Ambient Air Temperature Assisted Crystallization for Inorganic CsPbI2Br Perovskite Solar Cells
Abstract
1. Introduction
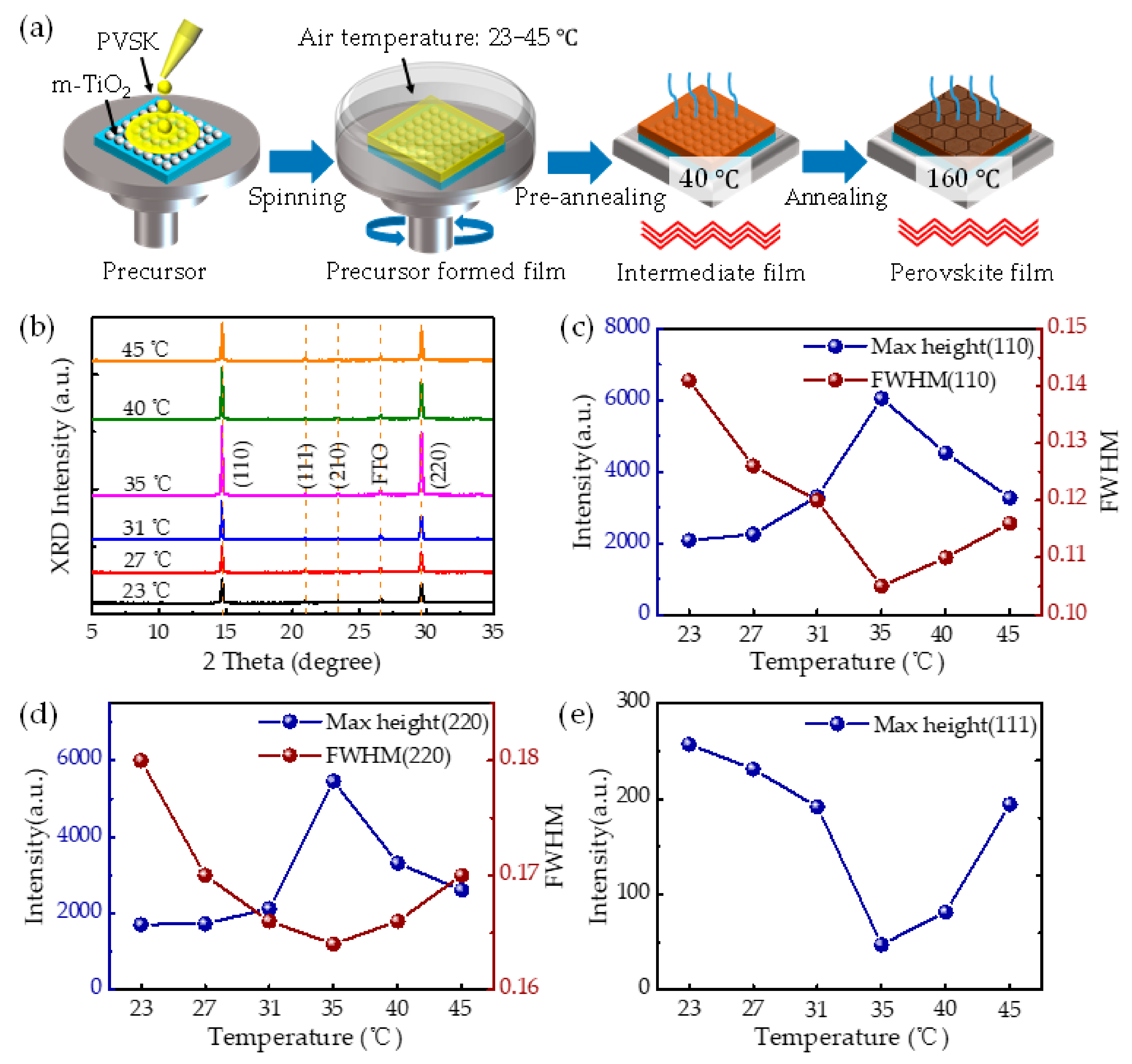
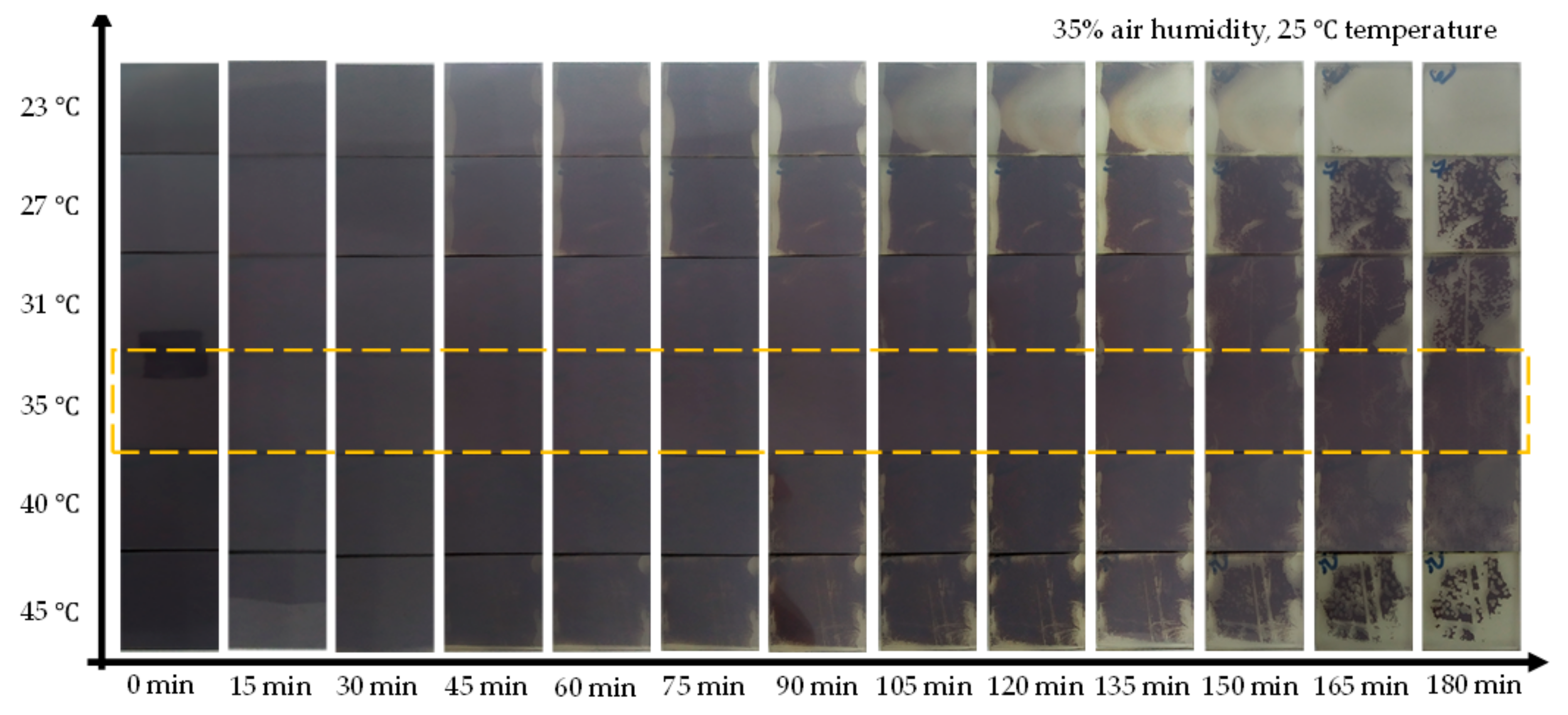
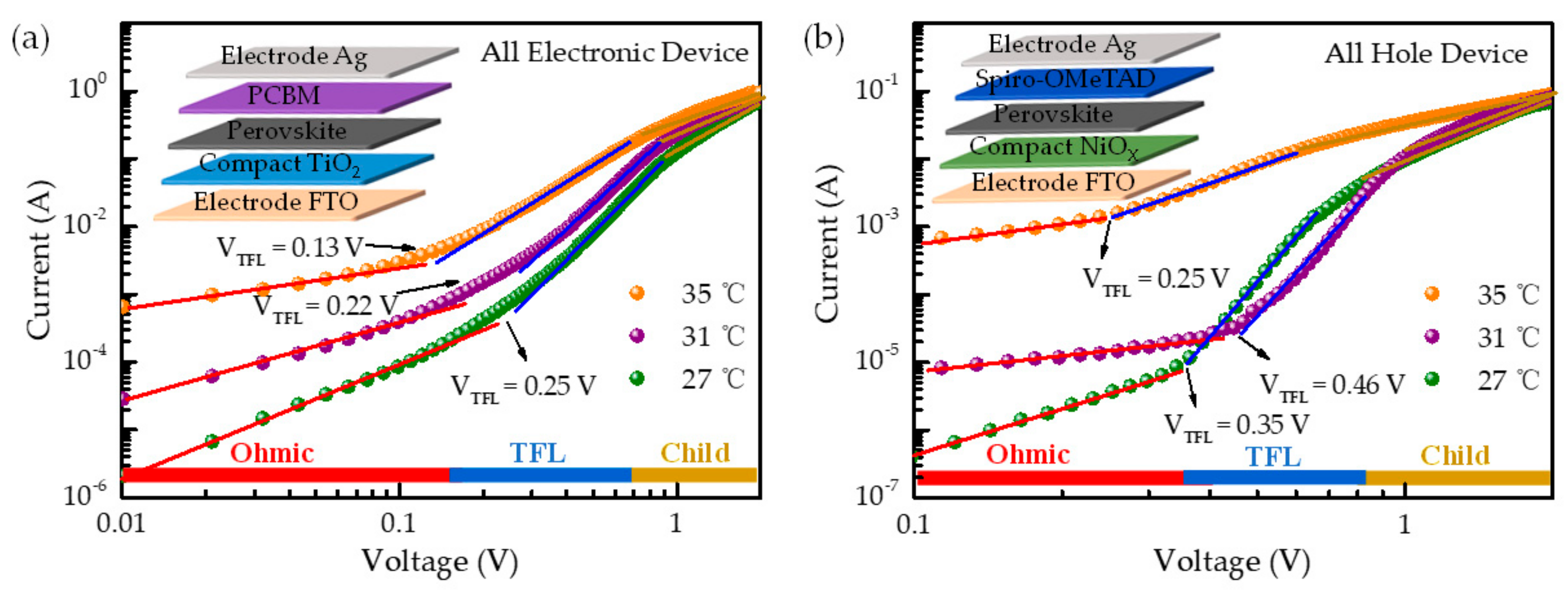
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Device Fabrication
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Im, J.-H.; Jang, I.-H.; Pellet, N.; Grätzel, M.; Park, N.-G. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 2014, 9, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide−based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiencies. Available online: www.nrel.gov/pv/cell-efficiency.html (accessed on 14 April 2021).
