Spruce Bark-Extracted Lignin and Tannin-Based Bioresin-Adhesives: Effect of Curing Temperatures on the Thermal Properties of the Resins
Abstract
1. Introduction
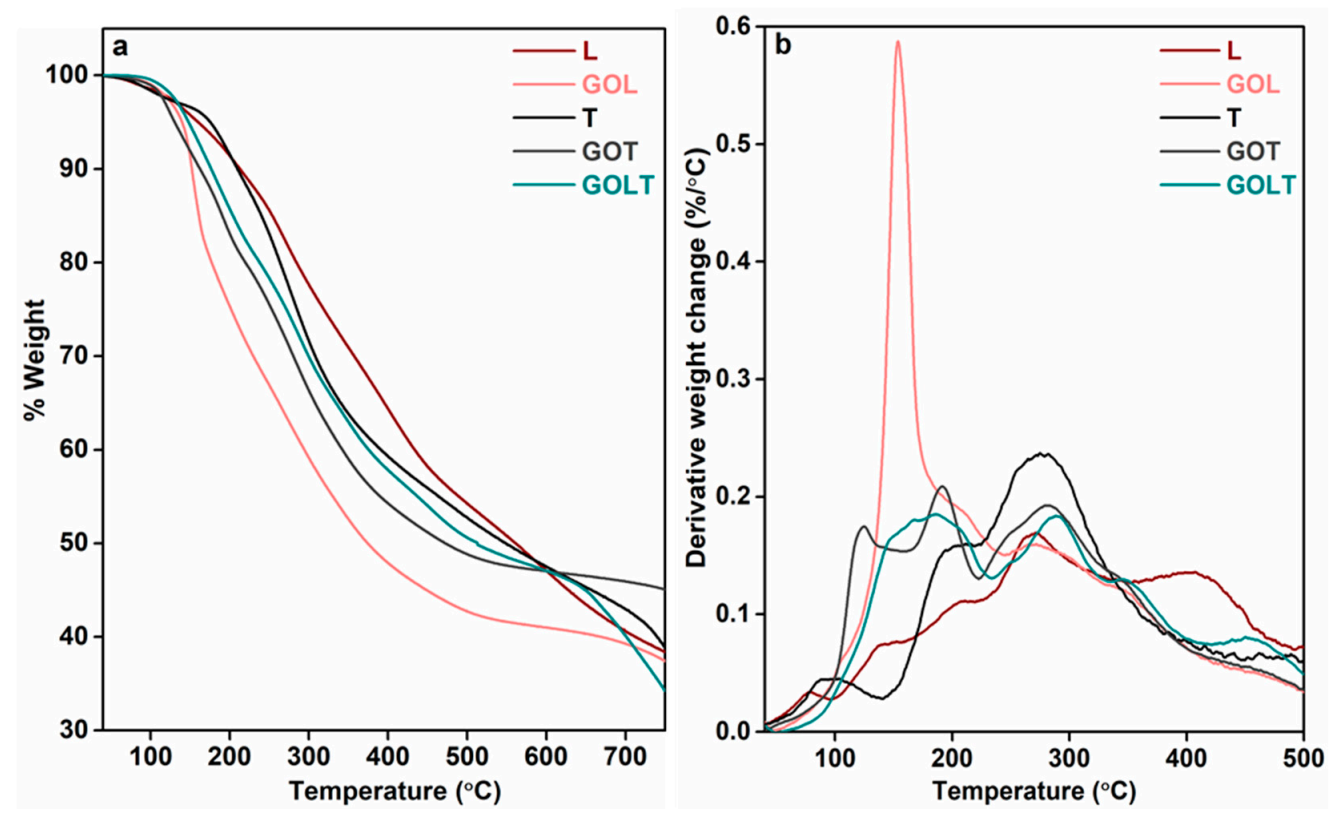
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Tannin and Lignin Extraction from Softwood Bark
3.3. Chemical Modifications
3.4. Resin Adhesive Formulation
3.5. Characterisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Moubarik, A.; Charrier, B.; Allal, A.; Charrier, F.; Pizzi, A. Development and optimization of a new formaldehyde-free cornstarch and tannin wood adhesive. Eur. J. Wood Wood Prod. 2010, 68, 167–177. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Moeller, B.C.; Lu, K.; Rager, J.E.; Fry, R.C.; Starr, T.B. Formaldehyde carcinogenicity research: 30 years and counting for mode of action, epidemiology, and cancer risk assessment. Toxicol. Pathol. 2013, 41, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Geng, X. Formaldehyde-free wood adhesives from decayed wood. Macromol. Rapid Commun. 2005, 26, 529–532. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Pu, Y.; Ragauskas, A. Cellulosic biorefineries—Unleashing lignin opportunities. Curr. Opin. Environ. Sustain. 2010, 2, 383–393. