Modified Exfoliated Carbon Nanoplatelets as Sorbents for Ammonium from Natural Mineral Waters
Abstract
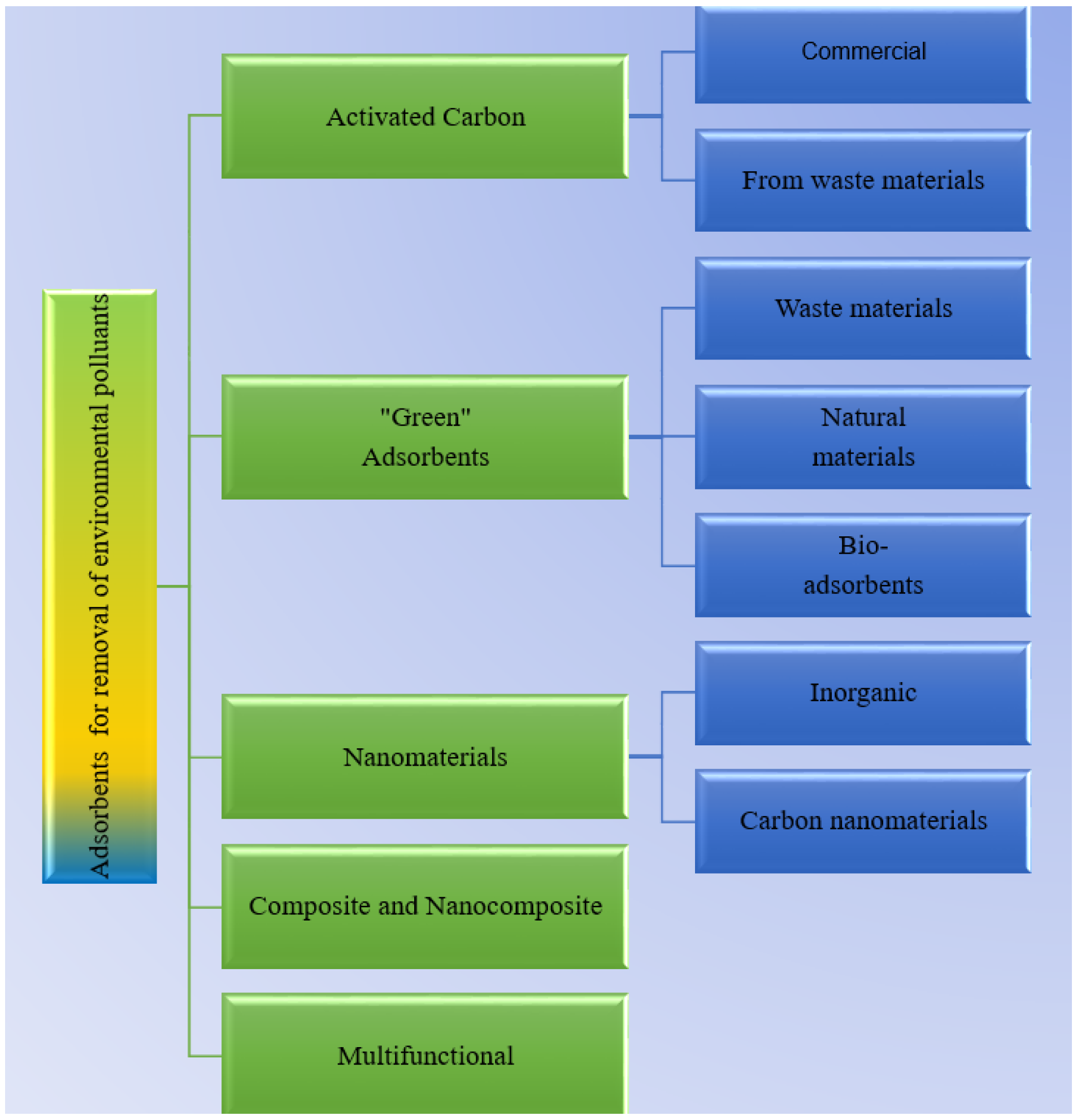
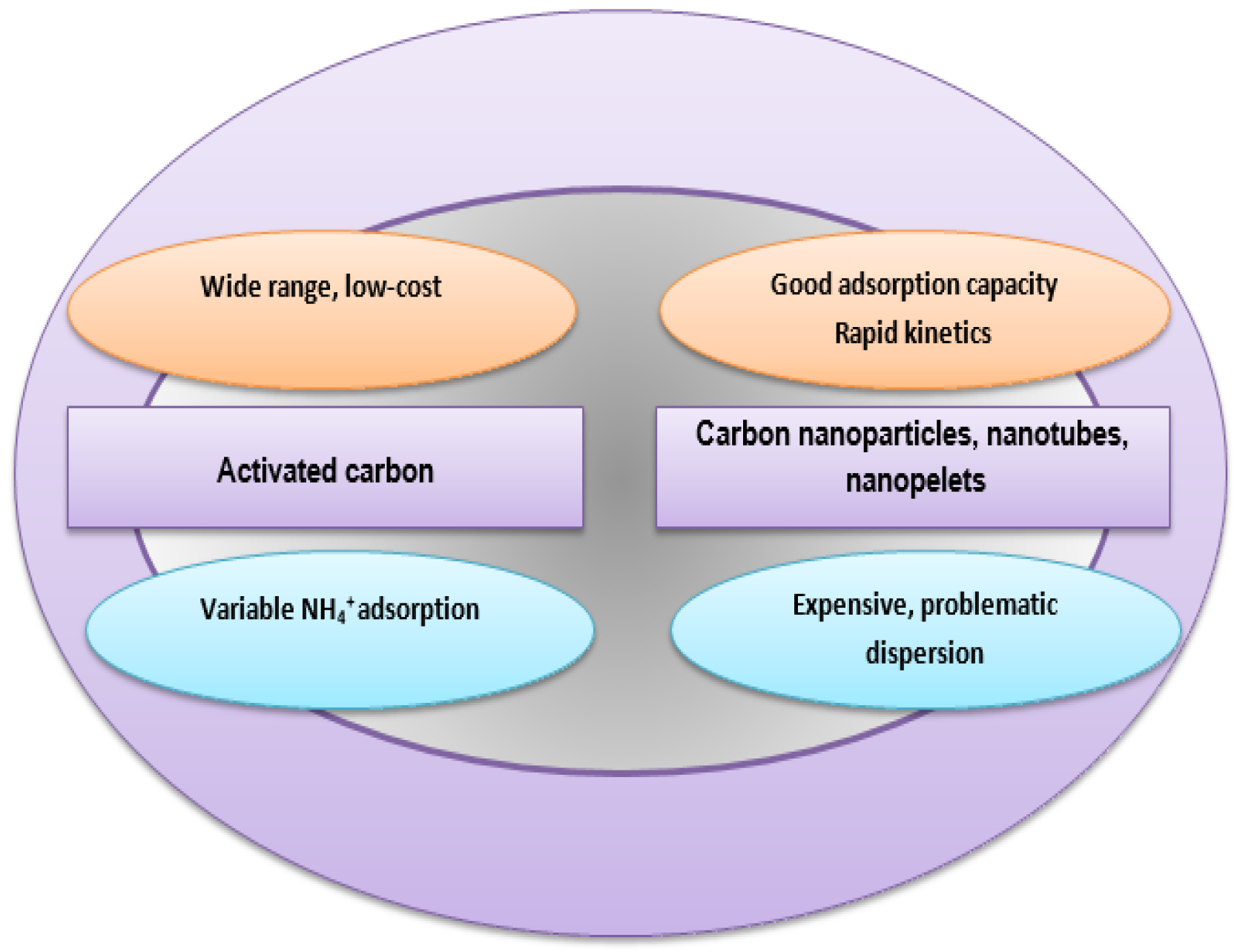
1. Introduction
2. Results
2.1. Characterization of Oxidized xGnP
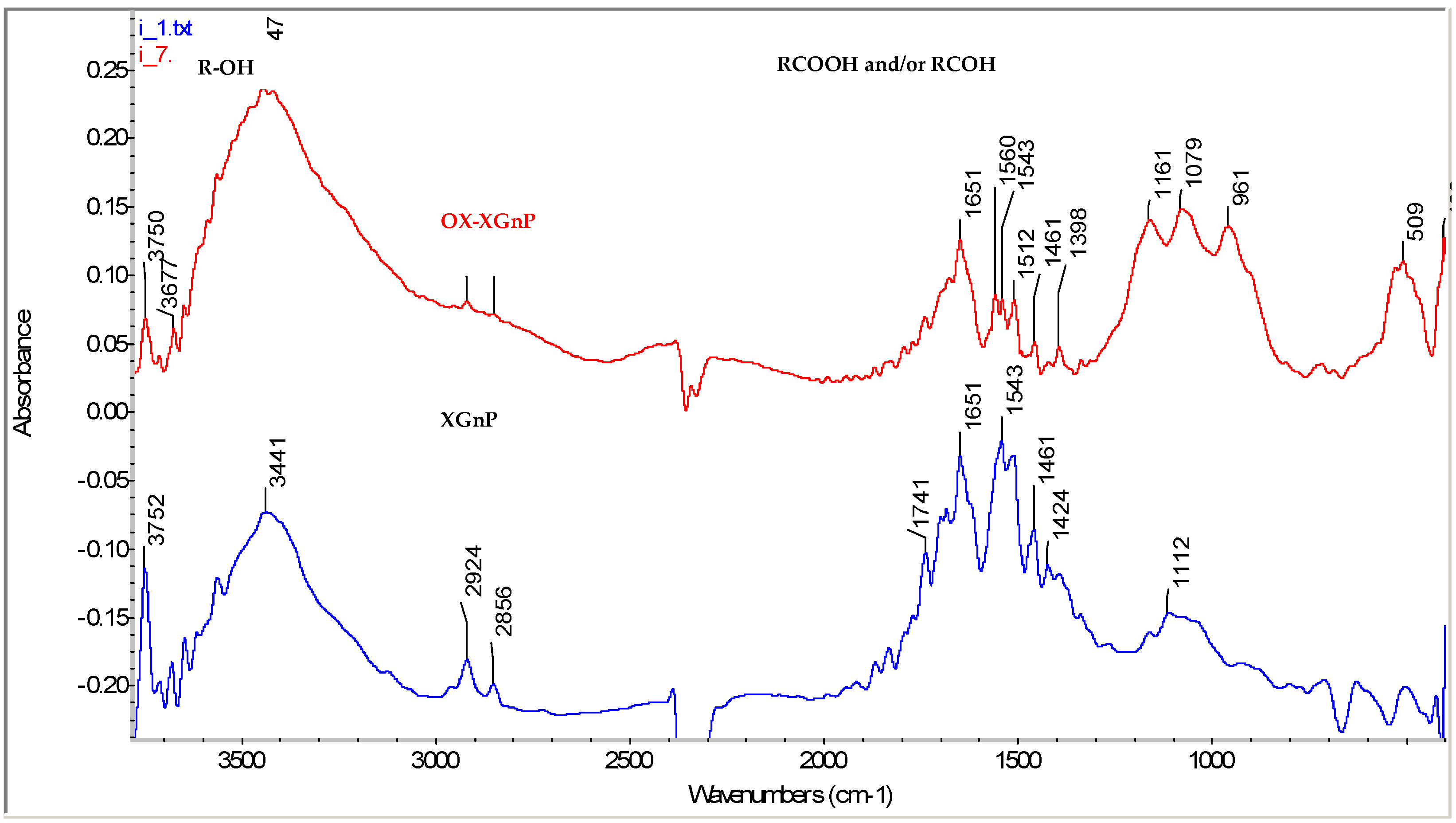
2.1.1. Fourier Transform Infrared Spectra (FTIR) Spectra
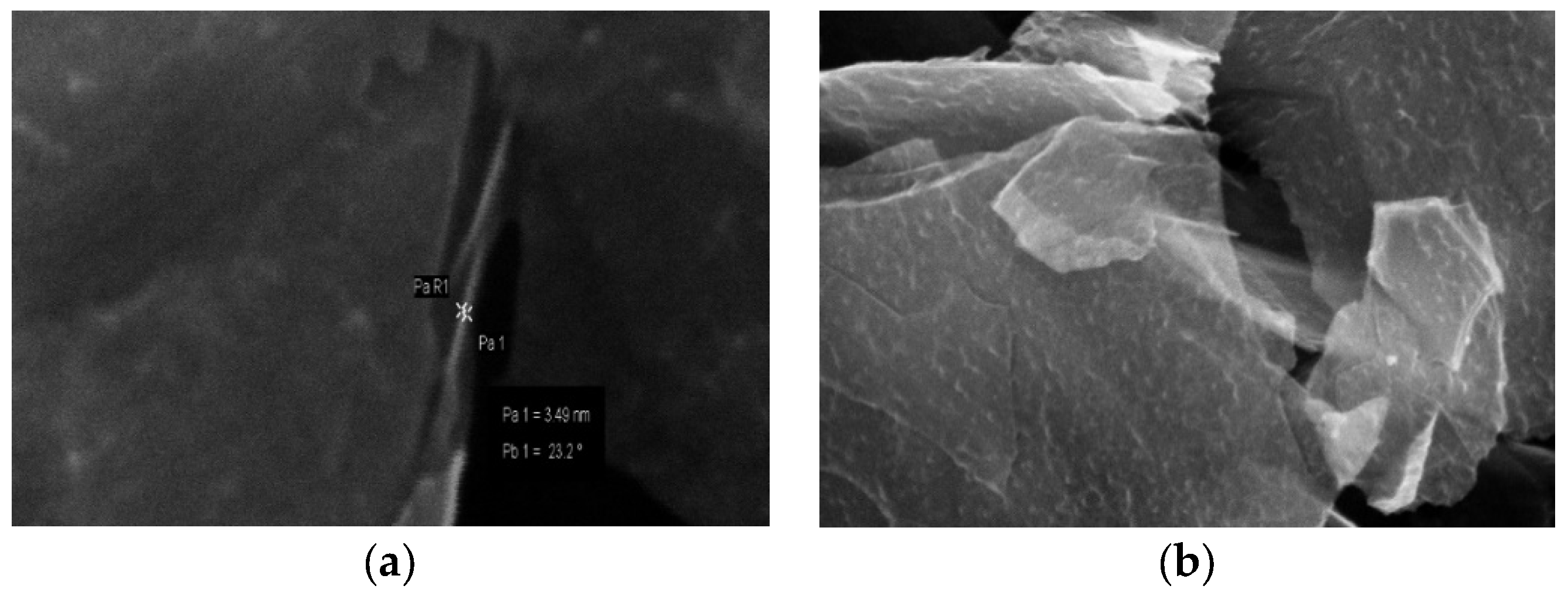
2.1.2. Scanning Electron Microscopy (SEM)
2.1.3. Thermogravimetric Analysis
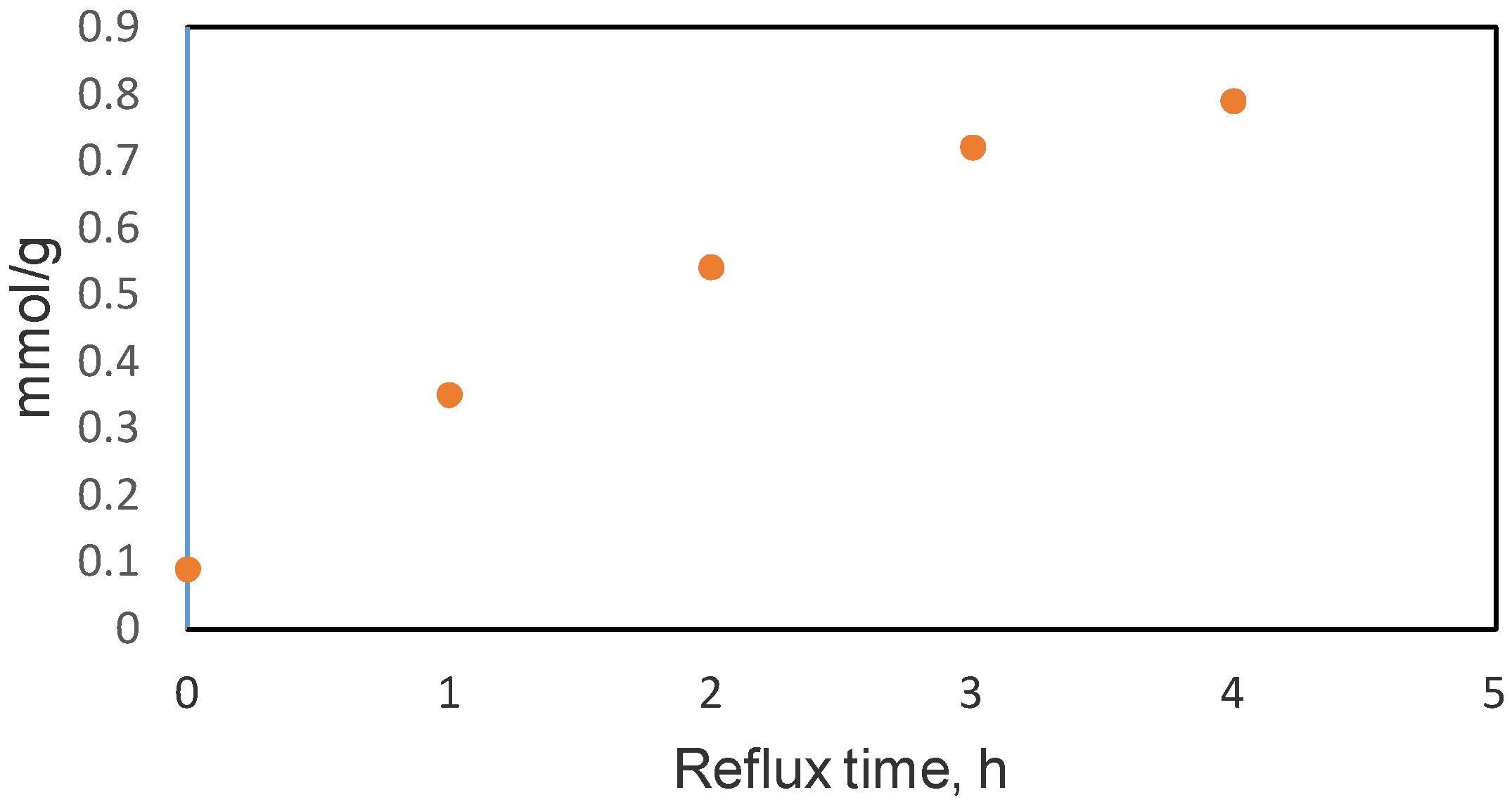
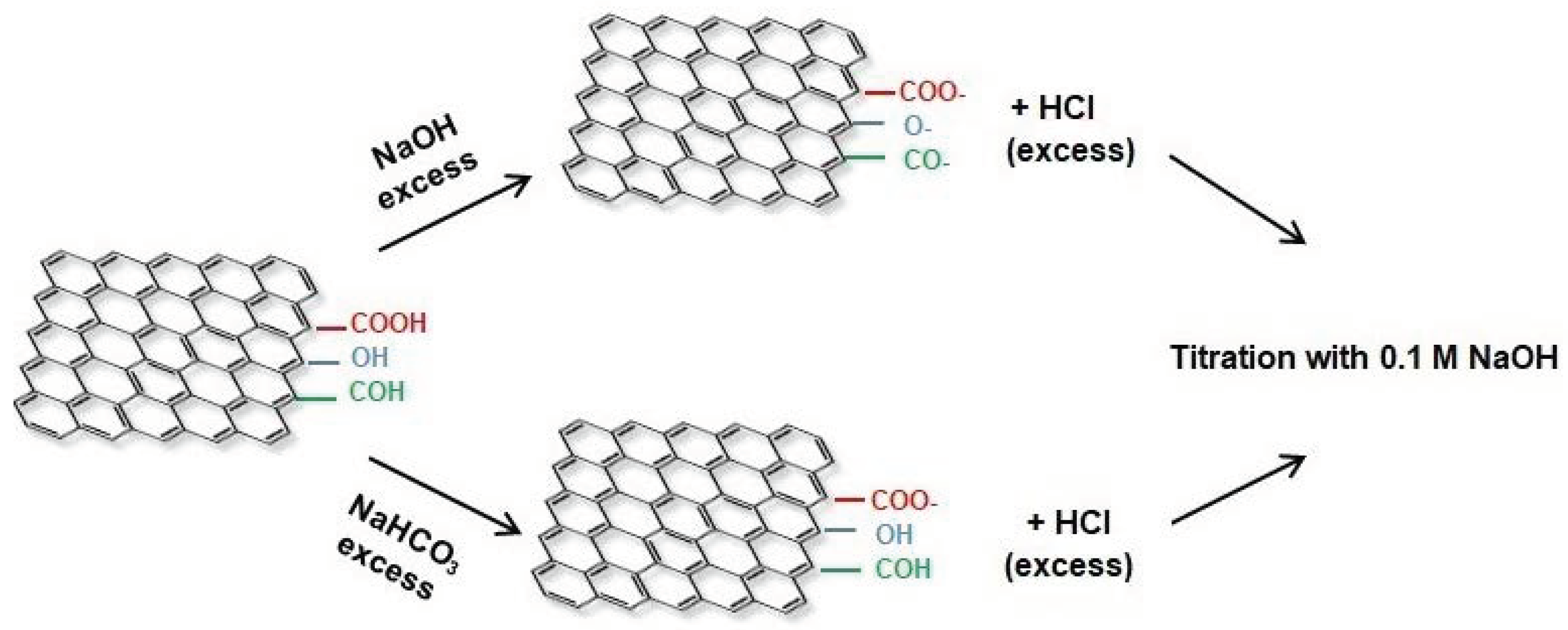
2.1.4. Boehm’s Titration
3. Discussion
3.1. Adsorption Studies
3.1.1. Kinetic Results
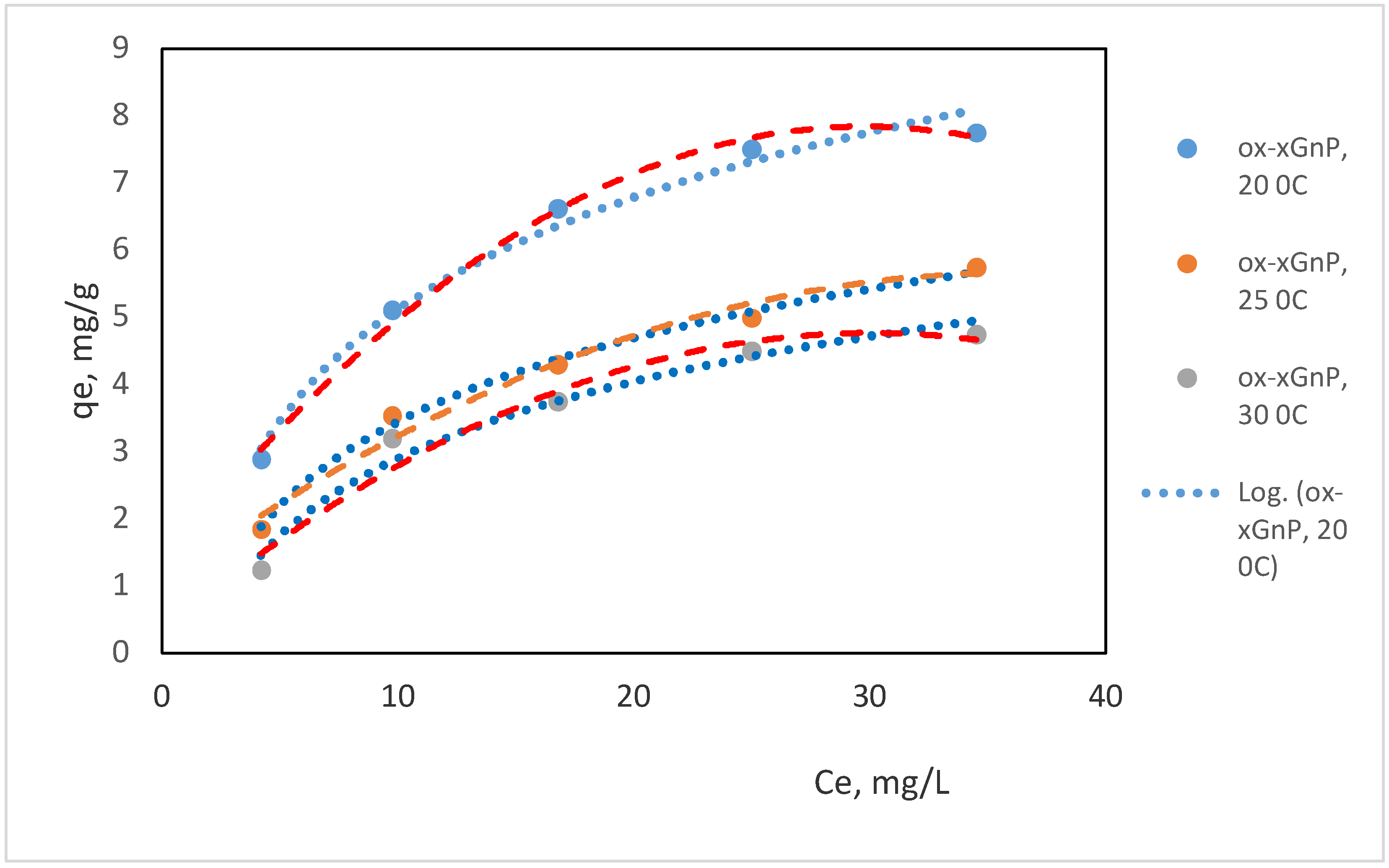
3.1.2. Adsorption Isotherm Study
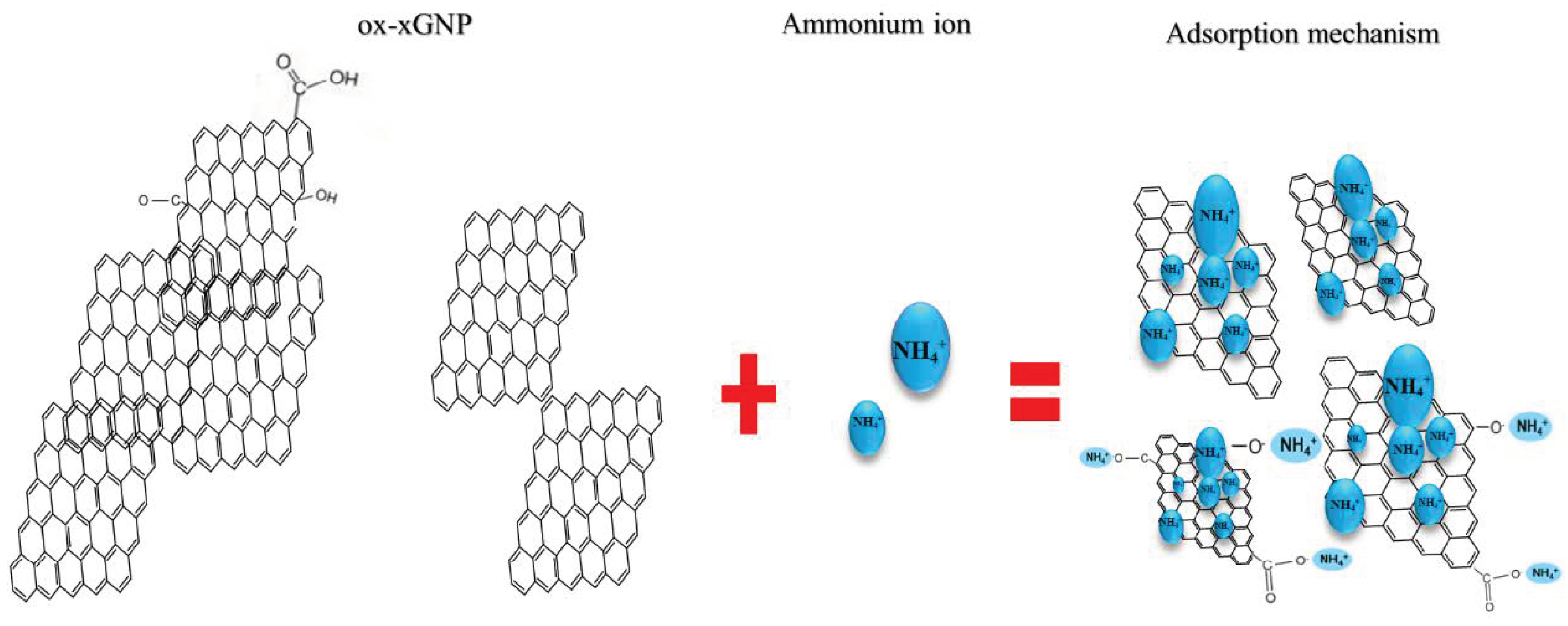
3.1.3. Adsorption Mechanism
4. Materials and Methods
4.1. Chemical Reagents and Instrumentation
4.2. Oxidation of xGnP
4.3. Batch Sorption Experiments
4.4. Analytical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Olariu, A.; Palcu, M. The origin of ammonium in carbonated mineral waters and its underground transport to one production well in Middle Ciuc Depression from Eastern Carpathians. Int. Symp. Environ. Ind. 2019, 2019, 259–278. [Google Scholar] [CrossRef]
- Katz, M.J.; Howarth, A.J.; Moghadam, P.Z.; DeCoste, J.B.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. High volumetric uptake of ammonia using Cu-MOF-74/Cu-CPO-27. Dalton Trans. 2016, 45, 4150–4153. [Google Scholar] [CrossRef]
- European Parliament, Council of the European Union. Directive 2009/54/EC of the European Parliament and of the Council of 18 June 2009 on the exploitation and marketing of natural mineral waters. Off. J. Eur. Union 2009, 164, 45–58. [Google Scholar]
- Singh, N.B.; Nagpal, G.; Agraval, S.; Rachna. Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Vu, M.T.; Chao, H.P.; Van Trinh, T.; Let, T.T.; Lin, C.C.; Tran, H.N. Removal of ammonium from groundwater using NaOH treated activated carbon derived from corncob wastes: Batch and column experiments. J. Clean. Prod. 2018, 180, 560–570. [Google Scholar] [CrossRef]
