Current Modulation of Guanylate Cyclase Pathway Activity—Mechanism and Clinical Implications
Abstract
1. Introduction
2. Guanylate Cyclases
3. NO Production
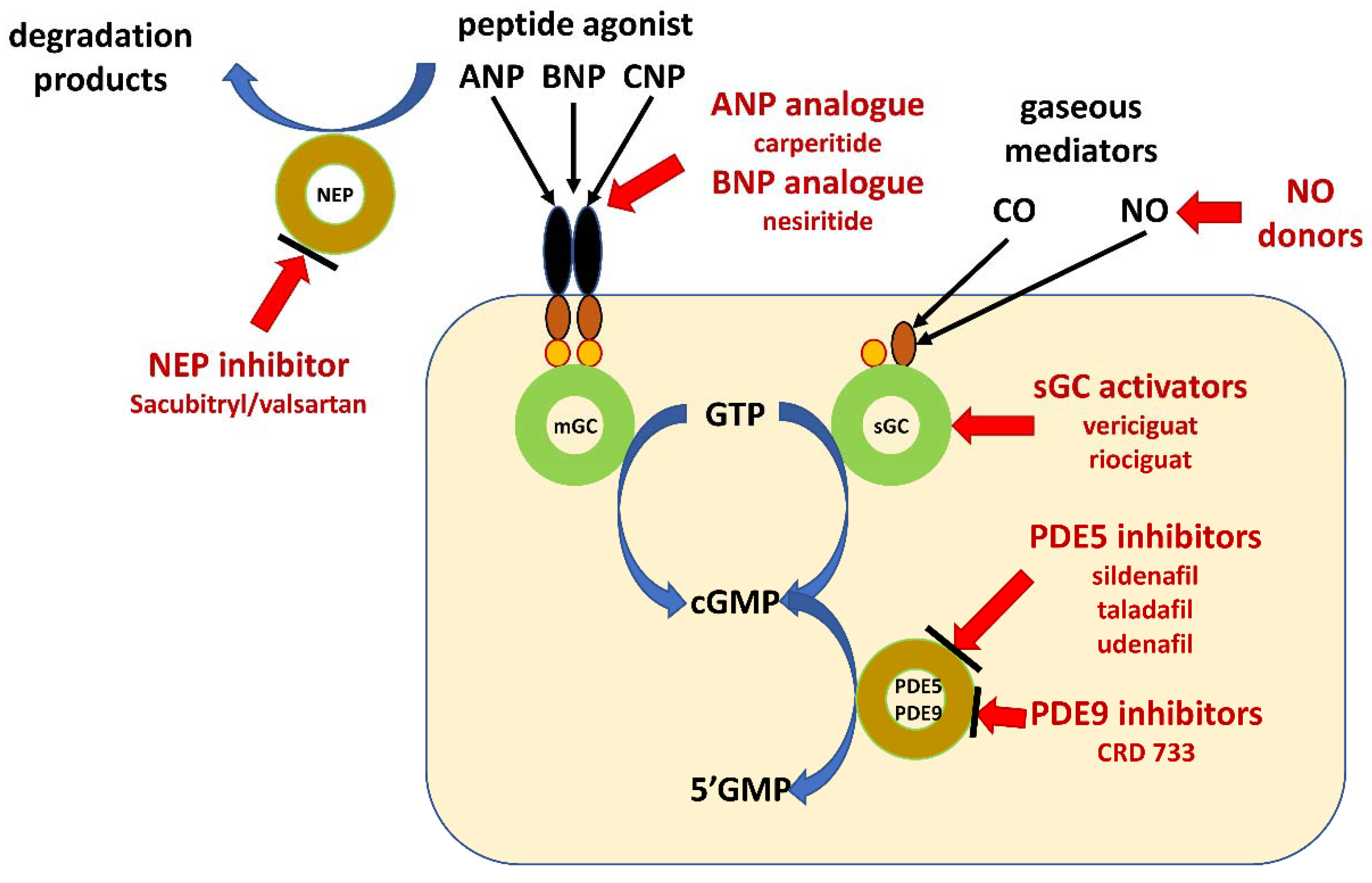
4. Pharmacological Intervention
4.1. Natriuretic Peptides Analogues
4.2. Inhibition of Neprilysin
4.3. Inhibition of Phosphodiesterases
4.4. Direct Activation of Soluble Guanylate Cyclase
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| cAMP | Cyclic Adenosine Monophosphate |
| CO | carbon monoxide |
| FDA | US Food and Drug Administration |
| GC | guanylate cyclase |
| GC-A | guanylate cyclase type A |
| GC-B | guanylate cyclase type B |
| GC-C | guanylate cyclase type C |
| GC-D | guanylate cyclase type D |
| GC-E | guanylate cyclase type E |
| GC-F | guanylate cyclase type F |
| GC-G | guanylate cyclase type G |
| sGC | soluble guanylate cyclase |
| mGC | guanylate cyclase-coupled receptor or membrane-bound guanylyl cyclase |
| GMP | guanosine monophosphate |
| cGMP | cyclic guanosine monophosphate |
| GTP | guanosine triphosphate |
| LPS | lipopolysaccharide/endotoxin |
| NP | natriuretic peptide |
| ANP | natriuretic peptide type A |
| BNP | natriuretic peptide type B |
| CNP | natriuretic peptide type C |
| NANC | non-adrenergic, non-cholinergic |
| NEP | neprilysin |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NOS-1 | neuronal nitric oxide synthase |
| NOS-2 | cytokine-inducible nitric oxide synthase |
| NOS-3 | endothelial nitric oxide synthase |
| PAH | pulmonary arterial hypertension |
| PDE5 | Phosphodiesterase type 5 |
| PDE9 | Phosphodiesterase type 9 |
| PKG | cGMP dependent protein kinase G |
| SNP | sodium nitroprusside |
| TNFα | tumor necrosis factor α |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| VASP | vasodilator-stimulated phosphoprotein |
References
- Dove, S. Mammalian Nucleotidyl Cyclases and Their Nucleotide Binding Sites. Handb. Exp. Pharmacol. 2017, 238, 49–66. [Google Scholar]
- Liu, Y.; Ruoho, A.E.; Rao, V.D.; Hurley, J.H. Catalytic mechanism of the adenylyl and guanylyl cyclases: Modeling and mutation-al analysis. Proc. Natl. Acad. Sci. USA 1997, 94, 13414–13419. [Google Scholar] [CrossRef]
- Thompson, D.K.; Garbers, D.L. Dominant negative mutations of the guanylyl cyclase-A receptor. Extracellular domain dele-tion and catalytic domain point mutations. J. Biol. Chem. 1995, 270, 425–430. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Potter, L.R. Guanylyl cyclase structure, function and regulation. Cell. Signal. 2011, 23, 1921–1926. [Google Scholar] [CrossRef]
- Giuili, G.; Scholl, U.; Bulle, F.; Guellaën, G. Molecular cloning of the cDNAs coding for the two subunits of soluble guanylyl cyclase from human brain. FEBS Lett. 1992, 304, 83–88. [Google Scholar] [CrossRef]
- Harteneck, C.; Wedel, B.; Koesling, D.; Malkewitz, J.; Böhme, E.; Schultz, G. Molecular cloning and expression of a new al-pha-subunit of soluble guanylyl cyclase. Interchangeability of the alpha-subunits of the enzyme. FEBS Lett. 1991, 292, 217–222. [Google Scholar]
- Mergia, E.; Friebe, A.; Dangel, O.; Russwurm, M.; Koesling, D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J. Clin. Investig. 2006, 116, 1731–1737. [Google Scholar] [CrossRef]
