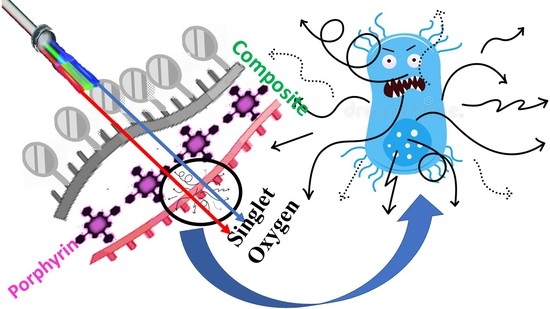
Development of Antimicrobial Laser-Induced Photodynamic Therapy Based on Ethylcellulose/Chitosan Nanocomposite with 5,10,15,20-Tetrakis(m-Hydroxyphenyl)porphyrin
Abstract
1. Introduction
2. Results and Discussion
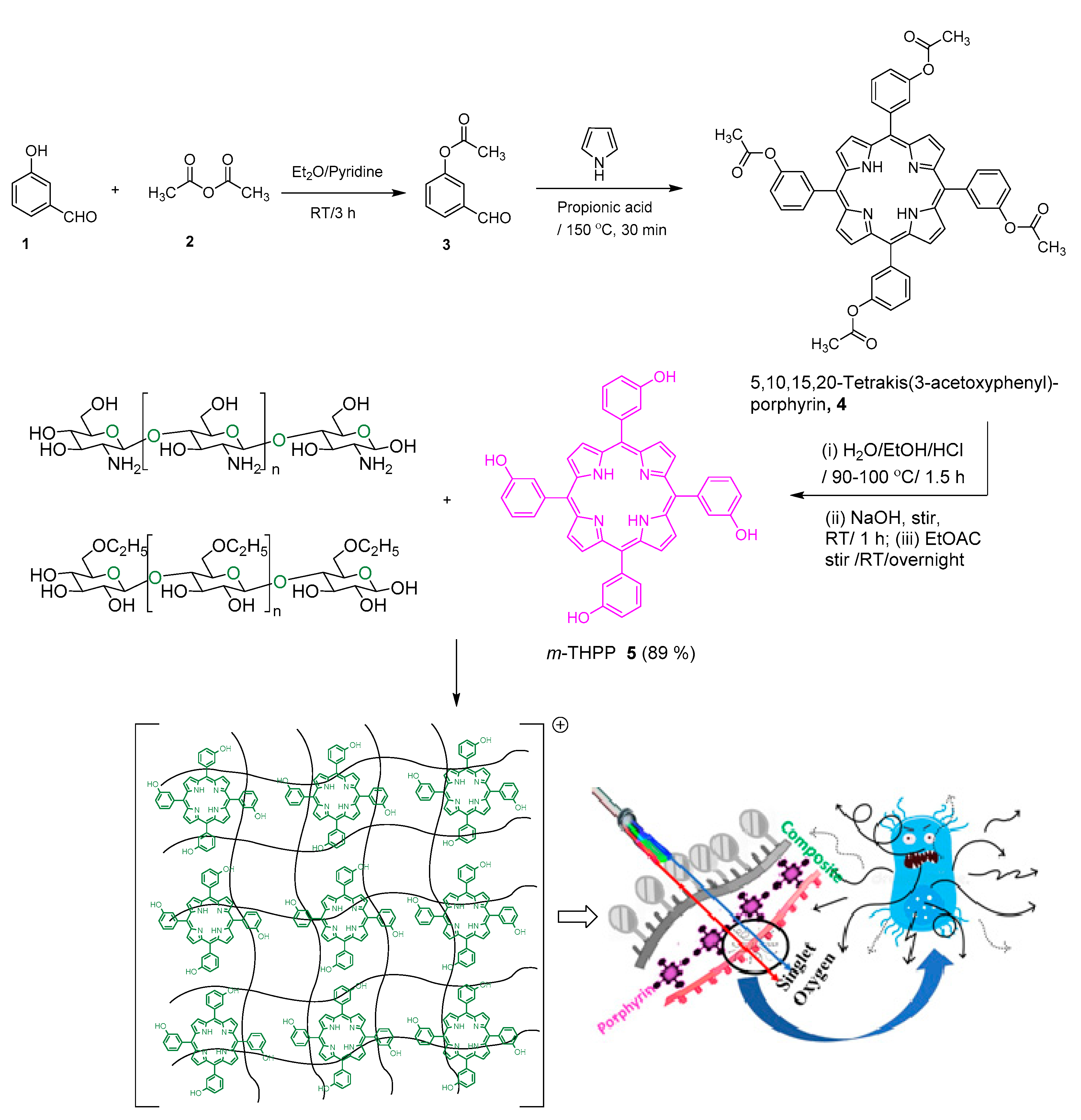
2.1. Preparation of mTHPP-Loaded Nanocomposite
2.2. Characterization of mTHPP and mTHPP-Loaded Nanocomposite
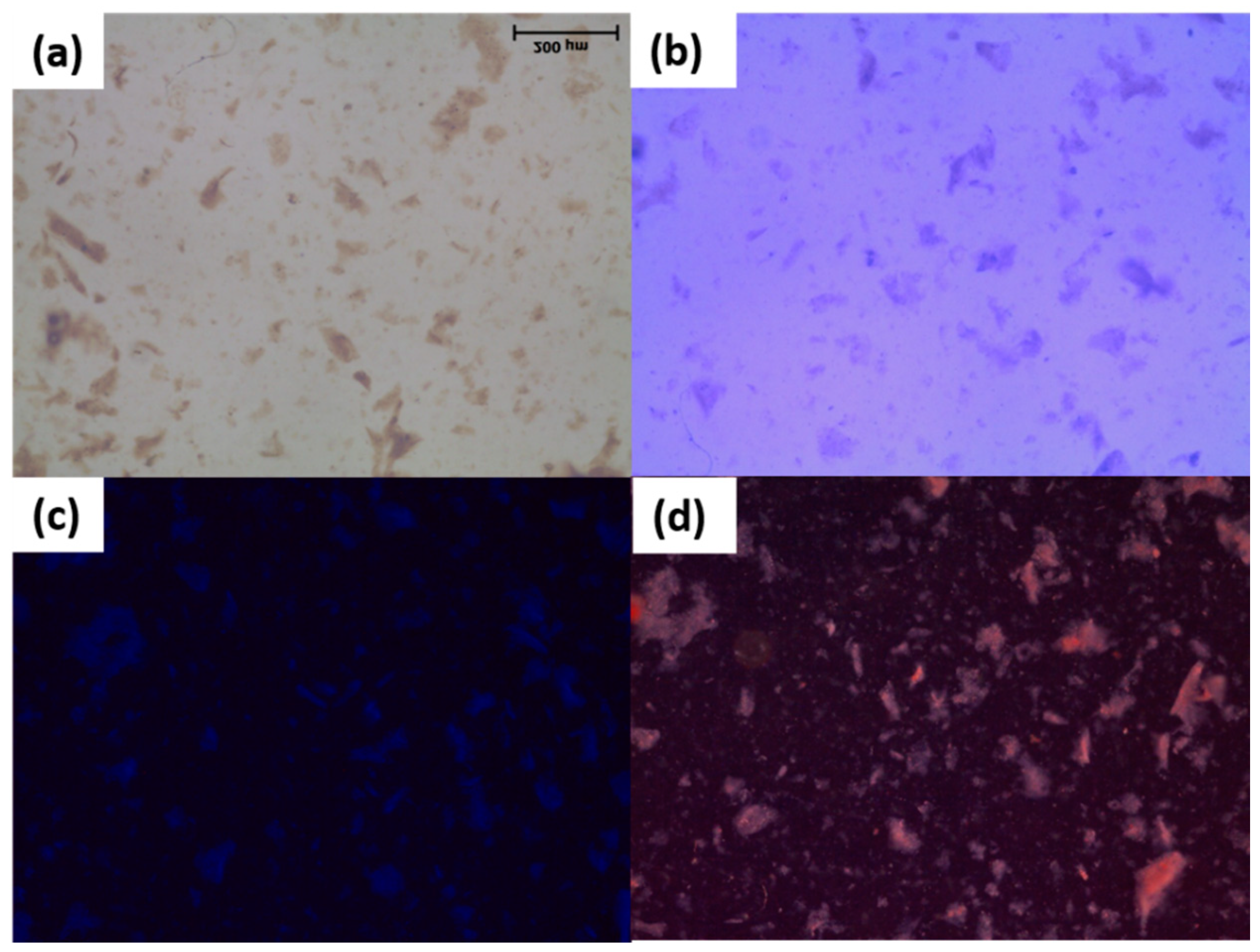
2.2.1. Polarized Light Microscopy
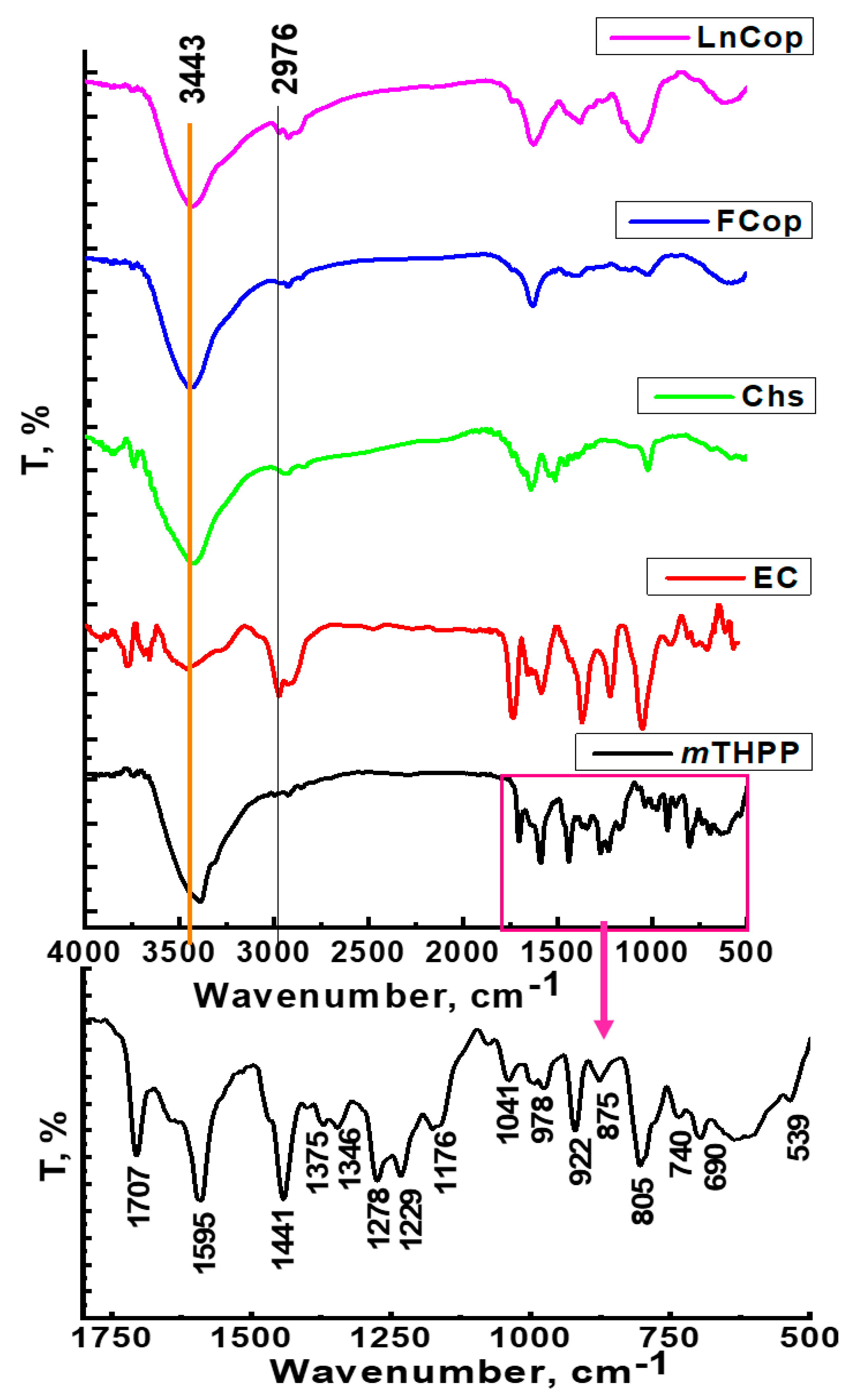
2.2.2. FT-IR Spectroscopy
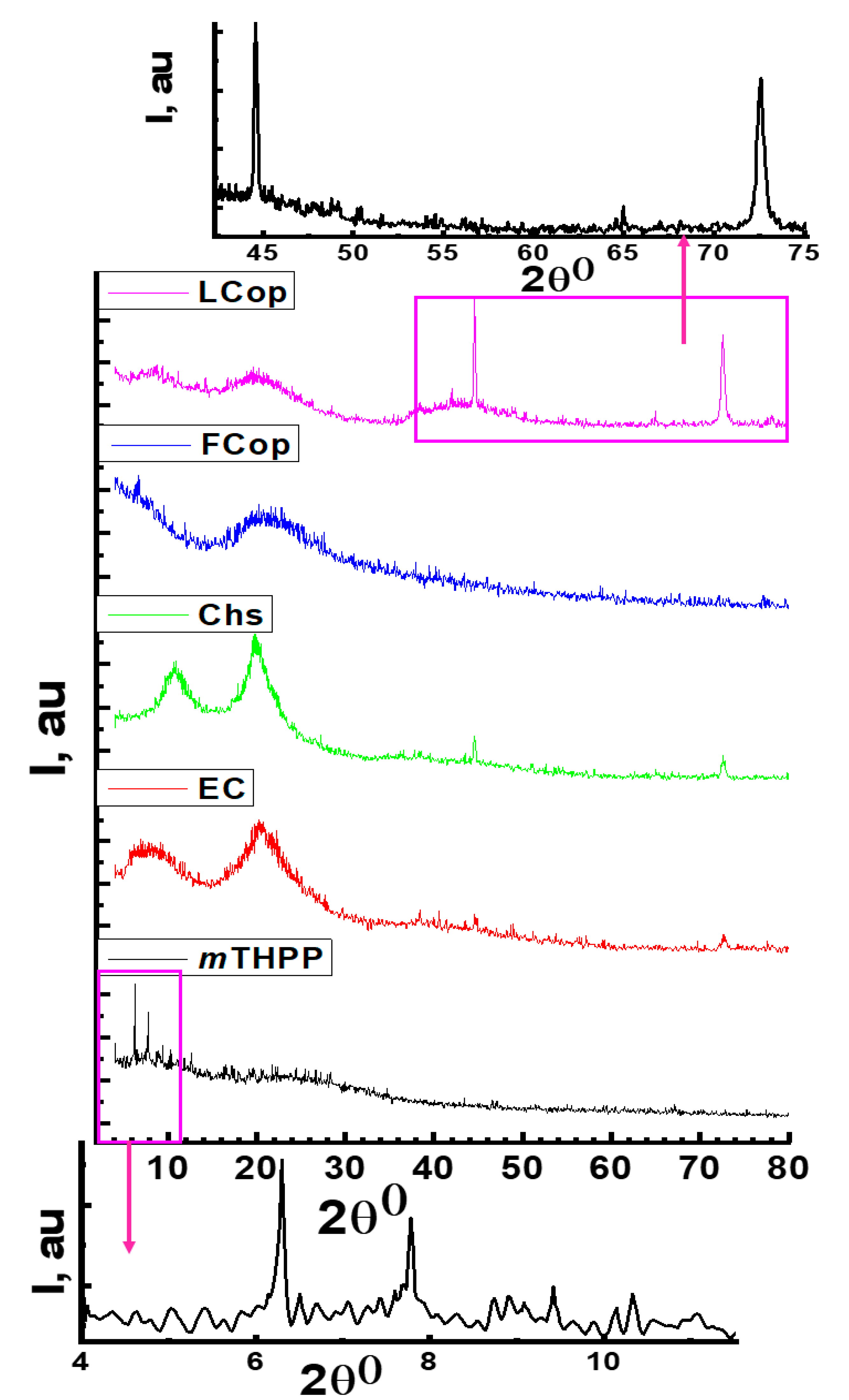
2.2.3. XRD Analysis
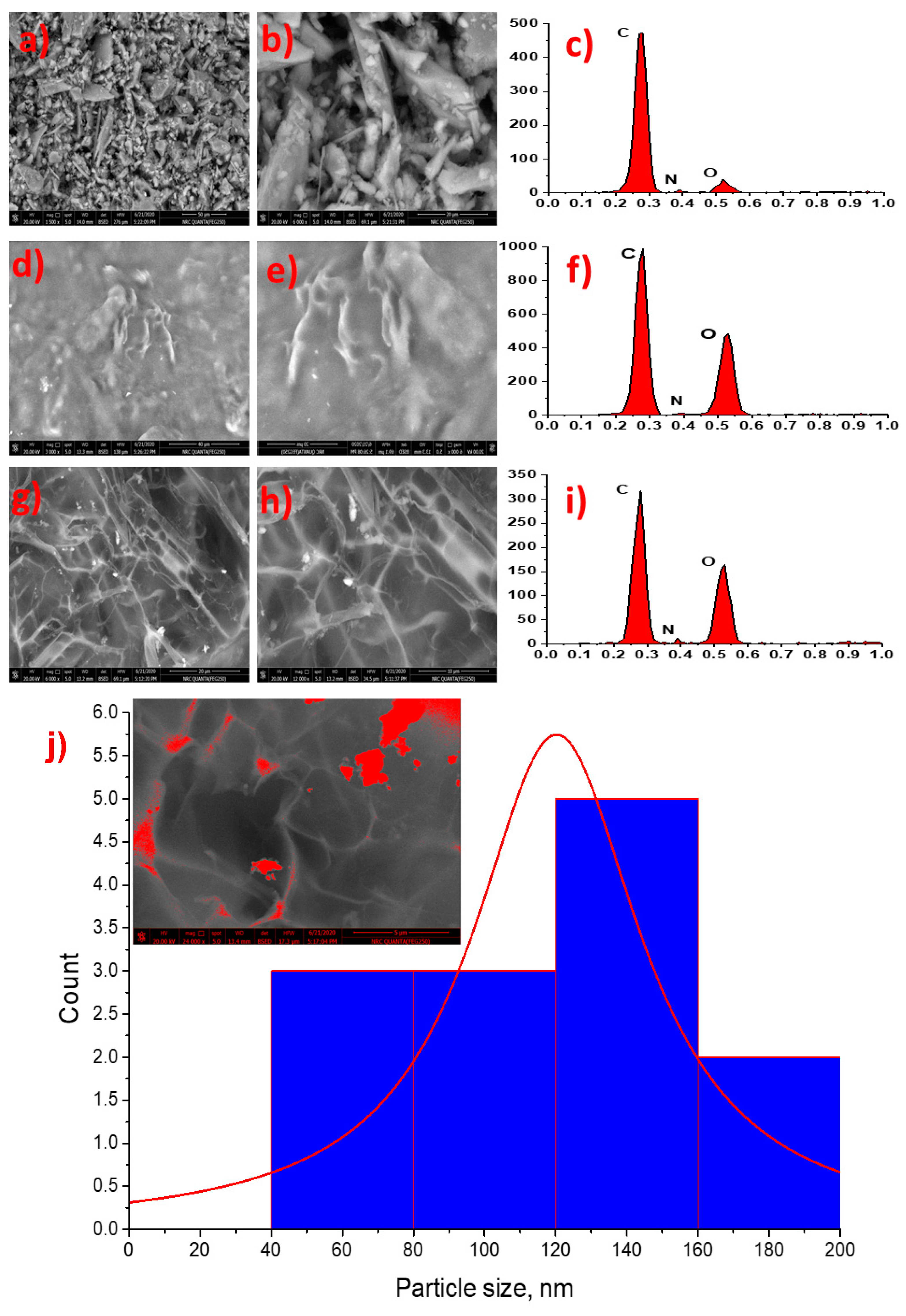
2.2.4. Scanning Electron and Energy Dispersive Electron Spectroscopy
2.2.5. Particle Size and Zeta Potential Measurements
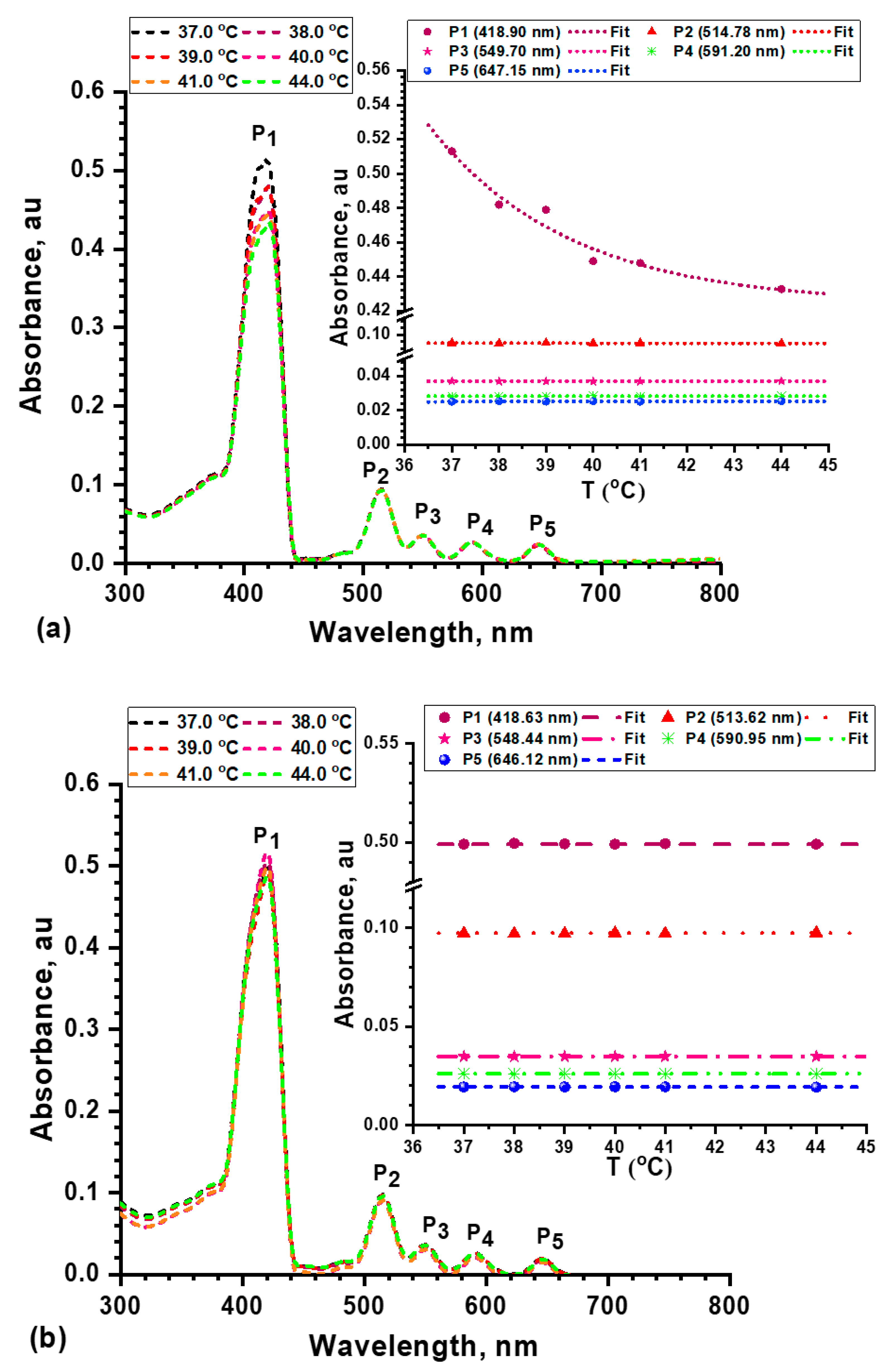
2.2.6. Thermal Stability and UV/Vis Absorption of mTHPP and Its Nanocomposite
2.2.7. Photodynamic Inactivation of Microbial Strains
2.2.8. Minimal Inhibitory Concentration and Minimal Bactericidal Concentration
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photosensitizer Nanocomposite
3.2.1. Synthesis of mTHPP
3.2.2. Composite Preparation
3.2.3. Loading of mTHPP onto the Nanocomposite
3.3. Characterizations and Instrumentation
3.4. Microorganisms and Culture Conditions
3.5. Photodynamic Inactivation of Microbial Strains
3.6. Determination of Microbial Survival
3.7. Determination of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
3.8. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting current photoactive materials for antimicrobial photodynamic therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Maragakis, L.L.; Perencevich, E.N.; Cosgrove, S.E. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti-infect. Ther. 2008, 6, 751–763. [Google Scholar] [CrossRef]
- Lopes, L.Q.S.; Ramos, A.P.; Copetti, P.M.; Acunha, T.V.; Iglesias, B.A.; Santos, R.C.V.; Machado, A.K.; Sagrillo, M.R. Antimicrobial activity and safety applications of meso-tetra (4-pyridyl) platinum(ii) porphyrin. Microb. Pathogen. 2019, 128, 47–54. [Google Scholar] [CrossRef]
- Bumah, V.V.; Morrow, B.N.; Cortez, P.M.; Bowman, C.R.; Rojas, P.; Masson-Meyers, D.S.; Suprapto, J.; Tong, W.G.; Enwemeka, C.S. The importance of porphyrins in blue light suppression of streptococcus agalactiae. J. Photochem. Photobiol. B Biol. 2020, 212, 111996. [Google Scholar] [CrossRef]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Sabry, R.; Fikry, M.; Ahmed, O.S.; Zekri, A.R.N.; Zedan, A.F. Laser-induced synthesis of pure zinc oxide nanoflakes. J. Phys. Conf. Ser. 2020, 1472, 012005. [Google Scholar] [CrossRef]