- Calin, M.R.; Ion, A.C.; Radulescu, I. Evaluation of quality parameters and of natural radionuclides concentrations in natural mineral water in Romania. J. Radioanal. Nucl. Chem. 2015, 303, 305–313. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Ai, Y.; Liu, Y.; Ji, Y.; Wang, H.; Hayat, T.; Alsaedi, A.; HU, W.; Wang, X. β-Cyclodextrin modified graphitic carbon nitride for the removal of pollutants from aqueous solution: Experimental and theoretical calculation study. J. Mater. Chem. A 2016, 4, 14170–14179. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Ai, Y.; Liu, Y.; Li, J.; Ji, Y.; Wang, X. Coagulation Behavior of Graphene Oxide on Nanocrystallined Mg/Al Layered Double Hydroxides: Batch Experimental and Theoretical Calculation Study. Environ. Sci. Technol. 2016, 50, 3658–3667. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Yan, T.; Li, T.X.; Wang, R.Z.; Jia, R. Experimental investigation on the ammonia adsorption and heat transfer characteristics of the packed multi-walled carbon nanotubes. Appl. Therm. Eng. 2015, 77, 20–29. [Google Scholar] [CrossRef]
- Yang, H.; Zuttel, H.; Kim, S.; Ko, Y.; Kim, W. Effect of Boron Doping On Graphene Oxide for Ammonia Adsorption. ChemNanoMat 2017, 3, 792–797. [Google Scholar] [CrossRef]
- Ion, I.; Culetu, A.; Gherase, D.; Sarbu, F.; Ion, A.C. Environmental applications of carbon based nanomaterials II. In Micro and Nanoengineering, 2nd ed.; Ion, A.C., Dascalu, D., Carja, C., Eds.; Romanian Academy, Publishing House of the Romanian Academy: Bucharest, Romania, 2014; Volume 22, pp. 33–51. [Google Scholar]
- Chim, Y.J.; Shin, T.S.; Choi, H.D.; Kwon, J.J.; Chung, Y.; Yoon, H.G. Electrical conductivity of chemically modified multiwalled carbon nanotube/epoxy composites. Carbon 2005, 43, 23–30. [Google Scholar] [CrossRef]
- Kim, Y.; Mitani, T. Competitive effect of carbon nanotubes oxidation on aqueous EDLC performance: Balancing hydrophilicity and conductivity. J. Power Sources 2006, 158, 1517–1522. [Google Scholar] [CrossRef]