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photo decomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Luchkin, S.Y.; Frolova, L.A.; Dremova, N.N.; Gerasimov, K.L.; Zhidkov, I.S.; Anokhin, D.V.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Probing the intrinsic thermal and photochemical stability of hybrid and inorganic lead halide perovskites. J. Phys. Chem. Lett. 2017, 8, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Kulbak, M.; Gupta, S.; Kedem, N.; Levine, I.; Bendikov, T.; Hodes, G.; Cahen, D. Cesium enhances long-term stability of lead bromide perovskite-based solar cells. J. Phys. Chem. Lett. 2016, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Li, W.; Li, J.; Liang, X.; Wang, L. Enhancement of thermal stability for perovskite solar cells through cesium doping. RSC Adv. 2017, 7, 17473–17479. [Google Scholar] [CrossRef]
- Xiang, T.; Zhang, Y.; Wu, H.; Li, J.; Yang, L.; Wang, K.; Xia, J.; Deng, Z.; Xiao, J.; Li, W.; et al. Universal defects elimination for high performance thermally evaporated CsPbBr3 perovskite solar cells. Sol. Energy Mater. Sol. Cells 2020, 206, 110317. [Google Scholar] [CrossRef]
- Rühle, S. Tabulated values of the Shockley−Queisser limit for single junction solar cells. Sol. Energy 2016, 130, 139–147. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Hörantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T.; et al. Bandgap-tunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Hong, C.K. A-Site Rubidium Cation-Incorporated CsPbI2Br All-Inorganic Perovskite Solar Cells Exceeding 17% Efficiency. Sol. RRL 2020, 4, 2000164. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Xu, F.; Li, Y.; Zhao, Y. A facile low temperature fabrication of high performance CsPbI2Br all-inorganic perovskite solar cells. Sol. RRL 2018, 2, 1700180. [Google Scholar] [CrossRef]
- Kulbak, M.; Cahen, D.; Hodes, G. How important is the organic part of lead halide perovskite photovoltaic cells? Efficient CsPbBr3 cells. J. Phys. Chem. Lett. 2015, 6, 2452–2456. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Deng, X.; Ma, Q.; Zheng, J.; Yun, J.S.; Green, M.A.; Huang, S.; Ho-Baillie, A.W.Y. CsPbIBr2 perovskite solar cell by spray-assisted deposition. ACS Energy Lett. 2016, 1, 573–577. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lin, H.-Y.; Chiang, K.-M.; Tsai, W.-L.; Huang, Y.-C.; Tsao, C.-S.; Lin, H.-W. All-Vacuum-Deposited Stoichiometrically Balanced Inorganic Cesium Lead Halide Perovskite Solar Cells with Stabilized Efficiency Exceeding 11%. Adv. Mater. 2017, 29, 1605290. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via Lewis base adduct of lead (II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Bai, D.; Bian, H.; Jin, Z.; Wang, H.; Meng, L.; Wang, Q.; Liu, S. Temperature-assisted crystallization for inorganic CsPbI2Br perovskite solar cells to attain high stabilized efficiency 14.81%. Nano Energy 2018, 52, 408–415. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Xu, G.; Xue, R.; Wang, S.; Li, Y.; Li, Y. Precise control of crystal growth for highly efficient CsPbI2Br perovskite solar cells. Joule 2019, 3, 191–204. [Google Scholar] [CrossRef]
- Duan, C.; Li, J.; Liu, Z.; Wen, Q.; Tang, H.; Yan, K. Highly electroluminescent and stable inorganic CsPbI2Br perovskite solar cell enabled by balanced charge transfer. Chem. Eng. J. 2021, 417, 128053. [Google Scholar] [CrossRef]