- Van Straten, D.; Mashayekhi, V.; De Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.C.; Manela-Azulay, M. Photodynamic therapy: A review of the literature and image documentation. An. Bras. Dermatol. 2010, 85, 501–511. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R. Photodynamic therapy: Update 2006. Part 2: Clinical results. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Alexiades-Armenakas, M. Laser-mediated photodynamic therapy. Clin. Dermatol. 2006, 24, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hanakova, A.; Bogdanova, K.; Tomankova, K.; Pizova, K.; Malohlava, J.; Binder, S.; Bajgar, R.; Langova, K.; Kolar, M.; Mosinger, J.; et al. The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiol. Res. 2014, 169, 163–170. [Google Scholar] [CrossRef]
- Xing, C.; Xu, Q.; Tang, H.; Liu, L.; Wang, S. Conjugated polymer/porphyrin complexes for efficient energy transfer and improving light-activated antibacterial activity. J. Am. Chem. Soc. 2009, 131, 13117–13124. [Google Scholar] [CrossRef]
- Senge, M.O. mTHPC--a drug on its way from second to third generation photosensitizer? Photodiagn. Photodyn. Ther. 2012, 9, 170–179. [Google Scholar] [CrossRef]
- Bonnett, R.; Krysteva, M.A.; Lalov, I.G.; Artarsky, S.V. Water disinfection using photosensitizers immobilized on chitosan. Water Res. 2006, 40, 1269–1275. [Google Scholar] [CrossRef]
- Shaker, Y.M.; Sweed, A.M.; Moylan, C.; Rogers, L.; Ryan, A.A.; Petitdemange, R.; Senge, M.O. Current developments in using meso-(tetra)substituted porphyrins for PDT. In Handbook of Photodynamic Therapy; Pandey, R.K., Kessel, D., Dougherty, T.J., Eds.; World Scientific: Singapore, 2016; pp. 95–149. [Google Scholar]
- Sobczyński, J.; Tønnesen, H.H.; Kristensen, S. Influence of aqueous media properties on aggregation and solubility of four structurally related meso-porphyrin photosensitizers evaluated by spectrophotometric measurements. Pharmazie 2013, 68, 100–109. [Google Scholar]
- Valkov, A.; Nakonechny, F.; Nisnevitch, M. Polymer-immobilized photosensitizers for continuous eradication of bacteria. Int. J. Mol. Sci. 2014, 15, 14984–14996. [Google Scholar] [CrossRef] [PubMed]
- Bastien, E.; Schneider, R.; Hackbarth, S.; Dumas, D.; Jasniewski, J.; Röder, B.; Bezdetnaya, L.; Lassalle, H.-P. PAMAM G4. 5-chlorin e6 dendrimeric nanoparticles for enhanced photodynamic effects. Photochem. Photobiol. Sci. 2015, 14, 2203–2212. [Google Scholar] [CrossRef]