- Allen, B.L.; Kichambare, P.D.; Star, A. Carbon Nanotube Field-Effect-Transistor-Based Biosensors. Adv. Mater. 2007, 19, 1439–1451. [Google Scholar] [CrossRef]
- Murphy, H.; Papakonstantinou, P.; Okpalugo, T.I.T. Raman study of multiwalled carbon nanotubes functionalized with oxygen groups. J. Vac. Sci. Technol. B 2006, 24, 715. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Naumenko, V.; Kuznetsova, A.; Yates, J.T., Jr.; Liu, J.; Smiley, R.E. Surface defect site density on single walled carbon nanotubes by titration. Chem. Phys. Lett. 2000, 324, 213–216. [Google Scholar] [CrossRef]
- Li, Y.; Pu, Z.; Sun, Q.; Pan, N. A review on novel activation strategy on carbonaceous materials with special morphology/texture for electrochemical storage. J. Energy Chem. 2021, 60, 572–590. [Google Scholar] [CrossRef]
- Gonzales-Guerrero, A.B.; Mendoza, E.; Pellicer, E.; Alsina, F.; Fernandez-Sanchez, C.; Lechuga, L.M. Discriminating the carboxylic groups from the total acidic sites in oxidized multi-wall carbon nanotubes by means of acid-base titration. Chem. Phys. Lett. 2008, 462, 256–259. [Google Scholar] [CrossRef]
- Khalil, A.; Seergeevich, S.N.; Borisova, V. Removal of ammonium from fish farms by biochar obtained from rice straw: Isotherm and kinetic studies for ammonium adsorption. Adsorpt. Sci. Technol. 2018, 36, 1–16. [Google Scholar] [CrossRef]
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.; Liu, Y. Influence of the pyrolysis temperature on production digested sludge biochar and its application for ammonium removal from municipal wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Fan, R.; Chen, C.L.; Li, Y.J.; Tzeng, J.H.; Huang, C.P.; Dong, C. Adsorption characteristics of ammonium ion onto hydrous biochars in dilute aqueous solutions. Bioresour. Technol. 2019, 272, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Butterly, C.; Zhang, W.; He, J.; Chen, D. Adsorbent materials for ammonium and ammonia removal: A review. J. Clean. Prod. 2021, 283, 124611. [Google Scholar] [CrossRef]