- Marronnier, A.; Roma, G.; Boyer-Richard, S.; Pedesseau, L.; Jancu, J.-M.; Bonnassieux, Y.; Katan, C.; Stoumpos, C.C.; Kanatzidis, M.G.; Even, J. Anharmonicity and disorder in the black phases of cesium lead iodide used for stable inorganic perovskite solar cells. ACS Nano 2018, 12, 3477–3486. [Google Scholar] [CrossRef]
- Bashir, A.K.H.; Furqan, C.M.; Bharuth-Ram, K.; Kaviyarasu, K.; Tchokonté, M.B.T.; Maaza, M. Structural, optical and Mössbauer investigation on the biosynthesized α-Fe2O3: Study on different precursors. Phys. E Low Dimens. Syst. Nanostructures 2019, 111, 152–157. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, H.; Duan, C.; Yang, S.; Yang, Z.; Liu, Z.; Liu, S. Controlled n-doping in air-stable CsPbI2Br perovskite solar cells with a record efficiency of 16.79%. Adv. Funct. Mater. 2020, 30, 1909972. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Li, H.; Wang, H.; Zhang, C.; Yang, Y.; Gao, X.; Xue, Q.; Yip, H.-L.; Fan, Z.; et al. Structurally reconstructed CsPbI2Br perovskite for highly stable and square-centimeter all-inorganic perovskite solar cells. Adv. Energy Mater. 2019, 9, 1803572. [Google Scholar] [CrossRef]
- Rezaie, A.; Fahrenholtz, W.G.; Hilmas, G.E. Effect of hot pressing time and temperature on the microstructure and mechanical properties of ZrB 2−SiC. J. Mater. Sci. 2007, 42, 2735–2744. [Google Scholar] [CrossRef]
- Gutierrez-Partida, E.; Hempel, H.; Caicedo-DáVila, S.N.; Raoufi, M.; Peña-Camargo, F.; Grischek, M.; Gunder, R.; Diekmann, J.; Caprioglio, P.; Brinkmann, K.O.; et al. Large-grain double cation perovskites with 18 μs lifetime and high luminescence yield for efficient inverted perovskite solar cells. ACS Energy Lett. 2021, 6, 1045–1054. [Google Scholar] [CrossRef]
- Lin, Z.Q.; Qiao, H.W.; Zhou, Z.R.; Hou, Y.; Li, X.; Yang, H.G.; Yang, S. Water assisted formation of highly oriented CsPbI2Br perovskite films with the solar cell efficiency exceeding 16%. J. Mater. Chem. A 2020, 8, 17670–17674. [Google Scholar] [CrossRef]
- Bube, R.H. Trap density determination by space-charge-limited currents. J. Appl. Phys. 1962, 33, 1733–1737. [Google Scholar] [CrossRef]
- Kiy, M.; Losio, P.; Biaggio, I.; Koehler, M.; Tapponnier, A.; Günter, P. Observation of the Mott–Gurney law in tris (8-hydroxyquinoline) aluminum films. Appl. Phys. Lett. 2002, 80, 1198–1200. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.G. Causes and solutions of recombination in perovskite solar cells. Adv. Funct. Mater. 2019, 31, 1803019. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Wolff, C.M.; Caprioglio, P.; Stolterfoht, M.; Neher, D. Nonradiative recombination in perovskite solar cells: The role of interfaces. Adv. Mater. 2019, 31, 1902762. [Google Scholar] [CrossRef]
- Buin, A.; Pietsch, P.; Xu, J.; Voznyy, O.; Ip, A.H.; Comin, R.; Sargent, E.H. Materials processing routes to trap-free halide perovskites. Nano Lett. 2014, 14, 6281–6286. [Google Scholar] [CrossRef] [PubMed]
- Parida, B.; Ryu, J.; Yoon, S.; Lee, S.; Seo, Y.; Cho, J.S.; Kang, D.-W. Two-step growth of CsPbI3−XBrX films employing dynamic CsBr treatment: Toward all-inorganic perovskite photovoltaics with enhanced stability. J. Mater. Chem. A 2019, 7, 18488–18498. [Google Scholar] [CrossRef]