- Shehabeldine, A.; Hasanin, M. Green synthesis of hydrolyzed starch–chitosan nano-composite as drug delivery system to gram negative bacteria. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100252. [Google Scholar] [CrossRef]
- Hasanin, M.; El-Henawy, A.; Eisa, W.H.; El-Saied, H.; Sameeh, M. Nano-amino acid cellulose derivatives: Eco-synthesis, characterization, and antimicrobial properties. Int. J. Biol. Macromol. 2019, 132, 963–969. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Moustafa, G.O. New potential green, bioactive and antimicrobial nanocomposites based on cellulose and amino acid. Int. J. Biol. Macromol. 2020, 144, 441–448. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; Farag, M.M.; Ahmed, H.Y. Green synthesis of bacterial cellulose/bioactive glass nanocomposites: Effect of glass nanoparticles on cellulose yield, biocompatibility and antimicrobial activity. Int. J. Biol. Macromol. 2019, 138, 975–985. [Google Scholar] [CrossRef]
- Abou Hammad, A.B.; Abd El-Aziz, M.E.; Hasanin, M.S.; Kamel, S. A novel electromagnetic biodegradable nanocomposite based on cellulose, polyaniline, and cobalt ferrite nanoparticles. Carbohydr. Polym. 2019, 216, 54–62. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Al Kiey, S.A. Environmentally benign corrosion inhibitors based on cellulose niacin nano-composite for corrosion of copper in sodium chloride solutions. Int. J. Biol. Macromol. 2020, 161, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial cellulose-based composite scaffolds for biomedical applications: A review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S. Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. Chem. Eng. J. 2009, 155, 892–898. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose–a pharmaceutical excipient with multidirectional application in drug dosage forms development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef]
- Salama, A.; Hasanin, M.; Hesemann, P. Synthesis and antimicrobial properties of new chitosan derivatives containing guanidinium groups. Carbohydr. Polym. 2020, 241, 116363. [Google Scholar] [CrossRef]
- Feese, E.; Sadeghifar, H.; Gracz, H.S.; Argyropoulos, D.S.; Ghiladi, R.A. Photobactericidal porphyrin-cellulose nanocrystals: Synthesis, Characterization, and Antimicrobial properties. Biomacromolecules 2011, 12, 3528–3539. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—state of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Kardumyan, V.V.; Aksenova, N.A.; Timofeeva, V.A.; Krivandin, A.V.; Shatalova, O.V.; Dubovik, A.S.; Plashchina, I.G.; Timashev, P.S.; Solovieva, A.B. Effect of Chitosan on the Activity of Water-Soluble and Hydrophobic Porphyrin Photosensitizers Solubilized by Amphiphilic Polymers. Polymers 2021, 13, 1007. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Dacrory, S.; Hasanin, M.; Saber, E.; Kamel, S. Biocompatible hydrogel based on aldehyde-functionalized cellulose and chitosan for potential control drug release. Sustain. Chem. Pharm. 2021, 21, 100419. [Google Scholar] [CrossRef]
- Dacrory, S.; Hashem, A.H.; Hasanin, M. Synthesis of cellulose based amino acid functionalized nano-biocomplex: Characterization, antifungal activity, molecular docking and hemocompatibility. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100453. [Google Scholar]
- Shehabeldine, A.; El-Hamshary, H.; Hasanin, M.; El-Faham, A.; Al-Sahly, M. Enhancing the Antifungal Activity of Griseofulvin by Incorporation a Green Biopolymer-Based Nanocomposite. Polymers 2021, 13, 542. [Google Scholar] [CrossRef]
- Bonnet, R.; McGarvey, D.J.; Harriman, A.; Land, E.J.; Truscott, T.G.; Winfield, U.-J. Photophysical properties of meso-tetraphenylporphyrin and some meso-tetra(hydroxyphenyl)porphyrins. Photochem. Photobiol. 1988, 48, 271–276. [Google Scholar] [CrossRef]
- Aydin, M.; Akins, D.L. Infrared and Raman spectroscopic characterization of porphyrin and its derivatives. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; Stauffer, M.T., Ed.; IntechOpen: London, UK, 2016; p. 141. [Google Scholar] [CrossRef]
- Castro, K.A.; Silva, S.; Pereira, P.M.; Simoes, M.M.; Neves, M.D.G.P.; Cavaleiro, J.A.; Wypych, F.; Tome, J.P.; Nakagaki, S. Galactodendritic porphyrinic conjugates as new biomimetic catalysts for oxidation reactions. Inorg. Chem. 2015, 54, 4382–4393. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R.; Jana, S. Characterization of physicochemical and thermal properties of biofield treated ethylcellulose and methyl cellulose. Int. J. Biomed. Mater. Res. 2015, 3, 83–91. [Google Scholar]
- Suthar, V.; Pratap, A.; Raval, H. Studies on poly (hydroxy alkanoates)/(ethylcellulose) blends. Bull. Mater. Sci. 2000, 23, 215–219. [Google Scholar] [CrossRef]
- Kang, S.-W.; Li, Q.; Chapman, B.D.; Pindak, R.; Cross, J.O.; Li, L.; Nakata, M.; Kumar, S. Microfocus x-ray diffraction study of the columnar phase of porphyrin-based mesogens. Chem. Mater. 2007, 19, 5657–5663. [Google Scholar] [CrossRef]
- Parida, P.; Mishra, S.C.; Sahoo, S.; Behera, A.; Nayak, B.P. Development and characterization of ethylcellulose based microsphere for sustained release of nifedipine. J. Pharm. Anal. 2016, 6, 341–344. [Google Scholar] [CrossRef]