- Tajdemir, A.; Cengiz, I.; Yildiz, E.; Bayhan, Y.K. Investigation of ammonia stripping with a hydrodynamic cavitation reactor. Ultrason. Sonochem. 2020, 60, 104741. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.T.; Liang, Y.; Han, X.; Liu, Y.; Wu, S.H.; Bai, H.; Jia, S.Y. Photocatalytic oxidation of aqueous ammonia by Ag2O/TiO2 (P25): New insights into selectivity and contributions of different oxidative species. Appl. Surf. Sci. 2020, 504, 144433. [Google Scholar] [CrossRef]
- Guadayol, M.; Cortina, M.; Guadayol, J.M.; Caixhah, J. Determination of dimethyl selenide and dimethyl sulphide compounds causing off-flavours in bottled mineral waters. Water Res. 2016, 92, 149–155. [Google Scholar] [CrossRef]
- Luo, Z.; He, Y.; Zhi, D.; Luo, L.; Sun, Y.; Khan, E.; Wang, L.; Peng, Y.; Zhou, Y.; Tsang, D.C.W. Current progress in treatment techniques of triclosan from wastewater: A review. Sci. Total Environ. 2019, 696, 133990. [Google Scholar] [CrossRef]
- Zhang, P.; Zeng, X.Z.; Wen, X.H.; Yang, C.K.; Ouyang, S.D.; Li, P.; Gu, Z.; Wu, D.S.; Frost, R.L. Insights into efficient removal and mechanisms for ammonium from aqueous solution on trcicalcium aluminate. Chem. Eng. J. 2019, 366, 11–20. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Gao, F.; Xue, Y.; Deng, P.; Cheng, X.; Yang, K. Removal of aqueous ammonium by biochars derived from agricultural residuals at different pyrolysis temperatures. Chem. Spec. Bioavailab. 2015, 27, 92–97. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hammed, B.H. Insights into the modelling of adsorption isotherms systems. Chem. Eng. J. 2018, 158, 2–10. [Google Scholar]
- Nazari, M.A.; Mohaddes, F.; Pramanik, B.K.; Othman, M.; Muster, T.; Bulyan, M.A. Application of victorian brown coal for removal of ammonium and organics from wastewater. Environ. Technol. 2018, 39, 1041–1051. [Google Scholar] [CrossRef]