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Tabarsa, T.; Jahanshahi, S.; Ashori, A. Mechanical and physical properties of wheat straw boards bonded with a tannin modified phenol–formaldehyde adhesive. Compos. Part B Eng. 2011, 42, 176–180. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H. Ionic liquid modified lignin-phenol-glyoxal resin: A green alternative resin for production of particleboards. J. Adhes. 2019, 95, 1075–1087. [Google Scholar] [CrossRef]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery review: Wide-reaching products through kraft lignin. BioResources 2019, 14, 7543–7581. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Matsakas, L.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. Lignin-first biomass fractionation using a hybrid organosolv—Steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour. Technol. 2019, 273, 521–528. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.; Yuan, Q.; Huang, F. Study of chemical modification of alkaline lignin by the glyoxalation reaction. BioResources 2011, 6, 4523–4536. [Google Scholar]
- Mancera, C.; Ferrando, F.; Salvadó, J.; El Mansouri, N.E. Kraft lignin behavior during reaction in an alkaline medium. Biomass Bioenergy 2011, 35, 2072–2079. [Google Scholar] [CrossRef]
- Ramires, E.C.; Megiatto, J.D.; Gardrat, C.; Castellan, A.; Frollini, E. Biobased composites from glyoxal-phenolic resins and sisal fibers. Bioresour. Technol. 2010, 101, 1998–2006. [Google Scholar] [CrossRef]