- de Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; de Oliveira Lima, V.A.; de Farias Rached, R.Í.; Lima, E.P.N.; da Silva Lima, R.J.; PenicheCovas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeusvannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef]
- Ban, Z.; Horev, B.; Rutenberg, R.; Danay, O.; Bilbao, C.; McHugh, T.; Rodov, V.; Poverenov, E. Efficient production of fungal chitosan utilizing an advanced freeze-thawing method; quality and activity studies. Food Hydrocoll. 2018, 81, 380–388. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Chang, S.-H.; Lin, H.-T.V.; Wu, G.-J.; Tsai, G.J. pH effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Aboulfotouh, A.; Fikry, M.; Mohamed, M.; Omar, M.; Rady, H.; Elbashar, Y. Spectroscopic study of oscillator strength and radiative decay time of colloidal CdSe quantum dots. Opt. Quant. Electron. 2018, 50, 115. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Nitzan, Y.; Ashkenazi, H. Photoinactivation of Acinetobacter baumannii and Escherichia coli B by a cationic hydrophilic porphyrin at various light wavelengths. Curr. Microbiol. 2001, 42, 408–414. [Google Scholar] [CrossRef]
- Wang, Y.; Harrington, O.D.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Dai, T. In vivo investigation of antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii burn infections using bioluminescence imaging. J. Vis. Exp. 2017, 122, 54997. [Google Scholar] [CrossRef]
- Plavskii, V.Y.; Mikulich, A.; Tretyakova, A.; Leusenka, I.; Plavskaya, L.; Kazyuchits, O.; Dobysh, I.; Krasnenkova, T. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J. Photochem. Photobiol. B Biol. 2018, 183, 172–183. [Google Scholar] [CrossRef]
- Staegemann, M.H.; Gitter, B.; Dernedde, J.; Kuehne, C.; Haag, R.; Wiehe, A. Mannose-functionalized hyperbranched polyglycerol loaded with zinc porphyrin: Investigation of the multivalency effect in antibacterial photodynamic therapy. Chem. Eur. J. 2017, 23, 3918–3930. [Google Scholar] [CrossRef]
- Le Guern, F.; Sol, V.; Ouk, C.; Arnoux, P.; Frochot, C.; Ouk, T.-S. Enhanced photobactericidal and targeting properties of a cationic porphyrin following the attachment of polymyxin b. Bioconj. Chem. 2017, 28, 2493–2506. [Google Scholar] [CrossRef] [PubMed]
- Moylan, C.; Sweed, A.M.; Shaker, Y.M.; Scanlan, E.M.; Senge, M.O. Lead structures for applications in photodynamic therapy 7. Efficient synthesis of amphiphilic glycosylated lipid porphyrin derivatives: Refining linker conjugation for potential pdt applications. Tetrahedron 2015, 71, 4145–4153. [Google Scholar] [CrossRef]