- Ion, A.C.; Ion, I.; Culetu, A. Lead adsorption onto exfoliated graphitic nanoplatelets in aqueous solutions. Mater. Sci. Eng. B 2011, 176, 504–509. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon-nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Quach, N.K.N.; Yang, W.D.; Chung, Z.J.; Tran, H.L. The influence of the activation temperature on the structural properties of the activated carbon xerogels and their electrochemical performance. Ann. Mater. Sci. Eng. 2017, 9. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure and anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Tu, Y.N.; Feng, P.; Ren, Y.G.; Cao, Z.H.; Wang, R.; Xu, Z.Q. Adsorption of ammonia nitrogen on lignite and its influence on coal water slurry preparation. Fuel 2019, 238, 34–43. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Li, C.; Cheng, C.; Zheng, Y. Adsorption of ammonium by graphene oxide based composites prepared by UV irradiation and using a slow-release fertilizer. J. Polym. Environ. 2018, 26, 4311–4320. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.P.; Zhu, J.X.; Xi, Y.F.; Zhu, R.L.; Ma, L.Y.; Tan, Q. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef]
- Rambabu, N.; Rao, B.V.S.K.; Surisetty, V.R.; Das, U.; Dalai, A.K. Production, characterization and evaluation of activated carbon from de-oiled canola meal for environmental applications. Ind. Crop. Prod. 2015, 65, 572–581. [Google Scholar] [CrossRef]
- Sumaraj, X.; Sarmah, A.K.; Padhye, L.P. Acidic surface functional groups control chemisorption of ammonium onto carbon materials in aqueous media. Sci. Total Environ. 2020, 698, 134193. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, D.; Rizea, G.A.; Ion, I.; Ion, A.C. Ammonium adsorption on exfoliated graphite nanoplatelets. Rev. Chim. 2016, 67, 2231–2236. [Google Scholar]
- Bogdan, D.; Muklive, A.J.S.; Ion, I.; Ion, A.C. Ammonium adsorption on oxidized exfoliated graphite nanoplatelets. Environ. Eng. Manag. J. 2017, 16, 543–552. [Google Scholar] [CrossRef]