- Yuen, P.S.T.; Potter, L.R.; Garbers, D.L. A new form of guanylyl cyclase is preferentially expressed in rat kidney. Biochemistry 1990, 29, 10872–10878. [Google Scholar] [CrossRef] [PubMed]
- Wedel, B.; Humbert, P.; Harteneck, C.; Foerster, J.; Malkewitz, J.; Bohme, E.; Schultz, G.; Koesling, D. Mutation of His-105 in the beta 1 subunit yields a nitric oxide-insensitive form of soluble guanylyl cyclase. Proc. Natl. Acad. Sci. USA 1994, 91, 2592–2596. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Mergia, E.; Dangel, O.; Lange, A.; Koesling, D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc. Natl. Acad. Sci. USA 2007, 104, 7699–7704. [Google Scholar] [CrossRef]
- Pfeifer, A.; Klatt, P.; Massberg, S.; Ny, L.; Sausbier, M.; Hirneiß, C.; Wang, G.; Korth, M.; Aszódi, A.; Andersson, K.; et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998, 17, 3045–3051. [Google Scholar] [CrossRef] [PubMed]
- Desch, M.; Sigl, K.; Hieke, B.; Salb, K.; Kees, F.; Bernhard, D.; Jochim, A.; Spiessberger, B.; Höcherl, K.; Feil, R.; et al. IRAG determines nitric oxide- and atrial natriuretic peptide-mediated smooth muscle relaxation. Cardiovasc. Res. 2010, 86, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, J.A.; Germain, A.M.; Huidobro-Toro, J.P.; Weiner, C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 2000, 184, 409–420. [Google Scholar] [CrossRef]
- Rajfer, J.; Aronson, W.J.; Bush, P.A.; Dorey, F.J.; Ignarro, L.J. Nitric Oxide as a Mediator of Relaxation of the Corpus Cavernosum in Response to Nonadrenergic, Noncholinergic Neurotransmission. N. Engl. J. Med. 1992, 326, 90–94. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, R.; Wu, J.-X.; Chen, L. Structural insights into the mechanism of human soluble guanylate cyclase. Nature 2019, 574, 206–210. [Google Scholar] [CrossRef]
- Seeger, F.; Quintyn, R.; Tanimoto, A.; Williams, G.J.; Tainer, J.A.; Wysocki, V.H.; Garcin, E.D. Interfacial Residues Promote an Optimal Alignment of the Catalytic Center in Human Soluble Guanylate Cyclase: Heterodimerization Is Required but Not Sufficient for Activity. Biochemistry 2014, 53, 2153–2165. [Google Scholar] [CrossRef]
- Suga, S.; Nakao, K.; Hosoda, K.; Mukoyama, M.; Ogawa, Y.; Shirakami, G.; Arai, H.; Saito, Y.; Kambayashi, Y.; Inouye, K.; et al. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 1992, 130, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Vesely, D.L. Atrial Natriuretic Peptide Prohormone Gene Expression: Hormones and Diseases That Upregulate its Expression. IUBMB Life 2002, 53, 153–159. [Google Scholar] [CrossRef]
- Gupta, D.K.; Wang, T.J. Natriuretic Peptides and Cardiometabolic Health. Circ. J. 2015, 79, 1647–1655. [Google Scholar] [CrossRef]
- Tamura, N.; Ogawa, Y.; Chusho, H.; Nakamura, K.; Nakao, K.; Suda, M.; Kasahara, M.; Hashimoto, R.; Katsuura, G.; Mukoyama, M.; et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 4239–4244. [Google Scholar] [CrossRef]
- Moyes, A.J.; Hobbs, A.J. C-Type Natriuretic Peptide: A Multifaceted Paracrine Regulator in the Heart and Vasculature. Int. J. Mol. Sci. 2019, 20, 2281. [Google Scholar] [CrossRef] [PubMed]