- Konai, N.; Raidandi, D.; Pizzi, A.; Girods, P.; Lagel, M.-C.; Kple, M. Thermogravimetric analysis of anningre tannin resin. Maderas Ciencia y Tecnologia 2016, 18, 245–252. [Google Scholar] [CrossRef]
- Zhou, X.; Du, G. Applications of Tannin Resin Adhesives in the Wood Industry. In Tannins—Structural Properties, Biological Properties and Current Knowledge; Intech Open: London, UK, 2019. [Google Scholar]
- Bianchi, S.; Gloess, A.N.; Kroslakova, I.; Mayer, I.; Pichelin, F. Analysis of the structure of condensed tannins in water extracts from bark tissues of Norway spruce (Picea abies [Karst.]) and Silver fir (Abies alba [Mill.]) using Maldi-TOF mass spectrometry. Ind. Crop. Prod. 2014, 61, 430–437. [Google Scholar] [CrossRef]
- Navarrete, P.; Pizzi, A.; Tapin-Lingua, S.; Benjelloun-Mlayah, B.; Pasch, H.; Rode, K.; Delmotte, L.; Rigolet, S. Low formaldehyde emitting biobased wood adhesives manufactured from mixtures of tannin and glyoxylated lignin. J. Adhes. Sci. Technol. 2012, 26, 1667–1684. [Google Scholar] [CrossRef]
- Hellström, J.K.; Mattila, P.H. HPLC determination of extractable and unextractable proanthocyanidins in plant materials. J. Agric. Food Chem. 2008, 56, 7617–7624. [Google Scholar] [CrossRef]
