cai, S.X.; Zhang, G.J.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Diorcinols B-E, new prenylated diphenyl ethers from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Antibiot. 2013, 66, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.Y.; Liu, X.H.; Miao, F.P.; Qiao, M.F. Aspeverin, a new alkaloid from an algicolous strain of Aspergillus versicolor. Org. Lett. 2013, 15, 2327–2329. [Google Scholar] [CrossRef]
- Wang, W.; Kim, H.; Nam, S.J.; Rho, B.J.; Kang, H. Antibacterial butenolides from the Korean tunicate Pseudodistoma antinboja. J. Nat. Prod. 2012, 75, 2049–2054. [Google Scholar] [CrossRef]
- Bai, Z.Q.; Lin, X.P.; Wang, Y.Z.; Wang, J.F.; Zhou, X.F.; Yang, B.; Liu, J.; Yang, X.W.; Wang, Y.; Liu, Y.H. New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia 2014, 95, 194–202. [Google Scholar] [CrossRef]
- Qin, J.J.; Zhu, J.X.; Zeng, Q.; Cheng, X.R.; Zhu, Y.; Zhang, S.D.; Shan, L.; Jin, H.Z.; Zhang, W.D. Pseudoguaianolides and guaianolides from Inula hupehensis as potential anti-inflammatory agents. J. Nat. Prod. 2011, 74, 1881–1887. [Google Scholar] [CrossRef]
- Dewi, R.T.; Tachibana, S.; Darmawan, A. Effect on a-glucosidase inhibition and antioxidant activities of butyrolactone derivatives from Aspergillus terreus MC751. Med. Chem. Res. 2014, 23, 454–460. [Google Scholar] [CrossRef]
- Lin, X.; Li, K.; Yang, L.; Peng, X.; Fang, W.; Tian, X.; Liu, Y.; Zhou, X. Dereplication and targeted isolation of bioactive Sulphur compounds from bacteria isolated from a hydrothermal field. Nat. Prod. Res. 2019, 33, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, N.; Nitta, K.; Sakaguchi, Y.; Taguchi, Y.; Yamamoto, Y. Studies on the metabolic products of Aspergillus terreus. III. Metabolites of the strain IFO 8835. Chem. Pharm. Bull. 1977, 25, 2593–2601. [Google Scholar] [CrossRef]
- Rao, K.V.; Sadhukhan, A.K.; Veerender, M.; Ravikumar, V.; Mohan, E.V.S.; Dhanvantri, S.D.; Sitaramkumar, M.; Babu, J.M.; Vyas, K.; Reddy, G.O. Butyrolactones from Aspergillus terreus. Chem. Pharm. Bull. 2000, 48, 559–562. [Google Scholar] [CrossRef]
- Niu, X.; Dahse, H.-M.; Menzel, K.-D.; Lozach, O.; Walther, G.; Meijer, L.; Grabley, S.; Sattler, I. Butyrolactone I derivatives from Aspergillus terreus carrying an unusual sulfate moiety. J. Nat. Prod. 2008, 71, 689–692. [Google Scholar] [CrossRef]
- Nitta, K.; Fujita, N.; Yoshimura, T.; Arai, K.; Yamamoto, Y. Metabolic products of Aspergillus terreus. IX. Biosynthesis of butyrolactone derivatives isolated from strains IFO 8835 and 4100. Chem. Pharm. Bull. 1983, 31, 1528–1533. [Google Scholar] [CrossRef]
- Chen, M.; Wang, K.-L.; Liu, M.; She, Z.-G.; Wang, C.-Y. Bioactive steroid derivatives and butryolactone derivatives from a gorgonian-derived fungus Aspergillus sp. fungus. Chem. Biodivers. 2015, 12, 1398–1406. [Google Scholar] [CrossRef]
- Springer, J.P.; Dorner, J.W.; Cole, R.J.; Cox, R.H. Terretonin, a toxic compound from Aspergillus terreus. J. Org. Chem. 1979, 44, 4852–4854. [Google Scholar] [CrossRef]
- Li, G.-Y.; Li, B.-G.; Yang, T.; Yin, J.-H.; Qi, H.-Y.; Liu, G.-Y.; Zhang, G.-L. Sesterterpenoids, terretonins A-D, and an alkaloid, asterrelenin, from Aspergillus terreus. J. Nat. Prod. 2006, 68, 1243–1246. [Google Scholar] [CrossRef]
- Hansson, D.; Menkis, A.; Olson, A.; Stenlid, J.; Broberg, A.; Karlsson, M. Biosynthesis of fomannoxin in the root rotting pathogen. Heterobasidion occidentale. Phytochemistry 2012, 84, 31–39. [Google Scholar] [CrossRef]