- Rogers, L.; Burke-Murphy, E.; Senge, M.O. Simple Porphyrin Desymmetrization: 5,10,15,20-Tetrakis(3-hydroxyphenyl)porphyrin (mTHPP) as a Gateway Molecule for Peripheral Functionalization. Eur. J. Org. Chem. 2014, 4283–4294. [Google Scholar] [CrossRef]
- Qader, M.M.; Hamed, A.A.; Soldatou, S.; Abdelraof, M.; Elawady, M.E.; Hassane, A.S.I.; Belbahri, L.; Ebel, R.; Rateb, M.E. Antimicrobial and Antibiofilm Activities of the Fungal Metabolites Isolated from the Marine Endophytes Epicoccum nigrum M13 and Alternaria alternata 13A. Mar. Drugs 2021, 19, 232. [Google Scholar] [CrossRef] [PubMed]
- El-Bendary, M.A.; Moharam, M.E.; Abdelraof, M.; Allam, M.A.; Roshdy, A.M.; Shaheen, M.N.F.; Elmahdy, E.M.; Elkomy, G.M. Multi-bioactive silver nanoparticles synthesized using mosquitocidal Bacilli and their characterization. Arch. Microbiol. 2020, 202, 63–75. [Google Scholar] [CrossRef]
- El-Bendary, M.A.; Abdelraof, M.; Moharam, M.E.; Elmahdy, E.M.; Allam, M.A. Potential of silver nanoparticles synthesized using low active mosquitocidal Lysinibacillus sphaericus as novel antimicrobial agents. Prep. Biochem. Biotechnol. 2021, 1–10. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.; Beelen, R.; Ossenkoppele, G.; Broekhoven, M.; Langenhuijsen, M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Khalil, A.; Abdelaziz, A.; Khaleil, M.; Hashem, A. Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett. Appl. Microbiol. 2021, 72, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as diagnostic and therapeutic agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed]

| Zeta Potential Measurements | Particle Size Measurements | |||||
|---|---|---|---|---|---|---|
| Sample | Cell Current, mA | Av. Phase Shift, rad/sec | Av. Mobility, M.U. | Av. Zeta Potential, mV | PDI | Average Particle Size, nm |
| mTHPP | 0.65 | 11.82 | 1.44 | −20.55 | 0.137 | 175 |
| Free composite | 7.34 | 11.02 | 1.67 | 35.5 | 0.245 | 292 |
| mTHPP-loaded nanocomposite | 1.65 | 18.2 | 2.48 | 23.84 | 0.442 | 595 |
| Sample | Inhibition in Microbial Survival, % | ||
|---|---|---|---|
| P. aeruginosa | S. aureus | C. albicans | |
| mTHPP | 3.29 ± 0.19 | 2.15 ± 0.20 | 6.28 ± 0.54 |
| mTHPP-loaded nanocomposite | 0.41 ± 0.02 | 0 ± 0 | 2.86 ± 0.11 |
| mTHPP (light) | 21.76 ± 1.7 | 14.16 ± 0.91 | 10.6 ± 0.56 |
| mTHPP-loaded nanocomposite(light) | 22.63 ± 1.5 | 16.5 ± 0.66 | 13.4 ± 0.40 |
| Control | 100 | ||
| Inhibition in C. albicans Survival % | ||||||
|---|---|---|---|---|---|---|
| Wavelength | Laser Only | mTHPP | mTHPP-loaded Nanocomposite | |||
