Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review
Abstract
:1. Introduction
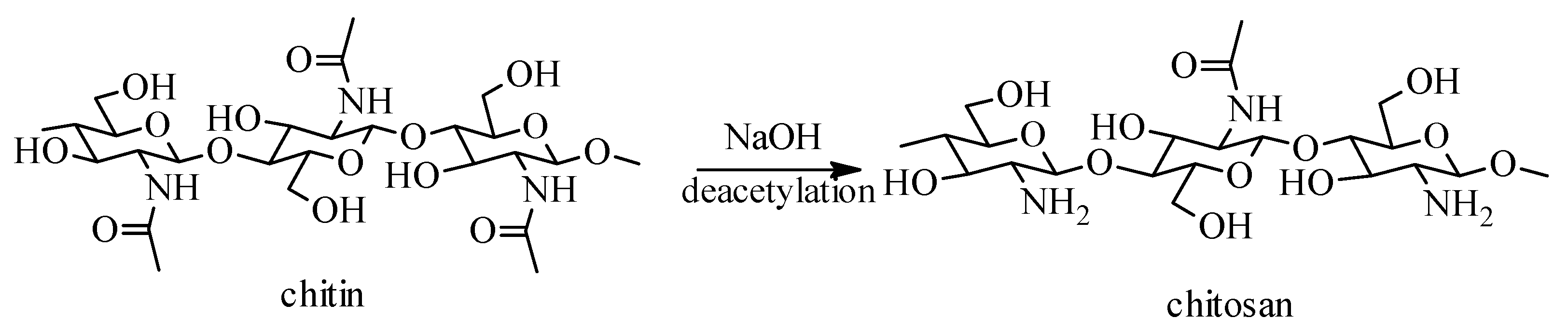
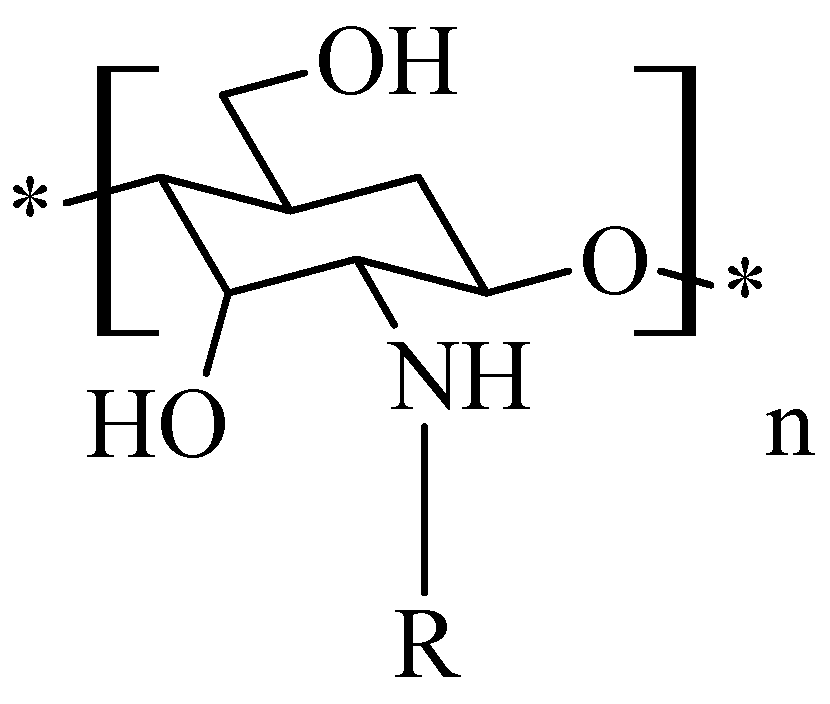
2. Sources, Preparation Methods and Physico-Chemical Properties of Chitosan and Its Derivatives
2.1. Chitosan Sulfate
- Obtaining the quaternary ammonium salt of chitosan. To an aqueous solution of N-(3-chloro-2-hydroxypropyl)-trimethylammonium chloride, 15% NaOH was added, to pH 8. The resulting mixture was stirred at room temperature for 48 h and another 24 h at 50 °C to form the quaternary ammonium salt of chitosan.