- Yasoda, A.; Ogawa, Y.; Suda, M.; Tamura, N.; Mori, K.; Sakuma, Y.; Chusho, H.; Shiota, K.; Tanaka, K.; Nakao, K. Natriuretic peptide regulation of endochondral ossification. Evidence for possible roles of the C-type natriuretic peptide/guanylyl cyclase-B pathway. J. Biol. Chem. 1998, 273, 11695–11700. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.-Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa Cell Ligand NPPC and Its Receptor NPR2 Maintain Meiotic Arrest in Mouse Oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef]
- He, M.; Zhang, T.; Yang, Y.; Wang, C. Mechanisms of Oocyte Maturation and Related Epigenetic Regulation. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Santhekadur, P.K.; Kumar, D.P.; Seneshaw, M.; Mirshahi, F.; Sanyal, A.J. The multifaceted role of natriuretic peptides in metabolic syndrome. Biomed. Pharmacother. 2017, 92, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Nagase, M.; Katafuchi, T.; Hirose, S.; Fujita, T. Tissue distribution and localization of natriuretic peptide receptor subtypes in stroke-prone spontaneously hypertensive rats. J. Hypertens. 1997, 15, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Chen, W.; Wang, J. Interventions in theB-type natriuretic peptide signalling pathway as a means of controlling chronic itch. Br. J. Pharmacol. 2020, 177, 1025–1040. [Google Scholar] [CrossRef]
- Hamra, F.K.; Forte, L.R.; Eber, S.L.; Pidhorodeckyj, N.V.; Krause, W.J.; Freeman, R.H.; Chin, D.T.; Tompkins, J.A.; Fok, K.F.; Smith, C.E. Uroguanylin: Structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc. Natl. Acad. Sci. USA 1993, 90, 10464–10468. [Google Scholar] [CrossRef]
- Amarachintha, S.; Harmel-Laws, E.; Steinbrecher, K.A. Guanylate cyclase C reduces invasion of intestinal epithelial cells by bacterial pathogens. Sci. Rep. 2018, 8, 1521. [Google Scholar] [CrossRef]
- Steinbrecher, K.A.; Wowk, S.A.; Rudolph, J.A.; Witte, D.P.; Cohen, M.B. Targeted Inactivation of the Mouse Guanylin Gene Results in Altered Dynamics of Colonic Epithelial Proliferation. Am. J. Pathol. 2002, 161, 2169–2178. [Google Scholar] [CrossRef]
- Lorenz, J.N.; Nieman, M.; Sabo, J.; Sanford, L.P.; Hawkins, J.A.; Elitsur, N.; Gawenis, L.R.; Clarke, L.L.; Cohen, M.B. Uroguanylin knock-out mice have increased blood pressure and impaired natriuretic response to enteral NaCl load. J. Clin. Investig. 2003, 112, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, J.A.; Waldman, S.A. An update on guanylyl cyclase C in the diagnosis, chemoprevention, and treatment of colorectal cancer. Expert Rev. Clin. Pharmacol. 2020, 13, 1125–1137. [Google Scholar] [CrossRef]
- Dye, F.S.; Larraufie, P.; Kay, R.; Darwish, T.; Rievaj, J.; Goldspink, D.A.; Meek, C.L.; Middleton, S.J.; Hardwick, R.H.; Roberts, G.; et al. Characterisation of proguanylin expressing cells in the intestine—Evidence for constitutive luminal secretion. Sci. Rep. 2019, 9, 15574. [Google Scholar] [CrossRef]
- Arakawa, H.; Kelliher, K.R.; Zufall, F.; Munger, S.D. The Receptor Guanylyl Cyclase Type D (GC-D) Ligand Uroguanylin Promotes the Acquisition of Food Preferences in Mice. Chem. Senses 2013, 38, 391–397. [Google Scholar] [CrossRef][Green Version]