- Mathurasa, S.; Damrongsiri, S. Low cost and easy rice husk modification to efficiently enhance ammonium and nitrate adsorption. Int. J. Recycl. Org. Waste Agric. 2018, 7, 143–151. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, Z.; Peng, H.; Qin, Y.; Li, G.; Chen, K. Nitrogen and oxygen-containing activated carbon nanotubes with improved capacitive properties. RSC Adv. 2014, 4, 5524–5530. [Google Scholar] [CrossRef]
- Valcarcel, M.; Cardenas, S.; Simonet, B.M.; Moliner-Martinez, Y.; Lucena, R. Carbon nanostructures as sorbent materials in analytical processes. Trends Anal. Chem. 2008, 27, 34–43. [Google Scholar] [CrossRef]
- Gerasimova, A.; Smolzky, G.; Melezhyc, A.; Galunin, E.; Memetov, N.; Tkakhev, A. Synthesis, Study and Applications of Graphene Materials. In Proceedings of the 4th World Congress on Recent Advances in Nanotechnology (RAN’19), ICNNFC 103, Rome, Italy, 14–16 April 2019. [Google Scholar] [CrossRef]
- Assis, L.K.; Damasceno, B.S.; Carvalho, M.N.; Oliveira, E.H.C.; Ghislandi, M.G. Adsorption capacity comparison between graphene oxide and graphene nanoplatelets for the removal of coloured textile dyes from wastewater. Environ. Technol. 2020, 41, 2360–2371. [Google Scholar] [CrossRef]
- Ion, I.; Bogdan, D.; Ion, A.C. Improvement in the determination of traces of nitrate and nitrite in natural mineral waters by ion chromatography. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2014, 76, 113–122. [Google Scholar]
- Chen, J.; Lu, H.; Chen, Y.; Tao, Z.; Shao, M. Stable aqueous dispersion of polymer functionalized graphene sheets from electrochemical exfoliation for anticorrosion application. Colloid Polym. Sci. 2017, 295, 1951–1959. [Google Scholar] [CrossRef]


| Approach | Conditions | Strengths | Weaknesses | Removal Efficiency, % | Ref. |
|---|---|---|---|---|---|
| Adsorption | Different temperatures and pH values | Simple equipment | Based on the adsorbent | 43–100 | [23] |
