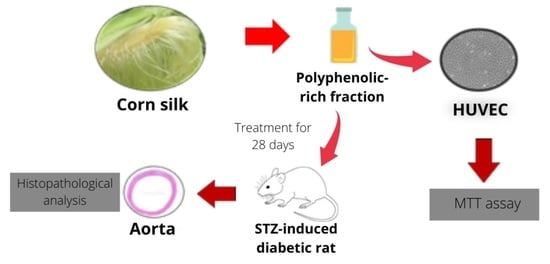
Potential Effect of Polyphenolic-Rich Fractions of Corn Silk on Protecting Endothelial Cells against High Glucose Damage Using In Vitro and In Vivo Approaches
Abstract
1. Introduction
2. Results
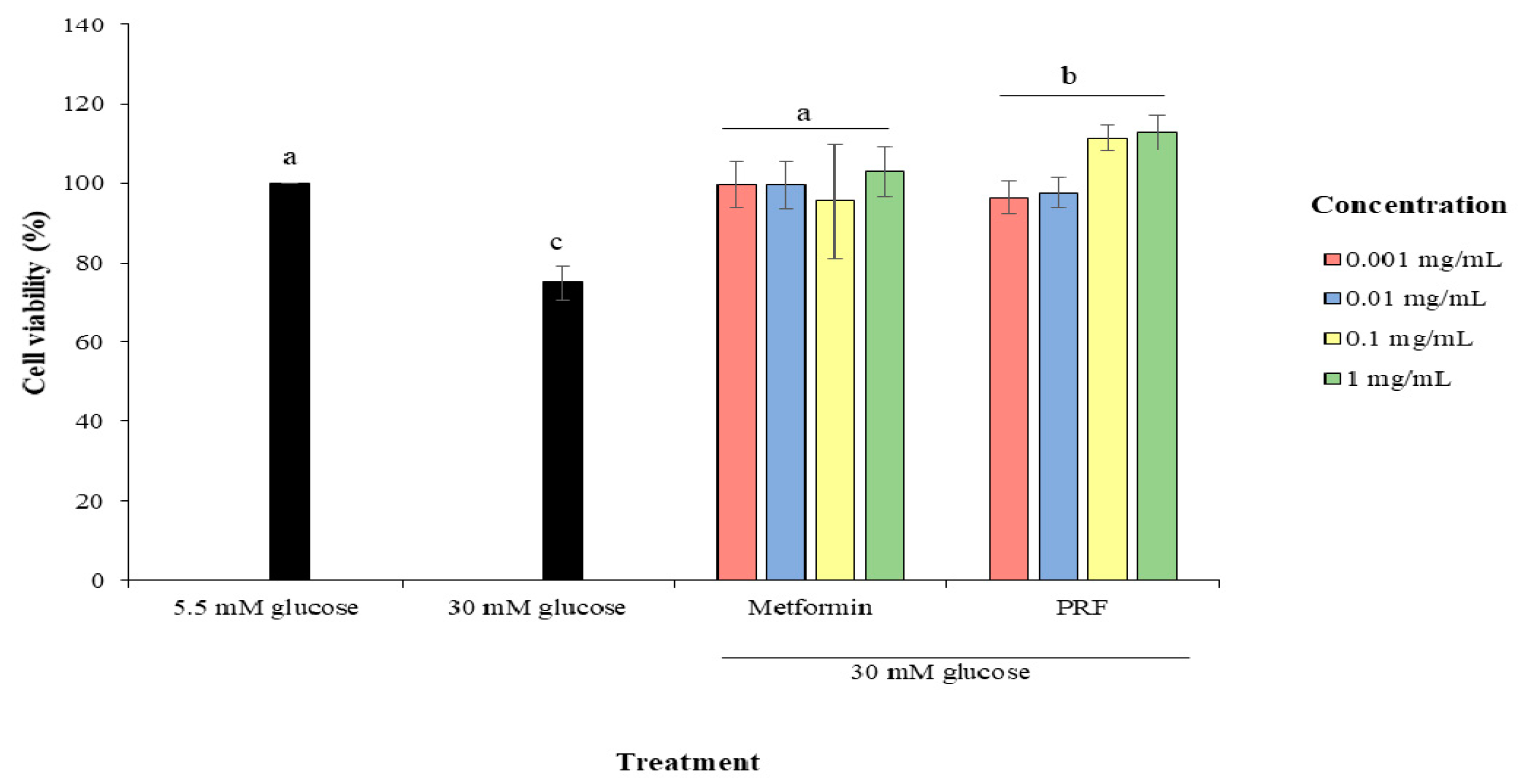
2.1. Protective Effect of PRF against High Glucose in Cytotoxicity in HUVECs
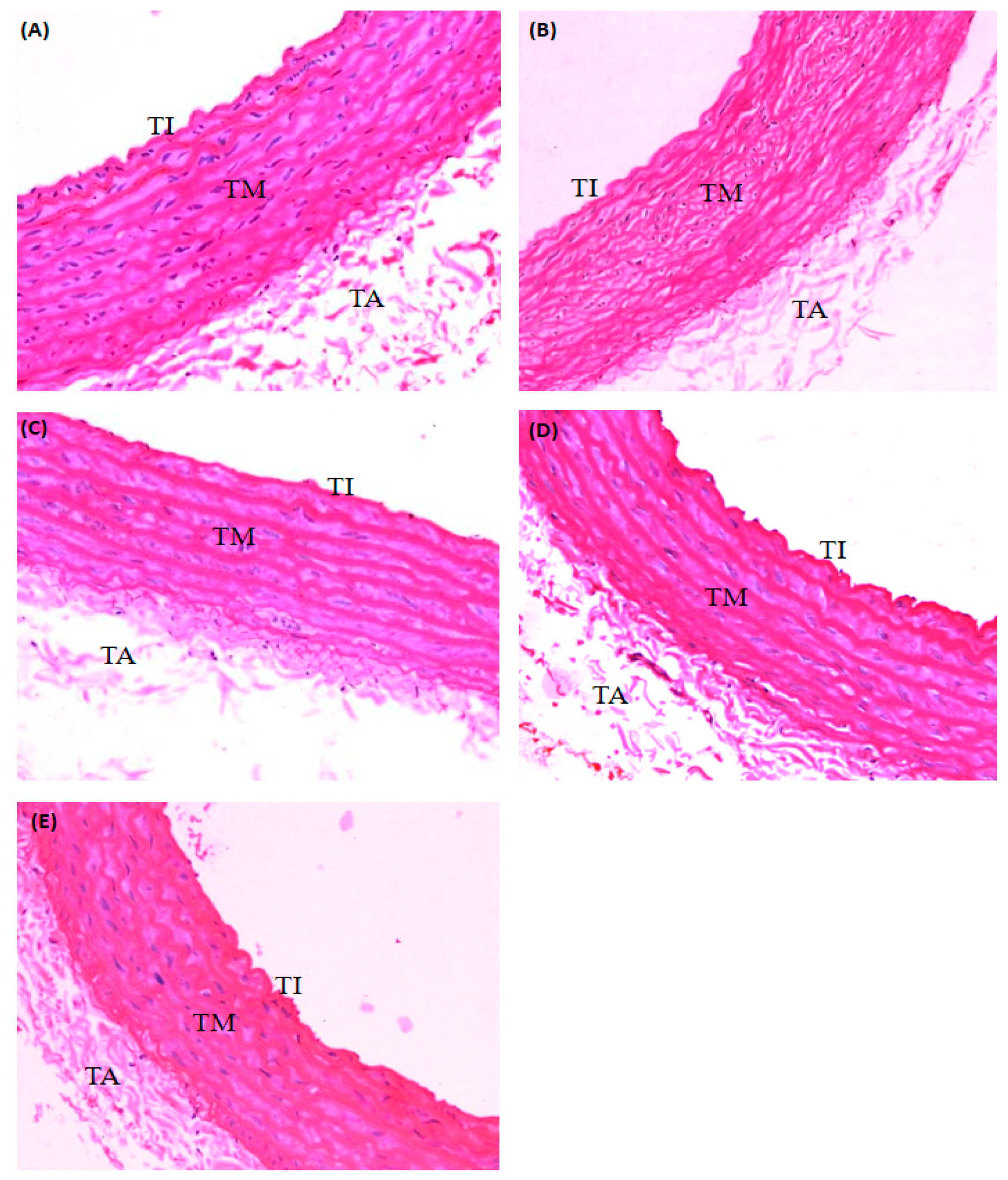
2.2. Effect of PRF on the Aorta of STZ-Induced Diabetic Rats
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation of Fractions
4.2. Protective Effect of PRF on Primary Cell Culture
4.2.1. Cell Culture
4.2.2. Protective Effect of PRF on HUVECs from High Glucose-Induced Cytotoxicity
4.3. Effect of PRF on the Aorta of STZ-Induced Diabetic Rats
4.3.1. Dosage Preparation of PRF and Drug
(mg/kg)]/Concentration (mg/mL).