- Kenemuth, J.K.; Hennessy, S.P.; Hanson, R.J.; Hensler, A.J.; Coates, E.L. Investigation of nasal CO2 receptor transduction mechanisms in wild-type and GC-D knockout mice. Chem. Senses. 2013, 38, 769–781. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zufall, F.; Munger, S.D. Receptor guanylyl cyclases in mammalian olfactory function. Mol. Cell. Biochem. 2010, 334, 191–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gill, J.S.; Georgiou, M.; Kalitzeos, A.; Moore, A.T.; Michaelides, M. Progressive cone and cone-rod dystrophies: Clinical features, molecular genetics and prospects for therapy. Br. J. Ophthalmol. 2019, 103, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Fain, G.L.; Sampath, A.P. Light responses of mammalian cones. Pflügers Arch. Eur. J. Physiol. 2021, 473, 1–14. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Cheng, C.-J.; Hsieh, H.-T.; Lin, C.-C.; Chen, C.-C.; Yang, R.-B. Guanylate cyclase-G, expressed in the Grueneberg ganglion olfactory subsystem, is activated by bicarbonate. Biochem. J. 2010, 432, 267–273. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Fleischer, J.; Yang, R. Guanylyl cyclase-G is an alarm pheromone receptor in mice. EMBO J. 2018, 37, 39–49. [Google Scholar] [CrossRef]
- Calvo-Ochoa, E.; Byrd-Jacobs, C.A.; Fuss, S.H. Diving into the streams and waves of constitutive and regenerative olfactory neurogenesis: Insights from zebrafish. Cell Tissue Res. 2021, 383, 227–253. [Google Scholar] [CrossRef]
- Grześk, E.; Tejza, B.; Wiciński, M.; Malinowski, B.; Szadujkis-Szadurska, K.; Baran, L.; Kowal, E.; Grześk, G. Effect of pertussis toxin on calcium influx in three contraction models. Biomed. Rep. 2014, 2, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Szadujkis-Szadurska, K.; Grzesk, G.; Szadujkis-Szadurski, L.; Gajdus, M.; Matusiak, G. Role of acetylcholine and calcium ions in three vascular contraction models: Angiotensin II, phenylephrine and caffeine. Exp. Ther. Med. 2012, 4, 329–333. [Google Scholar] [CrossRef]
- Grześk, E.; Darwish, N.; Stolarek, W.; Wiciński, M.; Malinowski, B.; Burdziński, I.; Grześk, G. Effect of reperfusion on vascular smooth muscle reactivity in three contraction models. Microvasc. Res. 2019, 121, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Szadujkis-Szadurski, L. Pharmacometric analysis of alpha1-adrenoceptor function in rat tail artery pretreated with lipopolysaccharides. Pol. J. Pharmacol. 2001, 53, 605–613. [Google Scholar]
- Bloch-Bogusławska, E.; Grześk, E.; Grześk, G. Comparison of the post-mortem interval on the effect of vascular responses to the activation of ionotropic and metabotropic receptors. Biomed. Rep. 2015, 3, 230–234. [Google Scholar] [CrossRef][Green Version]
- Grześk, G.; Szadujkis-Szadurski, L. Physiological antagonism of angiotensin II and lipopolysaccharides in early endotoxemia: Pharmacometric analysis. Pol. J. Pharmacol. 2003, 55, 753–762. [Google Scholar] [PubMed]
- Szadujkis-Szadurska, K.; Grzesk, G.; Szadujkis-Szadurski, L.; Gajdus, M.; Matusiak, G. Role of nitric oxide and cGMP in the mod-ulation of vascular contraction induced by angiotensin II and Bay K8644 during ischemia/reperfusion. Exp. Ther. Med. 2013, 5, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Slupski, M.; Szadujkis-Szadurski, L.; Grześk, G.; Wlodarczyk, Z.; Masztalerz, M.; Piotrowiak, I.; Jasinski, M.; Szadujkis-Szadurski, R.; Szadujkis-Szadurska, K. Guanylate Cyclase Activators Influence Reactivity of Human Mesenteric Superior Arteries Retrieved and Preserved in the Same Conditions as Transplanted Kidneys. Transplant. Proc. 2007, 39, 1350–1353. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Vo, P.A.; Lad, B.; Tomlinson, J.A.; Francis, S.; Ahluwalia, A. Autoregulatory Role of Endothelium-derived Nitric Oxide (NO) on Lipopolysaccharide-induced Vascular Inducible NO Synthase Expression and Function. J. Biol. Chem. 2005, 280, 7236–7243. [Google Scholar] [CrossRef]