- Tupa Esfandiyari, M.R.; Talaei-Pour, M.; Khademoleslam, H.; Mir Shokraei, S.A.; Bazyar, B. Investigating the possibility of making lignin-glyoxal resins as adhesives in the production of plywood. BioResources 2019, 14, 7122–7133. [Google Scholar] [CrossRef]
- Tondi, G. Tannin-based copolymer resins: Synthesis and characterization by solid state 13C NMR and FT-IR spectroscopy. Polymers 2017, 9, 223. [Google Scholar] [CrossRef]
- Stiefel, S.; Schmitz, A.; Peters, J.; Di Marino, D.; Wessling, M. An integrated electrochemical process to convert lignin to value-added products under mild conditions. Green Chem. 2016, 18, 4999–5007. [Google Scholar] [CrossRef]
- De Hoyos-Martínez, P.L.; Robles, E.; Khoukh, A.; Charrier-El Bouhtoury, F.; Labidi, J. Formulation of Multifunctional Materials Based on the Reaction of Glyoxalated Lignins and a Nanoclay/Nanosilicate. Biomacromolecules 2019, 20, 3535–3546. [Google Scholar] [CrossRef] [PubMed]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Fast curing bio-based phenolic resins via lignin demethylated under mild reaction condition. Polymers 2017, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Wood products and green chemistry. Ann. For. Sci. 2016, 73, 185–203. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Di, M. Green modification of corn stalk lignin and preparation of environmentally friendly lignin-based wood adhesive. Polymers 2018, 10, 631. [Google Scholar] [CrossRef]