- Obtaining the sulphating agent N(SO3Na)3. An aqueous solution of sodium nitrite was continuously stirred over a solution of sodium bisulfite, at 90 °C, over 90 min.
- Obtaining the quaternary ammonium salt of chitosan sulfate. The quaternary ammonium salt of chitosan was added over the solution containing the sulfating agent under mechanical agitation, at room temperature, then the reaction product obtained was concentrated and dried at 40 °C.
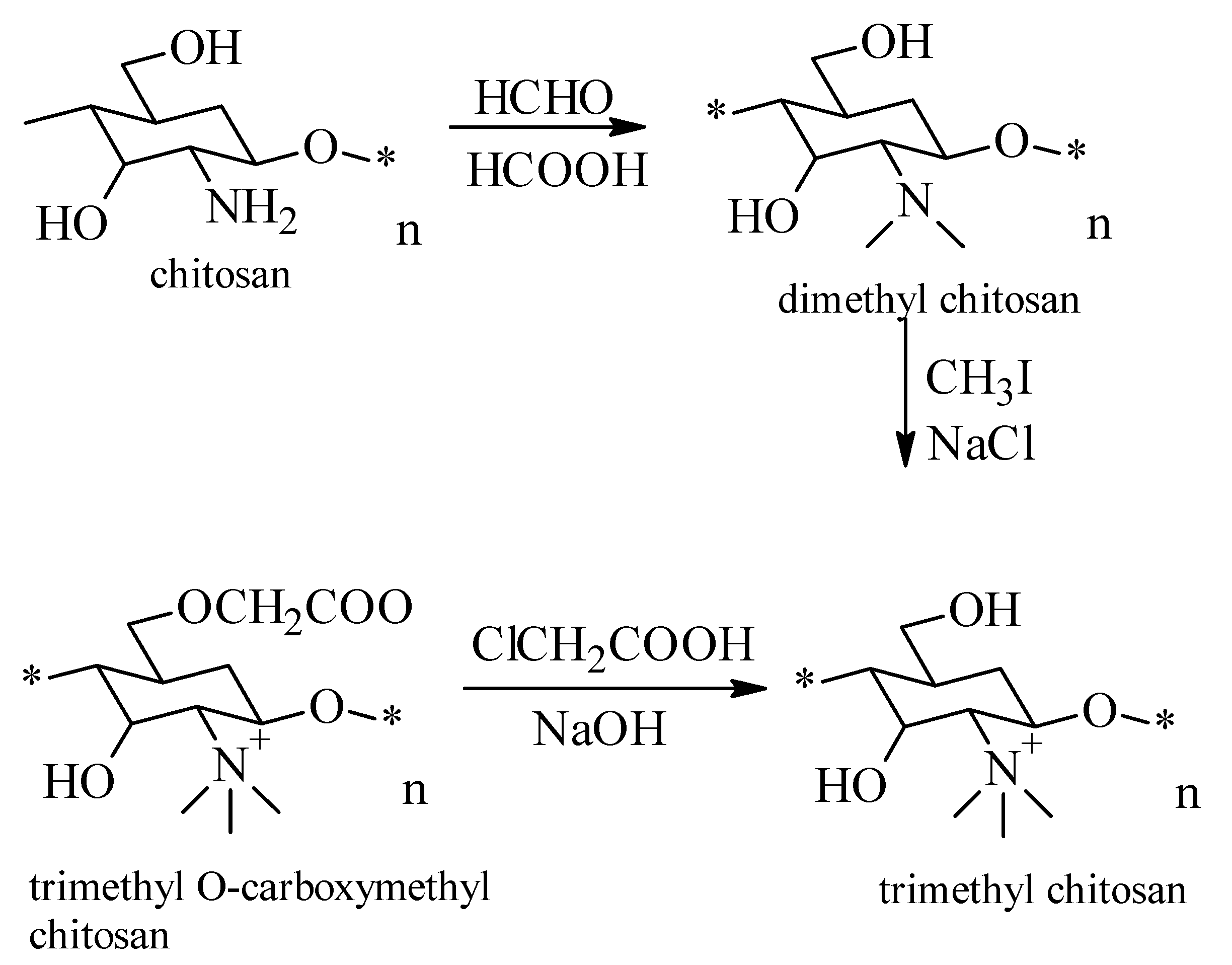
2.2. Chitosan Trimethylate
- obtaining the dimethyl chitosan by reaction of formic acid with formaldehyde at a temperature of 70 °C for 118 h;
- the reaction of dimethyl chitosan with iodoform under continuous stirring at a temperature of 70 °C, to obtain trimethyl chitosan;
- the last step is the reaction of trimethyl chitosan with monocloracetic acid in alkaline medium.