| Membrane Purification | pH: 8–12 temp. >15 °C | Applied in complex matrices | Energy and time consuming | 50–90 | [24] |
| Photocatalysis | In liquid phase | Sustainable method | Variable efficiency | 35–80 | [25] |
| Ion exchange | Waste materials | Variable efficiency | 50–100 | [26] | |
| Combined methods | Different reaction mechanisms are involved | Limitations of each mechanism are balanced by advantages of other components | Complex studies are developed by using new multi-functional nano-sorbents | Variable | [27,28] |
| Sample | Specific Surface 1 Area SBET, m2 g−1 | pH of the Point of Zero Charge, pHPZC 2 | Carboxyl 3 (mmol g−1) | Lactone 3 (mmol g−1) | Phenol 3 (mmolg−1) | Ref. |
|---|---|---|---|---|---|---|
| As-grown xGnP | 110 | 8.5 | - | - | - | [31] |
| HNO3 oxidized xGnP, reflux time one hour | 174 | 2.7 | 0.35 | 1.40 | 1.72 | [32] |
| KMnO4 oxidized xGnP | 153 | 4.3 | 0.87 | 1.26 | 1.09 | [33] |
| NaOH treated xGnP | 135 | 8.9 | 0.05 | 0.03 | 0.02 | [32] |
| 65% HNO3, reflux time 3 h, oxidized xGnP | 208 | 5.8 | 0.44 | 1.25 | 0.31 | This paper |
| Sorbent, (25 °C) | qeexp. (mg g−1) | The First-Order Kinetic Model | The Second-Order Kinetic Model | Intra-Particle Diffusion Model | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qecal. (mg g−1) | k1 (min−1) | R2 | qecal. (mg g−1) | k2 (g mg−1 min−1) | R2 | kid (mg g−1 min−1/2) | C (mg g−1) | R2 | |||
| Lignite | 3.43 | 1.31 | 0.533 | 0.7080 | 0.33 | 5.949 | 0.999 | 1.64 | 1.17 | 0.9780 | [37] |
| GO | 6.6 | 3.53 | 0.071 | 0.9574 | 6.95 | 0.037 | 0.9967 | [38] | |||
| Ox-xGnP | 6.15 | 1.76 | 0.035 | 0.9380 | 1.47 | 0.021 | 0.9900 | 0.2604 | 3.01 | 0.9750 | [32] |
| xGnP | 5.65 | 4.14 | 0.042 | 0.9826 | 2.79 | 0.001 | 0.9831 | [31] | |||
| Ox-xGnP This paper | 6.76 | 4.43 | 0.056 | 0.9238 | 5.26 | 0.046 | 0.9964 | 0.922 | 0.58 | 0.9976 | This work |
| T (K) | Freundlich | Langmuir | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Performance | Parameter | Performance | |||||
| KF | n | 1/n | R2 | KL (L mg−1) | qm (mg g−1) | RL | R2 | |