- Connelly, L.; Madhani, M.; Hobbs, A.J. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: A pro-inflammatory role for eNOS-derived no in vivo. J. Biol. Chem. 2005, 280, 10040–10046. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Cuspidi, C.; Tadic, M.; Grassi, G.; Mancia, G. Treatment of hypertension: The ESH/ESC guidelines recommendations. Pharmacol. Res. 2018, 128, 315–321. [Google Scholar] [CrossRef]
- Mitaka, C.; Kudo, T.; Haraguchi, G.; Tomita, M. Cardiovascular and renal effects of carperitide and nesiritide in cardiovascular surgery patients: A systematic review and meta-analysis. Crit. Care 2011, 15, R258. [Google Scholar] [CrossRef] [PubMed]
- Sezai, A.; Nakata, K.-I.; Iida, M.; Yoshitake, I.; Wakui, S.; Hata, H.; Shiono, M. Results of Low-Dose Carperitide Infusion in High-Risk Patients Undergoing Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2013, 96, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, D.Q.; Song, R.; Zhang, G. Nesiritide in patients with acute myocardial infarction and heart failure: A meta-analysis. J. Int. Med. Res. 2020, 48, 300060519897194. [Google Scholar] [CrossRef]
- O’Connor, C.; Starling, R.; Hernandez, A.; Armstrong, P.; Dickstein, K.; Hasselblad, V.; Heizer, G.; Komajda, M.; Massie, B.; McMurray, J.; et al. Effect of Nesiritide in Patients with Acute Decompensated Heart Failure. N. Engl. J. Med. 2011, 365, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, V.M.; Hernandez, A.F.; Stebbins, A.; Hasselblad, V.; Ezekowitz, J.A.; Califf, R.M.; Gottlieb, S.S.; O’Connor, C.M.; Starling, R.C.; Tang, W.H.; et al. Nesiritide, renal function, and associated outcomes during hospitaliza-tion for acute decompensated heart failure: Results from the Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure (ASCEND-HF). Circulation 2014, 130, 958–965. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Grodin, J.L.; Liebo, M.J.; Butler, J.; Metra, M.; Felker, G.M.; Hernandez, A.F.; Voors, A.A.; McMurray, J.J.; Armstrong, P.W.; O’Connor, C.; et al. Prognostic Implications of Changes in Amino-Terminal Pro–B-Type Natriuretic Peptide in Acute Decompensated Heart Failure: Insights From ASCEND-HF. J. Card. Fail. 2019, 25, 703–711. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; McCarthy, C.P.; Shrestha, S.; Gaggin, H.K.; Mukai, R.; Szymonifka, J.; Apple, F.S.; Burnett, J.C.; Iyer, S.; Januzzi, J.L. Effect of Neprilysin Inhibition on Various Natriuretic Peptide Assays. J. Am. Coll. Cardiol. 2019, 73, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Anand, I.S.; Ge, J.; Lam, C.S.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; PARAGON-HF Steering Committee and Investigators. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction: Reply. N. Engl. J. Med. 2020, 382, 1182–1183. [Google Scholar]