- Vu, M.; Herfindal, L.; Juvik, O.J.; Vedeler, A.; Haavik, S. Toxic aromatic compounds from fruits of Narthecium ossifragum L. Phytochemistry 2016, 132, 76–85. [Google Scholar] [CrossRef]
- Liu, Q.-M.; Xie, C.-L.; Gao, Y.-Y.; Liu, B.; Lin, W.-X.; Liu, H.; Cao, M.-J.; Su, W.-J.; Yang, X.-W.; Liu, G.-M. Deep-sea-derived butyrolactone I suppresses ovalbumin-induced anaphylaxis by regulating mast cell function in a murine model. J. Agric. Food Chem. 2018, 66, 5581–5592. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zaho, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Sacco, M.D.; Ma, C.; Lagarias, P.; Gao, A.; Townsend, J.A.; Meng, X.; Dube, P.; Zhang, X.; Hu, Y.; Kitamura, N.; et al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L. Sci. Adv. 2020, 6, eabe0751. [Google Scholar] [CrossRef]
- Zahran, E.M.; Albohy, A.; Khalil, A.; Ibrahim, A.H.; Ahmed, H.A.; El-hossary, E.M.; Bringmann, G.; Abdelmohsen, U.R. Bioactivity potential of marine natural products from scleractinia-associated microbes and in silico anti-SARS-CoV-2 evaluation. Mar. Drugs 2020, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Said, M.A.; Albohy, A.; Abdelrahman, M.A.; Ibrahim, H.S. Importance of glutamine 189 flexibility in SARS-CoV-2 main protease: Lesson learned from in silico virtual screening of ChEMBL database and molecular dynamics. Eur. J. Pharm. Sci. 2021, 160, 105744. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Al-Jawabri, N.A.; Youssef, F.S.; El-Kashef, D.H.; Knedel, T.-O.; Albohy, A.; Korinek, M.; Hwang, T.-L.; Chen, B.-H.; Lin, G.-H.; et al. Anti-inflammatory, antiallergic and COVID-19 protease inhibitory activities of phytochemicals from the Jordanian hawksbeard: Identification, structure-activity relationships, molecular modeling and impact on its folk medicinal uses. RSC Adv. 2020, 10, 38128–38141. [Google Scholar] [CrossRef]
- Macdonald, S.J.F.; Dowle, M.D.; Harrison, L.A.; Clarke, G.D.F.; Inglis, G.G.A.; Johnson, M.R.; Shah, P.; Smith, R.A.; Amour, A.; Fleetwood, G.; et al. Discovery of further pyrrolidine trans-lactams as inhibitors of human neutrophil elastase (HNE) with potential as development candidates and the crystal structure of HNE complexed with an inhibitor (GW475151). J. Med. Chem. 2002, 45, 3878–3890. [Google Scholar] [CrossRef] [PubMed]
- Korinek, M.; Wagh, V.D.; Lo, I.-W.; Hsu, Y.-M.; Hsu, H.-Y.; Hwang, T.-L.; Wu, Y.-C.; Cheng, Y.-B.; Chen, B.-H.; Chang, F.-R. Antiallergic phorbol ester from the seeds of Aquilaria malaccensis. Int. J. Mol. Med. 2016, 17, 398. [Google Scholar] [CrossRef]
- Korinek, M.; Tsai, Y.H.; El-Shazly, M.; Lai, K.H.; Backlund, A.; Wu, S.F.; Lai, W.C.; Wu, T.Y.; Chen, S.L.; Wu, Y.C.; et al. Antiallergic hydroxy fatty acids from Typhonium blumei explored through ChemGPS-NP. Front. Pharmacol. 2017, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.F.; Yu, H.P.; Chang, W.Y.; Liu, F.C.; Huang, Z.C.; Hwang, T.L. Sirtinol inhibits neutrophil elastase activity and attenuates lipopolysaccharide-mediated acute lung injury in mice. Sci. Rep. 2015, 5, 8347. [Google Scholar] [CrossRef]
- Ebada, S.S.; Al-Jawabri, N.A.; Youssef, F.S.; Albohy, A.; Aldalaien, S.M.; Disi, A.M.; Proksch, P. In vivo antiulcer activity, phytochemical exploration, and molecular modelling of the polyphenolic-rich fraction of Crepis sancta extract. Inflammopharmacology 2020, 28, 321–331. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Jheng, J.-R.; Lin, G.-H.; Chen, Y.-L.; Ho, J.Y.; Liu, C.-J.; Hsu, K.-Y.; Chen, Y.-S.; Chan, Y.F.; Yu, H.-M.; et al. Rosmarinic acid exhibits broad anti-enterovirus A71 activity by inhibiting the interaction between the five-fold axis of capsid VP1 and cognate sulfated receptors. Emerg. Microbes Infect. 2020, 9, 1194–1205. [Google Scholar] [CrossRef]