4.3.2. Experimental Animals
4.3.3. Induction of Experimental Diabetes
4.3.4. Study Design
4.3.5. Histopathological Analysis
4.3.6. Measurement of the Thickness of the Aorta
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gero, D. Hyperglycemia-Induced Endothelial Dysfunction. In Endothelial Dysfunction: Old Concepts and New Challenges; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/books/endothelial-dysfunction-old-concepts-and-new-challenges/hyperglycemia-induced-endothelial-dysfunction (accessed on 21 June 2019). [CrossRef]
- Sheu, M.L.; Ho, F.M.; Yang, R.S.; Chao, K.F.; Lin, W.W.; Lin-Shiau, S.Y.; Liu, S.H. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase–regulated cyclooxygenase-2 pathway. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 539–545. [Google Scholar] [CrossRef]
- Ho, F.M.; Liu, S.H.; Liau, C.S.; Huang, P.J.; Lin-Shiau, S.Y. High glucose–induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH2-terminal kinase and caspase-3. Circulation 2000, 101, 2618–2624. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Dhananjaya, B.L.; Prasad, N. Assessment of in vivo antidiabetic properties of umbelliferone and lupeol constituents of banana (Musa sp. var. Nanjangud Rasa Bale) flower in hyperglycaemic rodent model. PLoS ONE 2016, 11, e0151135. [Google Scholar] [CrossRef]
- Polovina, M.M.; Potpara, T.S. Endothelial dysfunction in metabolic and vascular disorders. Postgrad. Med. 2014, 126, 38–53. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Dragan, S.; Andrica, F.; Serban, M.-C.; Timar, R. Polyphenols-rich natural products for treatment of diabetes. Curr. Med. Chem. 2015, 22, 14–22. [Google Scholar] [CrossRef]
- Hasanudin, K.; Hashim, P.; Mustafa, S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715. [Google Scholar] [CrossRef]
- Nurhanan, A.R.; Wan Rosli, W.I. Evaluation of polyphenol content and antioxidant activities of some selected organic and aqueous extracts of cornsilk (Zea mays hairs). J. Med. Bioeng. 2012, 1, 48–51. [Google Scholar]
- de Gouveia, N.M.; Ramos, S.; Martín, M.; Espindola, F.; Goya, L.; Palomino, O. Vochysia rufa stem bark extract protects endothelial cells against high glucose damage. Medicines 2017, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Carling, D.; Ruderman, N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: Inhibition by the AMP-activated protein kinase activation. Diabetes 2002, 51, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Han, J.S. Effect of Polyopes lancifolia extract on oxidative stress in human umbilical vein endothelial cells induced by high glucose. Prev. Nutr. Food Sci. 2013, 18, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-A.; Song, Y.-O.; Jang, M.-S.; Han, J.-S. Effect of baechu kimchi added Ecklonia cava extracts on high glucose-induced oxidative stress in human umbilical vein endothelial cells. Prev. Nutr. Food Sci. 2014, 19, 170–177. [Google Scholar] [CrossRef]
- Nurraihana, H.; Rosli, W.I.W.; Sabreena, S.; Norfarizan-Hanoon, N.A. Optimisation extraction procedure and identification of phenolic compounds from fractional extract of corn silk (Zea mays hair) using LC-TOF/MS system. J. Food Meas. Charact. 2018, 12, 1852–1862. [Google Scholar] [CrossRef]
- Campos, J.; Schmeda-Hirschmann, G.; Leiva, E.; Guzman, L.; Orrego, R.; Fernandez, P.; González, M.; Radojkovic, C.; Zuñiga, F.; Lamperti, L.; et al. Lemon grass (Cymbopogon citratus (DC) Stapf) polyphenols protect human umbilical vein endothelial cell (HUVECs) from oxidative damage induced by high glucose, hydrogen peroxide and oxidised low-density lipoprotein. Food Chem. 2014, 151, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Angel-Morales, G.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenolics from acai (Euterpe oleracea Mart.) and red muscadine grape (Vitis rotundifolia) protect human umbilical vascular Endothelial cells (HUVEC) from glucose-and lipopolysaccharide (LPS)-induced inflammation and target microRNA-126. J. Agric. Food Chem. 2011, 59, 7999–8012. [Google Scholar] [CrossRef]
- Jittiporn, K.; Moongkarndi, P.; Samer, J.; Suvitayavat, W. Protective effect of α-mangostin on high glucose induced endothelial cell apoptosis. Walailak J. Sci. Technol. 2017, 15, 579–587. [Google Scholar] [CrossRef]
- Lee, S.-H.; Han, J.-S.; Heo, S.-J.; Hwang, J.-Y.; Jeon, Y.-J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. In Vitro 2010, 24, 375–381. [Google Scholar] [CrossRef]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Kato, N.; Kawabe, S.; Ganeko, N.; Yoshimura, M.; Amakura, Y.; Ito, H. Polyphenols from flowers of Magnolia coco and their anti-glycation effects. Biosci. Biotechnol. Biochem. 2017, 81, 1285–1288. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Wang, K.-J.; Zhao, J.-L. Corn silk (Zea mays L.), a source of natural antioxidants with α-amylase, α-glucosidase, advanced glycation and diabetic nephropathy inhibitory activities. Biomed. Pharmacother. 2019, 110, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Liang, Y.; Li, J.; Lin, Q.; Huang, P.; Zhang, L.; Wu, W.; Ma, Y. Research progress on signaling pathway-associated oxidative stress in endothelial cells. Oxidative Med. Cell. Longev. 2017, 2017, 7156941. [Google Scholar] [CrossRef]
- Shaw, A.; Doherty, M.K.; Mutch, N.J.; MacRury, S.M.; Megson, I.L. Endothelial cell oxidative stress in diabetes: A key driver of cardiovascular complications? Biochem. Soc. Trans. 2014, 42, 928–933. [Google Scholar] [CrossRef]
- NIH. Atherosclerosis 2019. Available online: https://www.nhlbi.nih.gov/health-topics/atherosclerosis (accessed on 13 May 2019).