- Solomon, S.D.; Claggett, B.; McMurray, J.J.; Hernandez, A.F.; Fonarow, G. Combined neprilysin and renin-angiotensin system inhibition in heart failure with reduced ejection fraction: A meta-analysis. Eur. J. Heart Fail. 2016, 18, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Claggett, B.; Packer, M.; Zile, M.R.; Rouleau, J.; Swedberg, K.; Teerlink, J.R.; Desai, A.S.; Lefkowitz, M.; Shi, V.; et al. Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) Investigators. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: The PARADIGM-HF trial. Eur. J. Heart Fail. 2016, 18, 1228–1234. [Google Scholar] [PubMed]
- Ruilope, L.M.; Dukat, A.; Böhm, M.; Lacourcière, Y.; Gong, J.; Lefkowitz, M.P. Blood-pressure reduction with LCZ696, a novel du-al-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010, 375, 1255–1266. [Google Scholar] [CrossRef]
- Voors, A.A.; Gori, M.; Liu, L.C.Y.; Claggett, B.; Zile, M.; Pieske, B.; McMurray, J.J.V.; Packer, M.; Shi, V.; Lefkowitz, M.P.; et al. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2015, 17, 510–517. [Google Scholar] [CrossRef]
- Campbell, D.J. Long-term neprilysin inhibition—Implications for ARNIs. Nat. Rev. Cardiol. 2017, 14, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Riddell, E.; Vader, J.M. Potential Expanded Indications for Neprilysin Inhibitors. Curr. Heart Fail. Rep. 2017, 14, 134–145. [Google Scholar] [CrossRef]
- Cote, R.H. Characteristics of Photoreceptor PDE (PDE6): Similarities and differences to PDE5. Int. J. Impot. Res. 2004, 16, S28–S33. [Google Scholar] [CrossRef]
- Maryam, A.; Vedithi, S.C.; Khalid, R.R.; Alsulami, A.F.; Torres, P.H.M.; Siddiqi, A.R.; Blundell, T.L. The Molecular Organization of Human cGMP Specific Phosphodiesterase 6 (PDE6): Structural Implications of Somatic Mutations in Cancer and Retinitis Pigmentosa. Comput. Struct. Biotechnol. J. 2019, 17, 378–389. [Google Scholar] [CrossRef]
- Ribaudo, G.; Memo, M.; Gianoncelli, A. A Perspective on Natural and Nature-Inspired Small Molecules Targeting Phos-phodiesterase 9 (PDE9): Chances and Challenges against Neurodegeneration. Pharmaceuticals 2021, 14, 58. [Google Scholar] [CrossRef]
- Montani, D.; Günther, S.; Dorfmüller, P.; Perros, F.; Girerd, B.; Garcia, G.; Jaïs, X.; Savale, L.; Artaud-Macari, E.; Price, L.C.; et al. Pulmonary arterial hypertension. Orphanet. J. Rare Dis. 2013, 8, 97. [Google Scholar] [CrossRef]
- Barnes, H.; Brown, Z.; Burns, A.; Williams, T. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst. Rev. 2019, 1, CD012621. [Google Scholar] [CrossRef]
- Mayeux, J.D.; Pan, I.Z.; Dechand, J.; Jacobs, J.A.; Jones, T.L.; McKellar, S.H.; Beck, E.; Hatton, N.D.; Ryan, J.J. Management of Pulmonary Arterial Hypertension. Curr. Cardiovasc. Risk Rep. 2021, 15, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.S.; Ivy, D.D. Diagnosis, Evaluation and Treatment of Pulmonary Arterial Hypertension in Children. Children 2018, 5, 44. [Google Scholar] [CrossRef]
- Galiè, N.; Ghofrani, A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; Parpia, T.; Burgess, G.; Branzi, A.; et al. Sildenafil Citrate Therapy for Pulmonary Arterial Hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Brundage, B.H.; Ghofrani, H.A.; Oudiz, R.J.; Simonneau, G.; Safdar, Z.; Shapiro, S.; White, R.J.; Chan, M.; Beardsworth, A.; et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009, 119, 2894–2903. [Google Scholar] [CrossRef]