| Compound. | Cell Viability, RBL-2H3 a | Inhibition of A23187-Induced Degranulation b | Inhibition of Antigen-Induced Degranulation c |
|---|---|---|---|
| % (100 μM) | IC50 (μM) d | IC50 (μM) d | |
| Butyrolactone I (1) | >90% | 39.7 e | 41.6 f |
| Butyrolactone III (2) | >90% | >100 | >100 |
| Terretonin (3) | >90% | >100 | >100 |
| 4-Hydroxy-3-(3-methylbut-2-enyl)benzaldehyde (4) | >90% | >100 | >100 |
| Compound | Elastase Release in Human Neutrophils a | Cell viability, Human Neutrophils c | Elastase Enzymatic Activity (Cell-Free) d |
|---|---|---|---|
| IC50 (μM) b | % (10 μM) | IC50 (μM) b | |
| Butyrolactone I (1) | 2.30 ± 0.27 | 94.13 ± 2.31 | 16.70 ± 2.64 |
| Butyrolactone III (2) | >10 | 98.25 ± 1.77 | >30 |
| Terretonin (3) | >10 | n.t. | n.t. |
| 4-Hydroxy-3-(3-methylbut-2-enyl)benzaldehyde (4) | >10 | n.t. | n.t. |
| Ligand | 1H1B (Elastase) | 6LU7 (Mpro) | ||
|---|---|---|---|---|
| Binding Affinity (kcal/mol) | Interacting Residues | Binding Affinity (kcal/mol) | Interacting Residues | |
| Butyrolactone I (1) | −7.3 | Ser195-Arg147 | −7.3 | Gly143-Ser144- His163-Glu166 |
| Butyrolactone III (2) | −6.7 | Ser195-Arg147 | −7.8 | Thr26- Leu141-Gly143- Ser144- Cys145- His163- Glu166 |
| Terretonin (3) | −6.7 | Ser195- Val216 | −7.8 | Asn142- Gly143- His163 |
| 4-Hydroxy-3-(3-methylbut-2-enyl)benzaldehyde (4) | −5.1 | Ser195- Cyc191 | −5.6 | Leu141-Gly143-Ser144-Cys145- Glu166 |
| 1H1B-Ligand | −6.9 | Ser195 | -- | -- |
| 6lu7-Ligand | -- | -- | −7.1 (3rd pose) | Phe140, Gly143, His163, His164, Glu166, Gln189, Thr190 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uras, I.S.; Ebada, S.S.; Korinek, M.; Albohy, A.; Abdulrazik, B.S.; Wang, Y.-H.; Chen, B.-H.; Horng, J.-T.; Lin, W.; Hwang, T.-L.; et al. Anti-Inflammatory, Antiallergic, and COVID-19 Main Protease (Mpro) Inhibitory Activities of Butenolides from a Marine-Derived Fungus Aspergillus terreus. Molecules 2021, 26, 3354. https://doi.org/10.3390/molecules26113354
Uras IS, Ebada SS, Korinek M, Albohy A, Abdulrazik BS, Wang Y-H, Chen B-H, Horng J-T, Lin W, Hwang T-L, et al. Anti-Inflammatory, Antiallergic, and COVID-19 Main Protease (Mpro) Inhibitory Activities of Butenolides from a Marine-Derived Fungus Aspergillus terreus. Molecules. 2021; 26(11):3354. https://doi.org/10.3390/molecules26113354
Chicago/Turabian StyleUras, Ibrahim Seyda, Sherif S. Ebada, Michal Korinek, Amgad Albohy, Basma S. Abdulrazik, Yi-Hsuan Wang, Bing-Hung Chen, Jim-Tong Horng, Wenhan Lin, Tsong-Long Hwang, and et al. 2021. "Anti-Inflammatory, Antiallergic, and COVID-19 Main Protease (Mpro) Inhibitory Activities of Butenolides from a Marine-Derived Fungus Aspergillus terreus" Molecules 26, no. 11: 3354. https://doi.org/10.3390/molecules26113354
APA StyleUras, I. S., Ebada, S. S., Korinek, M., Albohy, A., Abdulrazik, B. S., Wang, Y.-H., Chen, B.-H., Horng, J.-T., Lin, W., Hwang, T.-L., & Konuklugil, B. (2021). Anti-Inflammatory, Antiallergic, and COVID-19 Main Protease (Mpro) Inhibitory Activities of Butenolides from a Marine-Derived Fungus Aspergillus terreus. Molecules, 26(11), 3354. https://doi.org/10.3390/molecules26113354