- Elbe, H.; Vardı, N.; Orman, D.; Taşlıdere, E.; Yıldız, A. Ameliorative effects of aminoguanidine on rat aorta in Streptozotocin-induced diabetes and evaluation of α-SMA expression. Anatol. J. Cardiol. 2014, 14, 679–684. [Google Scholar] [CrossRef]
- Zainalabidin, S.; Budin, S.B.; Anuar, N.N.M.; Yusoff, N.A.; Yusof, N.L.M. Hibiscus sabdariffa Linn. improves the aortic damage in diabetic rats by acting as antioxidant. J. Appl. Pharm. Sci. 2018, 8, 108–114. [Google Scholar]
- Abbasi, N.; Akhavan, M.M.; Rahbar-Roshandel, N.; Shafiei, M. The effects of low and high concentrations of luteolin on cultured human endothelial cells under normal and glucotoxic conditions: Involvement of integrin-linked kinase and cyclooxygenase-2. Phytother. Res. 2014, 28, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Erhirhie, E.; Ekene, N.; Ajaghaku, D. Guidelines on dosage calculation and stock solution preparation in experimental animals’ studies. J. Nat. Sci. Res. 2014, 4, 100–106. [Google Scholar]
- Nayak, I.M.N.; Chinta, R.; Jetti, R. Anti-atherosclerotic potential of aqueous extract of Cinnamomum Zeylanicum bark against glucocorticoid induced atherosclerosis in Wistar rats. J. Clin. Diagn. Res. 2017, 11, FC19–FC23. [Google Scholar] [CrossRef]

| Sample (n = 6) | EC50 (ng/mL) ± S.E.M. |
|---|---|
| PRF | 108.00 ± 45.22 a |
| Metformin | 261.23 ± 112.18 b |
| Histopathological Changes | Evaluation of Deposition of Foamy Cell in Tunica | Evaluation of Density of Endothelial Cells at Tunica Intima | Evaluation of Thickness of Tunica Media | |
|---|---|---|---|---|
| Treatment Groups (n = 6) | ||||
| Normal control | − | − | − | |
| Diabetic control | ++ | ++ | ++ | |
| Diabetic-PRF treated (100 mg/kg) | + | + | + | |
| Diabetic-PRF treated (200 mg/kg) | + | + | + | |
| Diabetic-metformin treated (150 mg/kg) | + | + | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzah, N.; Safuan, S.; Wan Ishak, W.R. Potential Effect of Polyphenolic-Rich Fractions of Corn Silk on Protecting Endothelial Cells against High Glucose Damage Using In Vitro and In Vivo Approaches. Molecules 2021, 26, 3665. https://doi.org/10.3390/molecules26123665
Hamzah N, Safuan S, Wan Ishak WR. Potential Effect of Polyphenolic-Rich Fractions of Corn Silk on Protecting Endothelial Cells against High Glucose Damage Using In Vitro and In Vivo Approaches. Molecules. 2021; 26(12):3665. https://doi.org/10.3390/molecules26123665
Chicago/Turabian StyleHamzah, Nurraihana, Sabreena Safuan, and Wan Rosli Wan Ishak. 2021. "Potential Effect of Polyphenolic-Rich Fractions of Corn Silk on Protecting Endothelial Cells against High Glucose Damage Using In Vitro and In Vivo Approaches" Molecules 26, no. 12: 3665. https://doi.org/10.3390/molecules26123665
APA StyleHamzah, N., Safuan, S., & Wan Ishak, W. R. (2021). Potential Effect of Polyphenolic-Rich Fractions of Corn Silk on Protecting Endothelial Cells against High Glucose Damage Using In Vitro and In Vivo Approaches. Molecules, 26(12), 3665. https://doi.org/10.3390/molecules26123665