| 293 | 1.91 | 1.45 | 0.41 | 0.9851 | 0.0736 | 12.04 | 0.49 | 0.9166 |
| 298 | 0.79 | 1.51 | 0.54 | 0.9992 | 0.0346 | 10.51 | 0.45 | 0.9922 |
| 303 | 0.58 | 1.50 | 0.58 | 0.9652 | 0.0373 | 8.17 | 0.42 | 0.9768 |
| Sorbent | T °C | Freundlich | Langmuir | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| 1/n | KF (mg g−1) | R2 | qm (mg g−1) | KL (L mg−1) | R2 | |||
| NaOH Lignite | 20 | 0.43 | 0.17 | 0.9570 | 0.67 | 0.392 | 0.997 | [32] |
| NaOH AC | 20 | 2.05 | 1.81 | 0.9650 | 17.03 | 0.039 | 0.9800 | [5] |
| Coconut shell AC | 25 | 0.75 | 0.04 | 0.9800 | 5.47 | 0.003 | 0.9800 | [41] |
| AC | 20 | 1.34 | 0.03 | 0.9800 | 5.47 | 0.003 | 0.9800 | [42] |
| ox xGnP | 25 | 0.3891 | 1.44 | 0.9649 | 9.41 | 0.051 | 0.9903 | [12] |
| xGnP | 25 | 1.6037 | 1.06 | 0.9897 | 1.66 | 0.025 | 0.9987 | [43] |
| HNO3 ox xGnP | 25 | 0.64 | 0.12 | 0.9940 | 10.00 | 0.079 | 0.9920 | [44] |
| NaOH xGnP | 0.57 | 0.58 | 0.9764 | 8.17 | 0.037 | 0.9743 | [33,45] | |
| 65% HNO3, reflux time 3 h ox-xGnP | 20 | 0.41 | 1.91 | 0.9851 | 12.04 | 0.076 | 0.9768 | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, I.; Bogdan, D.; Mincu, M.M.; Ion, A.C. Modified Exfoliated Carbon Nanoplatelets as Sorbents for Ammonium from Natural Mineral Waters. Molecules 2021, 26, 3541. https://doi.org/10.3390/molecules26123541
Ion I, Bogdan D, Mincu MM, Ion AC. Modified Exfoliated Carbon Nanoplatelets as Sorbents for Ammonium from Natural Mineral Waters. Molecules. 2021; 26(12):3541. https://doi.org/10.3390/molecules26123541
Chicago/Turabian StyleIon, Ion, Daniela Bogdan, Monica Maria Mincu, and Alina Catrinel Ion. 2021. "Modified Exfoliated Carbon Nanoplatelets as Sorbents for Ammonium from Natural Mineral Waters" Molecules 26, no. 12: 3541. https://doi.org/10.3390/molecules26123541
APA StyleIon, I., Bogdan, D., Mincu, M. M., & Ion, A. C. (2021). Modified Exfoliated Carbon Nanoplatelets as Sorbents for Ammonium from Natural Mineral Waters. Molecules, 26(12), 3541. https://doi.org/10.3390/molecules26123541