- Wetzelaer, G.A.H.; Kuik, M.; Lenes, M.; Blom, P.W.M. Origin of the dark-current ideality factor in polymer: Fullerene bulk heterojunction solar cells. J. Mater. Chem. A 2011, 99, 153506. [Google Scholar]
- Brus, V.V.; Proctor, C.M.; Ran, N.A.; Nguyen, T.-Q. Capacitance spectroscopy for quantifying recombination losses in nonfullerene small-molecule bulk heterojunction solar cells. Adv. Energy Mater. 2016, 6, 1502250. [Google Scholar] [CrossRef]
- Wetzelaer, G.-J.A.H.; Scheepers, M.; Sempere, A.M.; Momblona, M.; Ávila, J.; Bolink, H.J. Trap-assisted non-radiative recombination in organic−inorganic perovskite solar cells. Adv. Energy Mater. 2015, 27, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, J.; Brus, V.V.; Ko, S.J.; Lee, J.; Karki, A.; Cao, D.X.; Cho, K.; Bazan, G.C.; Nguyen, T.-Q. Quantifying the nongeminate recombination dynamics in nonfullerene bulk heterojunction organic solar cells. Adv. Energy Mater. 2019, 9, 1901438. [Google Scholar] [CrossRef]
- Proctor, C.M.; Nguyen, T.-Q. Effect of leakage current and shunt resistance on the light intensity dependence of organic solar cells. Appl. Phys. Lett. 2015, 106, 083301. [Google Scholar] [CrossRef]
- Yin, G.; Zhao, H.; Jiang, H.; Yuan, S.; Niu, T.; Zhao, K.; Liu, Z.; Liu, S. Precursor engineering for all-inorganic CsPbI2Br perovskite solar cells with 14.78% efficiency. Adv. Funct. Mater. 2018, 28, 1803269. [Google Scholar] [CrossRef]
- Tress, W.; Yavari, M.; Domanski, K.; Yadav, P.; Niesen, B.; Correa-Baena, J.P.; Hagfeldt, A.; Graetzel, M. Interpretation and evolution of open-circuit voltage, recombination, ideality factor and subgap defect states during reversible light-soaking and irreversible degradation of perovskite solar cells. Energy Environ. Sci. 2018, 11, 151–165. [Google Scholar] [CrossRef]




| Device | JSC (mA cm−2) | |||
|---|---|---|---|---|
| 23 °C-For | 14.6 ± 0.3 | 0.76 ± 0.05 | 0.51 ± 0.03 | 5.5 ± 0.8 |
| 23 °C-ReV | 14.6 ± 0.3 | 0.88 ± 0.05 | 0.65 ± 0.03 | 8.4 ± 0.8 |
| 35 °C-For | 15.4 ± 0.4 | 1.21 ± 0.03 | 0.72 ± 0.02 | 13.5 ± 0.9 |
| 35 °C-Rev | 15.4 ± 0.4 | 1.24 ± 0.03 | 0.76 ± 0.02 | 14.5 ± 1.0 |
| 45 °C-For | 15.0 ± 0.4 | 1.09 ± 0.06 | 0.59 ± 0.02 | 9.7 ± 0.6 |
| 45 °C-Rev | 15.0 ± 0.4 | 1.14 ± 0.06 | 0.68 ± 0.02 | 11.7 ± 0.6 |
| Device. | Rs (Ω cm−2) | Rsh (Ω cm−2) | VOC Slop (KT/q) |
|---|---|---|---|
| 23 °C | 2.9 | 1214 | 2.6 |
| 35 °C | 4.2 | 8484 | 1.6 |
| 45 °C | 3.0 | 2160 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Liu, K.; Zhang, Y.; Li, W. Ambient Air Temperature Assisted Crystallization for Inorganic CsPbI2Br Perovskite Solar Cells. Molecules 2021, 26, 3398. https://doi.org/10.3390/molecules26113398
Long Y, Liu K, Zhang Y, Li W. Ambient Air Temperature Assisted Crystallization for Inorganic CsPbI2Br Perovskite Solar Cells. Molecules. 2021; 26(11):3398. https://doi.org/10.3390/molecules26113398
Chicago/Turabian StyleLong, Yi, Kun Liu, Yongli Zhang, and Wenzhe Li. 2021. "Ambient Air Temperature Assisted Crystallization for Inorganic CsPbI2Br Perovskite Solar Cells" Molecules 26, no. 11: 3398. https://doi.org/10.3390/molecules26113398
APA StyleLong, Y., Liu, K., Zhang, Y., & Li, W. (2021). Ambient Air Temperature Assisted Crystallization for Inorganic CsPbI2Br Perovskite Solar Cells. Molecules, 26(11), 3398. https://doi.org/10.3390/molecules26113398