- Lewis, G.D.; Lachmann, J.; Camuso, J.; Lepore, J.J.; Shin, J.; Martinovic, M.E.; Systrom, D.M.; Bloch, K.D.; Semigran, M.J. Sildenafil im-proves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation 2007, 115, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M.; Chen, H.H.; Borlaug, B.A.; Semigran, M.J.; Lee, K.L.; Lewis, G.; LeWinter, M.M.; Rouleau, J.L.; Bull, D.A.; Mann, D.L.; et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2013, 309, 1268–1277. [Google Scholar] [CrossRef]
- Pons, J.; Leblanc, M.-H.; Bernier, M.; Cantin, B.; Bourgault, C.; Bergeron, S.; Proulx, G.; Morin, J.; Nalli, C.; O’Connor, K.; et al. Effects of chronic sildenafil use on pulmonary hemodynamics and clinical outcomes in heart transplantation. J. Heart Lung Transplant. 2012, 31, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.A.; Aronovitz, M.J.; Liu, P.; Martin, G.L.; Tam, K.; Pande, S.; Karas, R.H.; Bloomfield, D.M.; Mendelsohn, M.E.; Blanton, R.M. CRD-733, a Novel PDE9 (Phosphodiesterase 9) Inhibitor, Reverses Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2021, 14, e007300. [Google Scholar] [CrossRef]
- Pinilla-Vera, M.; Hahn, V.S.; Kass, D.A. Leveraging Signaling Pathways to Treat Heart Failure with Reduced Ejection Fraction. Circ. Res. 2019, 124, 1618–1632. [Google Scholar] [CrossRef]
- Kokkonen-Simon, K.M.; Saberi, A.; Nakamura, T.; Ranek, M.J.; Zhu, G.; Bedja, D.; Kuhn, M.; Halushka, M.K.; Lee, D.I.; Kass, D.A. Marked disparity of microRNA modulation by cGMP-selective PDE5 versus PDE9 inhibitors in heart disease. JCI Insight. 2018, 3, e121739. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Klett, J.; Pilarzyk, K.; Lee, D.I.; Kass, D.; Menniti, F.S.; Kelly, M.P. Identification of new PDE9A isoforms and how their expression and subcellular compartmentalization in the brain change across the life span. Neurobiol. Aging 2018, 65, 217–234. [Google Scholar] [CrossRef]
- Stasch, J.-P.; Hobbs, A. NO-Independent, Haem-Dependent Soluble Guanylate Cyclase Stimulators. Handb. Exp. Pharmacol. 2009, 191, 277–308. [Google Scholar]
- Schmidt, H.H.H.W.; Schmidt, P.M.; Stasch, J.-P. NO- and Haem-Independent Soluble Guanylate Cyclase Activators. Handb. Exp. Pharmacol. 2009, 191, 309–339. [Google Scholar]
- Stasch, J.P.; Evgenov, O.V. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb. Exp. Pharmacol. 2013, 218, 279–313. [Google Scholar] [PubMed]
- Ghofrani, H.-A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.-C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.-A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Frey, R.; Mück, W.; Unger, S.; Artmeier-Brandt, U.; Weimann, G.; Wensing, G. Single-Dose Pharmacokinetics, Pharmacodynamics, Tolerability, and Safety of the Soluble Guanylate Cyclase Stimulator BAY 63-2521: An Ascending-Dose Study in Healthy Male Volunteers. J. Clin. Pharmacol. 2008, 48, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Greene, S.J.; Butler, J.; Filippatos, G.; Lam, C.S.; Maggioni, A.P.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Kraigher-Krainer, E.; et al. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Pep-tide Levels in Patients with Worsening Chronic Heart Failure and Reduced Ejection Fraction: The Socrates-Reduced Randomized Trial. JAMA 2015, 314, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Maggioni, A.P.; Lam, C.S.; Pieske-Kraigher, E.; Butler, J.; Spertus, J.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Scalise, A.V.; et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: Results of the Soluble Guanylate Cyclase Stimulator in Heart Failure Patients with Preserved EF (Socrates-Preserved) Study. Eur. Heart J. 2017, 38, 1119–1127. [Google Scholar]