- Ang, A.; Ashaari, Z.; Bakar, E.S.; Ibrahim, N.A. Characterization and Optimization of the Glyoxalation of a Methanol-Fractionated Alkali Lignin using Response Surface Methodology. BioResources 2015, 10, 16. [Google Scholar] [CrossRef]
- Yubo, T.; Shujun, L.; Peng, L.; Qinglin, W. Thermogravimetric analyses (TGA) of lignins isolated from the residue of corn stover bioethanol (CSB) production. Holzforschung 2016, 70, 1175–1182. [Google Scholar] [CrossRef]
- Faris, A.H.; Rahim, A.A.; Ibrahim, M.N.M.; Alkurdi, A.M.; Shah, I. Combination of lignin polyol-tannin adhesives and polyethylenimine for the preparation of green water-resistant adhesives. J. Appl. Polym. Sci. 2016, 133, 43437. [Google Scholar] [CrossRef]
- Yadav, P.; Athanassiadis, D.; Antonopoulou, I.; Rova, U.; Christakopoulos, P.; Tysklind, M.; Matsakas, L. Environmental impact and cost assessment of a novel lignin production method. J. Clean. Prod. 2021, 279, 123515. [Google Scholar] [CrossRef]
- Elgailani, I.E.H.; Ishak, C.Y. Determination of tannins of three common Acacia species of Sudan. Adv. Chem. 2014, 2014, 192708. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012; pp. 1–18. [Google Scholar]