| (nm) | Time Exposure (min) | |||||
| 15 | 30 | 15 | 30 | 15 | 30 | |
| 458 | 13.3 ± 0.5 | 23.66 ± 1.45 | 44.03 ± 0.75 | 58.73 ± 1.11 | 21.8 ± 1.02 | 27.26 ± 1.39 |
| 476 | 10.36 ± 0.49 | 15.73 ± 0.87 | 36.03 ± 1.54 | 44.13 ± 0.71 | 21.93 ± 0.89 | 25.96 ± 0.77 |
| 488 | 13.93 ± 1.05 | 18.56 ± 1.14 | 38.33 ± 0.41 | 51.86 ± 0.49 | 17.53 ± 1.23 | 33.16 ± 0.26 |
| 515 | 7.33 ± 0.59 | 13.26 ± 1.47 | 40.1 ± 0.86 | 50.46 ± 0.40 | 31.26 ± 1.01 | 43.33 ± 0.74 |
| 635 | 3.46 ± 0.54 | 7.33 ± 0.94 | 35.06 ± 0.82 | 54.3 ± 0.64 | 59.1 ± 0.21 | 71.13 ± 1.5 |
| Control | 100 | |||||
| Inhibition in S. aureus Survival % | ||||||
| Wavelength | Laser Only | mTHPP | mTHPP-loaded Nanocomposite | |||
| (nm) | Time Exposure (min) | |||||
| 15 | 30 | 15 | 30 | 15 | 30 | |
| 458 | 16.4 ± 0.60 | 21.7 ± 0.94 | 40.6 ± 1.22 | 74.4 ± 1.73 | 23.6 ± 1.47 | 32.6 ± 1.46 |
| 476 | 17.8 ± 0.32 | 22.7 ± 0.95 | 54.06 ± 0.82 | 83.4 ± 1.44 | 30.8 ± 1.04 | 38.3 ± 2.02 |
| 488 | 22.1 ± 1.3 | 27.7 ± 1.16 | 47.2 ± 0.55 | 70.6 ± 0.94 | 42.2 ± 2.17 | 56.3 ± 0.65 |
| 515 | 4.4 ± 0.45 | 10.3 ± 0.49 | 49.9 ± 0.91 | 56.7 ± 0.94 | 61.6 ± 0.75 | 72.8 ± 1 |
| 635 | 2.06 ± 0.12 | 7.8 ± 0.32 | 52.3 ± 0.49 | 58.1 ± 3.1 | 74.2 ± 1.26 | 81 ± 2.23 |
| Control | 100 | |||||
| Inhibition in P. aeruginosa Survival % | ||||||
| Wavelength | Laser Only | mTHPP | mTHPP-loaded Nanocomposite | |||
| (nm) | Time Exposure (min) | |||||
| 15 | 30 | 15 | 30 | 15 | 30 | |
| 458 | 12.9 ± 1.06 | 34.2 ± 2.99 | 51 ± 2.94 | 79.3 ± 1.6 | 33 ± 1.51 | 36.5 ± 1.89 |
| 476 | 12.1 ± 0.84 | 32.8 ± 0.94 | 53.1 ± 2.31 | 75.9 ± 1.85 | 37.9 ± 2.07 | 43.8 ± 0.94 |
| 488 | 8.2 ± 0.28 | 27.3 ± 1.6 | 65.8 ± 2.23 | 82.2 ± 2.44 | 41.7 ± 1.02 | 53.7 ± 1.40 |
| 515 | 6.5 ± 0.74 | 20.3 ± 1.47 | 52.6 ± 1.94 | 63.8 ± 2.25 | 54.6 ± 1.32 | 63.5 ± 2.2 |
| 635 | 5.5 ± 0.97 | 10.5 ± 0.62 | 36.6 ± 2.27 | 59 ± 2.86 | 71.7 ± 1.72 | 83.1 ± 2.82 |
| Control | 100 | |||||
| Power (mW/cm2 at 458 nm) | Inhibition in C. albicans Survival % | |||||
|---|---|---|---|---|---|---|
| Laser Only | mTHPP | mTHPP-loaded Nanocomposite | ||||
| Time Exposure (min) | ||||||
| 15 | 30 | 15 | 30 | 15 | 30 | |
| 10 | 12.63 ± 0.70 | 23.36 ± 0.49 | 41.36 ± 1.14 | 60.96 ± 0.78 | 21.8 ± 0.86 | 27.6 ± 1.07 |
| 20 | 19.73 ± 0.65 | 29.2 ± 0.57 | 61.3 ± 0.49 | 72.3 ± 1.02 | 27.93 ± 0.65 | 32.33 ± 0.47 |
| 40 | 22.46 ± 1.1 | 33.26 ± 0.97 | 75.96 ± 0.85 | 77.33 ± 1.69 | 37 ± 1.10 | 41.33 ± 0.49 |
| 70 | 34.4 ± 0.43 | 47.96 ± 0.78 | 83.66 ± 1.24 | 85.2 ± 1.06 | 56.13 ± 0.69 | 60.43 ± 0.40 |
| Control | 100 | |||||