- Armstrong, P.W.; Lam, C.S.P.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; O’Connor, C.M.; Pieske, B.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; et al. Effect of Vericiguat vs Placebo on Quality of Life in Patients with Heart Failure and Preserved Ejection Fraction: The Vitality-HFpEF Randomized Clinical Trial. JAMA 2020, 324, 1512–1521. [Google Scholar] [CrossRef] [PubMed]

| Guanylyl Cyclase | Tissue Expression | Physiological Activator | Key Effects |
|---|---|---|---|
| Soluble α1 | Cardiovascular system, platelets, brain | NO, CO | Vasodilation, angiogenesis, inhibition of platelet aggregation |
| Soluble α2 | Cardiovascular system, brain | NO, CO | Vasodilation, angiogenesis |
| Soluble β1 | Cardiovascular system, platelets, brain | NO, CO | Vasodilation, angiogenesis inhibition of platelet aggregation, intestinal motility |
| Soluble β2 | Gastrointestinal tract, liver, kidney | NO, CO | Apoptosis, inhibition of anti-apoptotic endothelin pathway |
| GC-A | Cardiovascular system (vascular smooth muscle, heart), lung, kidney, adrenal, adipose tissue | ANP, BNP | Vasodilation, angiogenesis, regulation of hypertrophy, remodeling processes |
| GC-B | Cardiovascular system (vascular smooth muscle, endothelium, heart), lung, bone, brain, liver, uterus, follicle | CNP | Vasodilation, angiogenesis. regulation of hypertrophy, remodeling processes, cartilage homeostasis and endochondral bone formation, regulation of female fertility |
| GC-C | Intestinal epithelium | Guanylin, uroguanylin and bacterial heat-stable enterotoxin | Regulation of colonic epithelial cell proliferation |
| GC-D | Olfactory bulb | Guanylin, uroguanylin, CO2/HCO3 | guanylin- and uroguanylin-dependent olfactory signaling, food and odor preference response (mices) |
| GC-E | Retina, pineal gland | guanylyl cyclase activator proteins | Vision process |
| GC-F | Retina | guanylyl cyclase activator proteins | Vision process |
| GC-G | Olfactory bulb, lung, intestine, skeletal muscle, testes | Pheromones, CO2/HCO3 | detection of the volatile alarm pheromones, kidney, ischemia/reperfusion preconditioning |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grześk, G.; Nowaczyk, A. Current Modulation of Guanylate Cyclase Pathway Activity—Mechanism and Clinical Implications. Molecules 2021, 26, 3418. https://doi.org/10.3390/molecules26113418
Grześk G, Nowaczyk A. Current Modulation of Guanylate Cyclase Pathway Activity—Mechanism and Clinical Implications. Molecules. 2021; 26(11):3418. https://doi.org/10.3390/molecules26113418
Chicago/Turabian StyleGrześk, Grzegorz, and Alicja Nowaczyk. 2021. "Current Modulation of Guanylate Cyclase Pathway Activity—Mechanism and Clinical Implications" Molecules 26, no. 11: 3418. https://doi.org/10.3390/molecules26113418
APA StyleGrześk, G., & Nowaczyk, A. (2021). Current Modulation of Guanylate Cyclase Pathway Activity—Mechanism and Clinical Implications. Molecules, 26(11), 3418. https://doi.org/10.3390/molecules26113418