- Trubetskaya, A.; Lange, H.; Wittgens, B.; Brunsvik, A.; Crestini, C.; Rova, U.; Christakopoulos, P.; Leahy, J.J.; Matsakas, L. Structural and Thermal Characterization of Novel Organosolv Lignins from Wood and Herbaceous Sources. Processes 2020, 8, 860. [Google Scholar] [CrossRef]

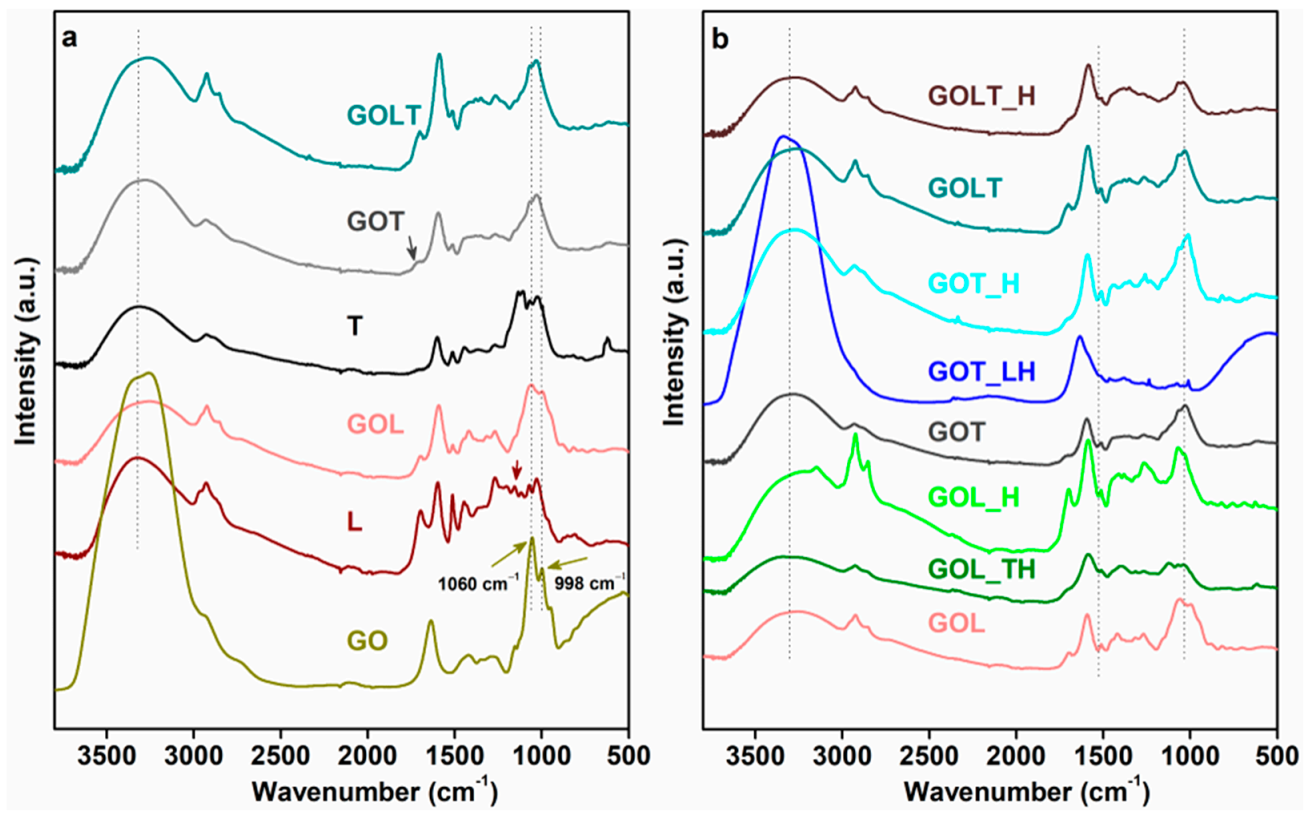
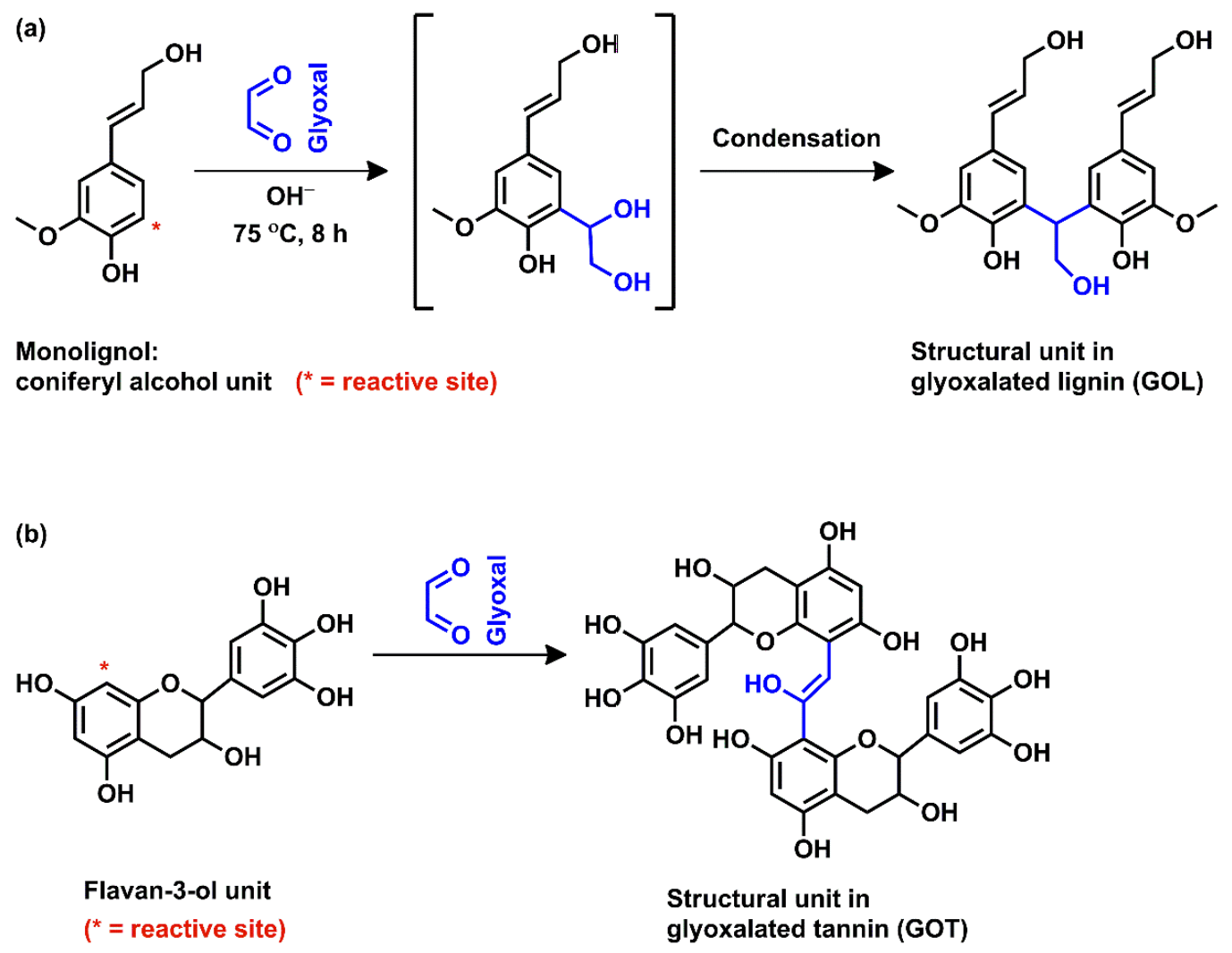

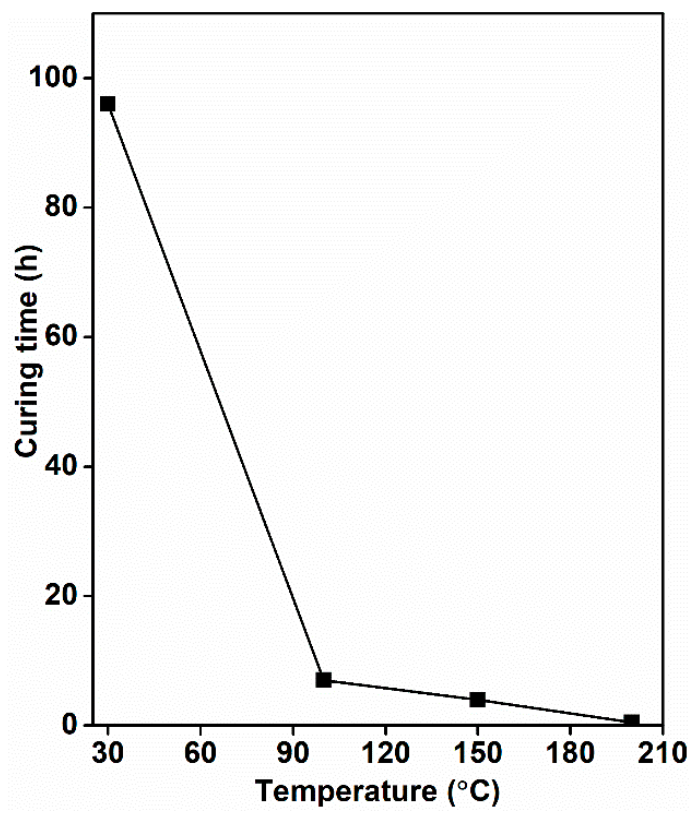
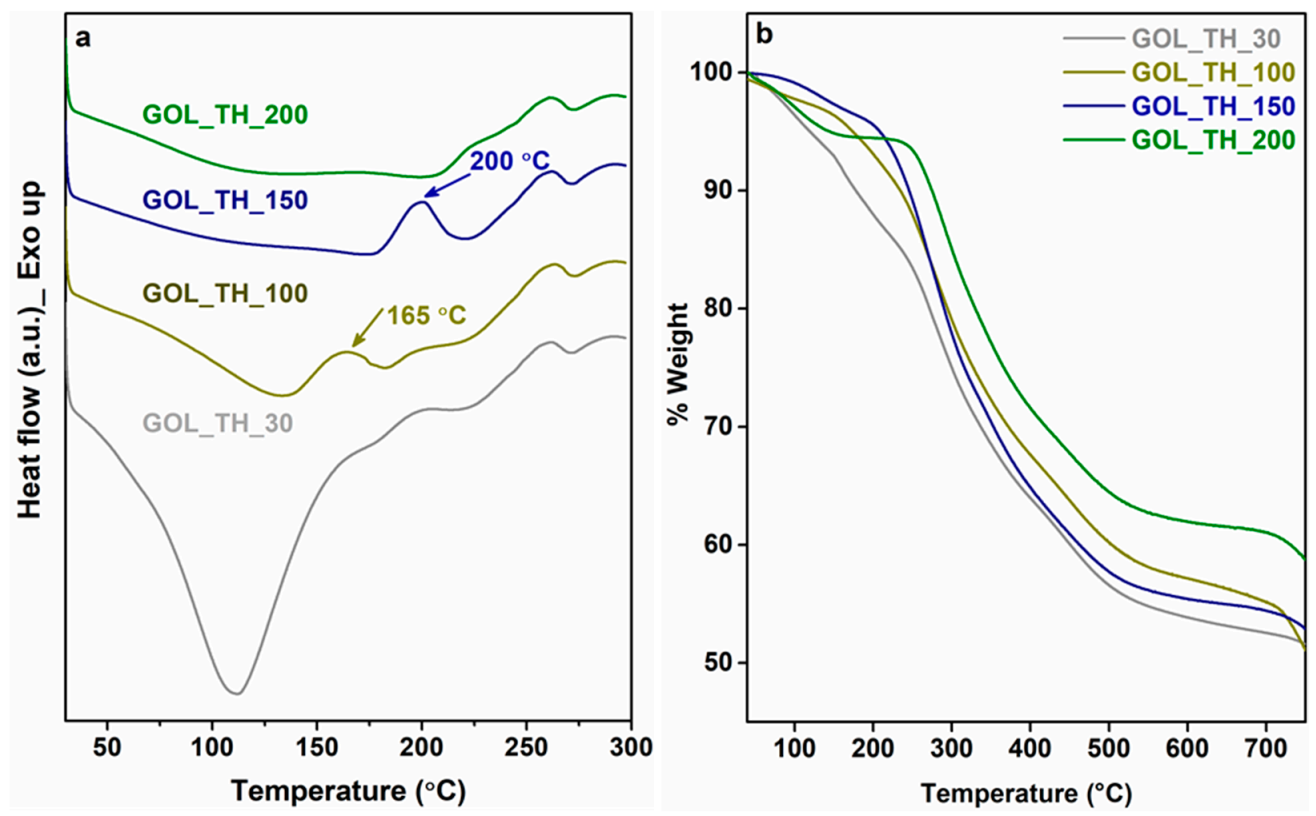


| Samples | T10 (°C) | T30 (°C) | T50 (°C) | % Residue |
|---|---|---|---|---|
| L | 211 | 358 | 561 | 39 |
| T | 209 | 308 | 545 | 45 |
| GOL | 150 | 231 | 374 | 37 |
| GOT | 164 | 279 | 471 | 38 |
| GOLT | 177 | 298 | 509 | 34 |
| GOL_TH | 276 | 416 | - | 58 |
| GOL_H | 172 | 291 | 472 | 28 |
| GOT_LH | 160 | 344 | 711 | 48 |
| GOT_H | 232 | 346 | 762 | 46 |
| GOLT_H | 259 | 397 | 800 | 51 |
| Sample Names | Compositions (wt%) | Hexamine (H) (wt%) | Curing Temperature (°C) | Curing Time (h) |
|---|---|---|---|---|
| GOL_TH_30 | GOL (40%), T (60%) | 6 | 30 | 96 |
| GOL_TH_100 | GOL (40%), T (60%) | 6 | 100 | 7 |
| GOL_TH_150 | GOL (40%), T (60%) | 6 | 150 | 4 |
| GOL_TH_200 | GOL (40%), T (60%) | 6 | 200 | 0.5 |
| GOL_H_200 | GOL | 6 | 200 | 0.5 |
| GOT_LH_200 | GOT (40%), L (60%) | 6 | 200 | 0.5 |
| GOT_H_200 | GOT | 6 | 200 | 0.5 |