| Power (mW/cm2 at 476 nm) | Inhibition in S. aureus Survival % | |||||
| Laser Only | mTHPP | mTHPP-loaded Nanocomposite | ||||
| Time Exposure (min) | ||||||
| 15 | 30 | 15 | 30 | 15 | 30 | |
| 10 | 18.13 ± 0.69 | 23.5 ± 0.57 | 34.71 ± 0.95 | 84.16 ± 0.70 | 23.46 ± 1.30 | 32.73 ± 0.74 |
| 20 | 21.06 ± 0.16 | 30.43 ± 0.75 | 56.3 ± 0.91 | 77.76 ± 1.5 | 35.96 ± 1.36 | 43.36 ± 1.08 |
| 40 | 23.9 ± 0.90 | 31.53 ± 1.12 | 76 ± 0.61 | 81.96 ± 1.36 | 41.26 ± 0.61 | 58.4 ± 0.43 |
| 70 | 30.5 ± 0.57 | 35.16 ± 0.30 | 87.06 ± 0.87 | 88.8 ± 0.69 | 55.56 ± 0.80 | 56.86 ± 1.29 |
| Control | 100 | |||||
| Power (mW/cm2 at 488 nm) | Inhibition in P. aeruginosa survival % | |||||
| Laser Only | mTHPP | mTHPP-loaded Nanocomposite | ||||
| Time Exposure (min) | ||||||
| 15 | 30 | 15 | 30 | 15 | 30 | |
| 10 | 7.9 ± 0.24 | 26.96 ± 1 | 66.5 ± 0.5 | 82.96 ± 1.45 | 31.96 ± 0.85 | 36.8 ± 0.88 |
| 20 | 23.3 ± 0.57 | 36.83 ± 0.93 | 69.9 ± 0.82 | 88.13 ± 0.89 | 42.8 ± 0.88 | 44.43 ± 1.26 |
| 40 | 23.23 ± 1.01 | 37.73 ± 1.06 | 75.9 ± 0.77 | 87.86 ± 0.93 | 50.86 ± 0.69 | 59.4 ± 0.58 |
| 70 | 31.86 ± 0.93 | 44.03 ± 1.29 | 83.4 ± 0.53 | 90.26 ± 0.57 | 59.2 ± 0.90 | 62.26 ± 0.83 |
| Control | 100 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanin, M.S.; Abdelraof, M.; Fikry, M.; Shaker, Y.M.; Sweed, A.M.K.; Senge, M.O. Development of Antimicrobial Laser-Induced Photodynamic Therapy Based on Ethylcellulose/Chitosan Nanocomposite with 5,10,15,20-Tetrakis(m-Hydroxyphenyl)porphyrin. Molecules 2021, 26, 3551. https://doi.org/10.3390/molecules26123551
Hasanin MS, Abdelraof M, Fikry M, Shaker YM, Sweed AMK, Senge MO. Development of Antimicrobial Laser-Induced Photodynamic Therapy Based on Ethylcellulose/Chitosan Nanocomposite with 5,10,15,20-Tetrakis(m-Hydroxyphenyl)porphyrin. Molecules. 2021; 26(12):3551. https://doi.org/10.3390/molecules26123551
Chicago/Turabian StyleHasanin, Mohamed S., Mohamed Abdelraof, Mohamed Fikry, Yasser M. Shaker, Ayman M. K. Sweed, and Mathias O. Senge. 2021. "Development of Antimicrobial Laser-Induced Photodynamic Therapy Based on Ethylcellulose/Chitosan Nanocomposite with 5,10,15,20-Tetrakis(m-Hydroxyphenyl)porphyrin" Molecules 26, no. 12: 3551. https://doi.org/10.3390/molecules26123551
APA StyleHasanin, M. S., Abdelraof, M., Fikry, M., Shaker, Y. M., Sweed, A. M. K., & Senge, M. O. (2021). Development of Antimicrobial Laser-Induced Photodynamic Therapy Based on Ethylcellulose/Chitosan Nanocomposite with 5,10,15,20-Tetrakis(m-Hydroxyphenyl)porphyrin. Molecules, 26(12), 3551. https://doi.org/10.3390/molecules26123551