| GOLT_H_200 | GOLT | 6 | 200 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sain, S.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Öman, T.; Skrifvars, M. Spruce Bark-Extracted Lignin and Tannin-Based Bioresin-Adhesives: Effect of Curing Temperatures on the Thermal Properties of the Resins. Molecules 2021, 26, 3523. https://doi.org/10.3390/molecules26123523
Sain S, Matsakas L, Rova U, Christakopoulos P, Öman T, Skrifvars M. Spruce Bark-Extracted Lignin and Tannin-Based Bioresin-Adhesives: Effect of Curing Temperatures on the Thermal Properties of the Resins. Molecules. 2021; 26(12):3523. https://doi.org/10.3390/molecules26123523
Chicago/Turabian StyleSain, Sunanda, Leonidas Matsakas, Ulrika Rova, Paul Christakopoulos, Tommy Öman, and Mikael Skrifvars. 2021. "Spruce Bark-Extracted Lignin and Tannin-Based Bioresin-Adhesives: Effect of Curing Temperatures on the Thermal Properties of the Resins" Molecules 26, no. 12: 3523. https://doi.org/10.3390/molecules26123523
APA StyleSain, S., Matsakas, L., Rova, U., Christakopoulos, P., Öman, T., & Skrifvars, M. (2021). Spruce Bark-Extracted Lignin and Tannin-Based Bioresin-Adhesives: Effect of Curing Temperatures on the Thermal Properties of the Resins. Molecules, 26(12), 3523. https://doi.org/10.3390/molecules26123523