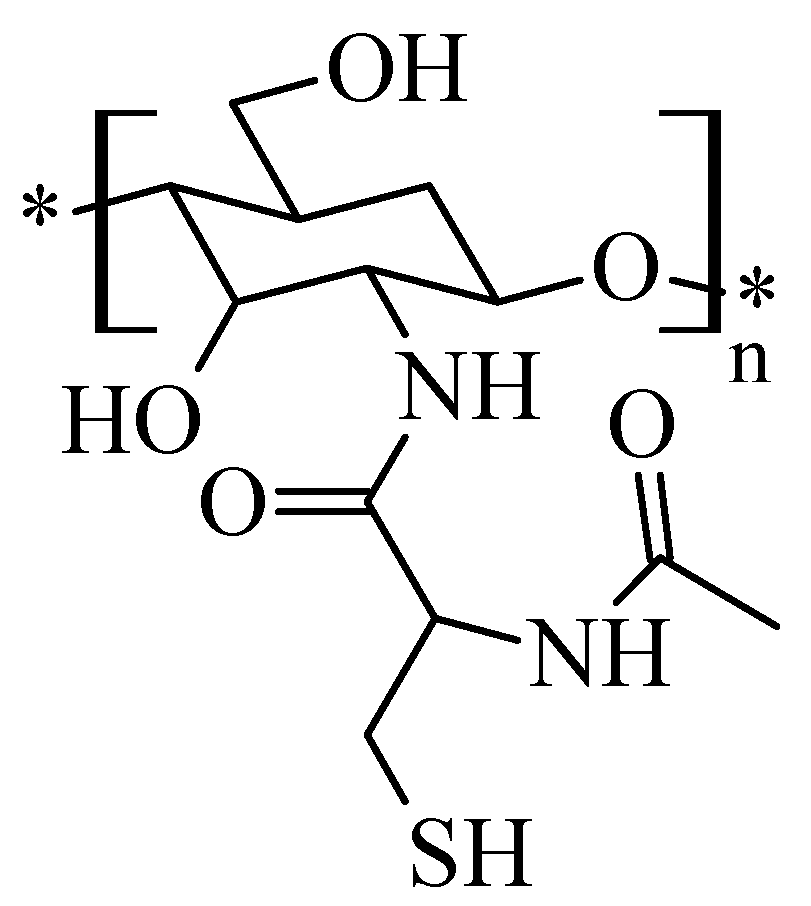
2.3. Chitosan Thiolate
2.4. Chitosan-N-hydroxy-propyl Derivatives
2.5. Chitosan N-Alkyl Derivatives
2.6. Chitosan N-Carboxymethyl Derivatives
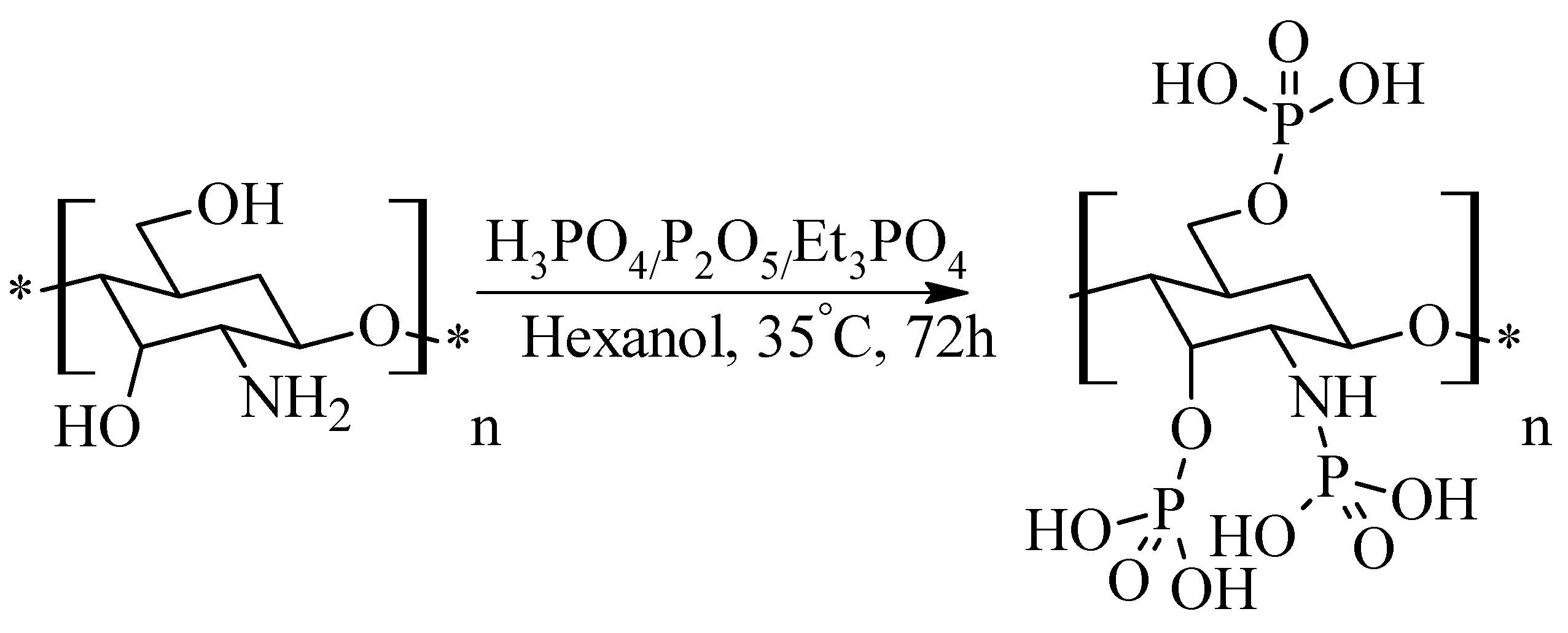
2.7. Phosphorylated Chitosan
3. Pharmacological Potential of Chitosan and Its Derivatives. Applications in Current Pharmacotherapy
3.1. Antimicrobial Activity
3.1.1. Antibacterial Activity
Mechanism of Action
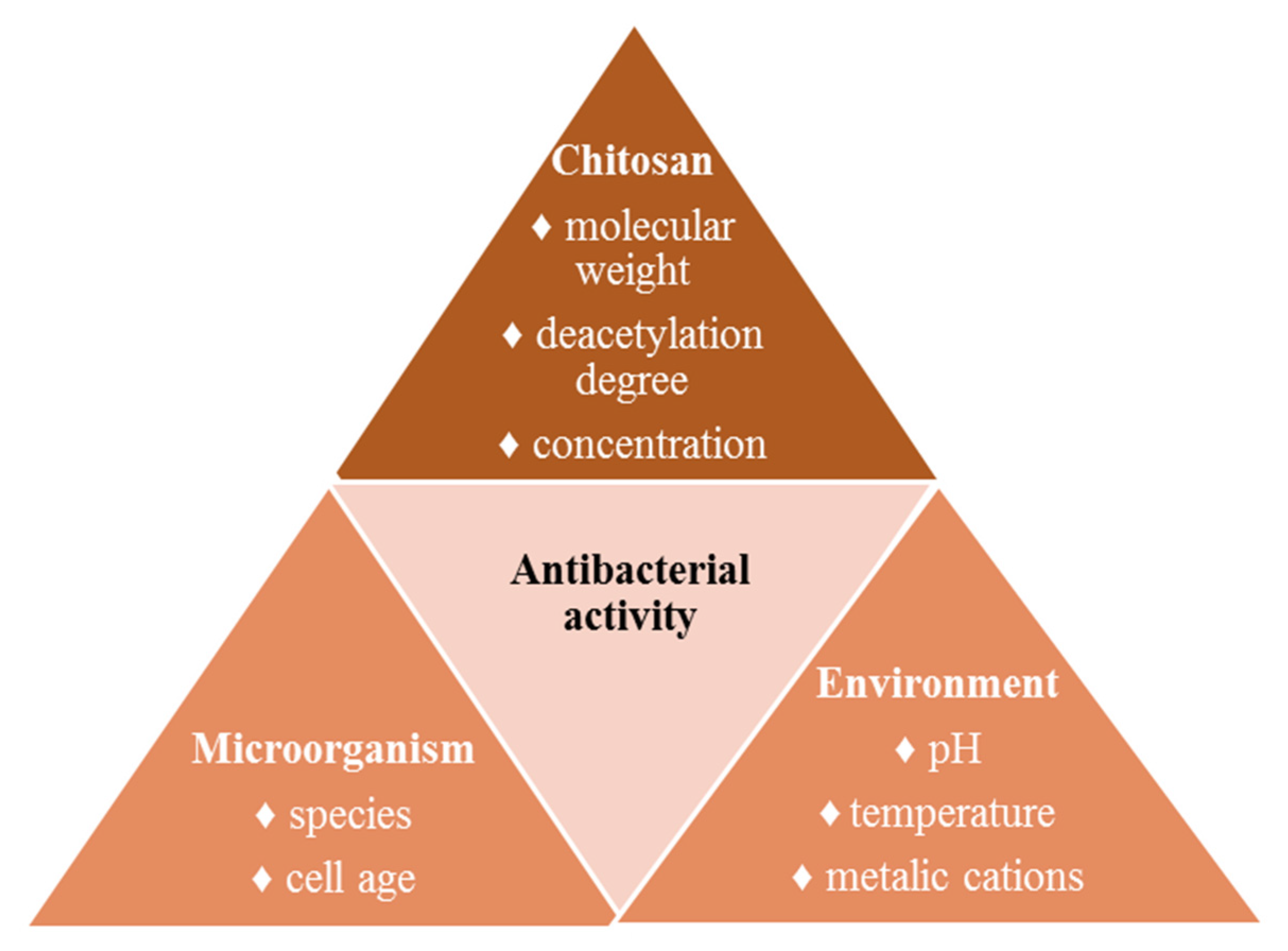
Factors That Influence the Antibacterial Activity
Antibacterial Activity against Resistant Strains
3.1.2. Antiviral Activity
3.1.3. Antifungal Activity
3.2. Other Pharmacological Effects
3.2.1. Healing Activity
3.2.2. Hemostatic Effect
3.2.3. Antioxidant Activity
3.3. Drug Delivery Systems
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonia, T.A.; Sharma, C.P. 6–Polymers in Oral Insulin Delivery. In Oral Delivery of Insulin; Woodhead Publishing: Cambridge, UK, 2014; pp. 257–310. [Google Scholar] [CrossRef]
- Fonte, P.; Araújo, F.; Silva, C.; Pereira, C.; Reis, S.; Santos, H.A.; Sarmento, B. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnol. Adv. 2015, 33, 1342–1354. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mat. Sci. Eng. C Mater. 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Agrawal, U.; Sharma, R.; Gupta, M.; Vyas, S.P. Is nanotechnology a boon for oral drug delivery? Drug Discov. Today 2014, 19, 1530–1549. [Google Scholar] [CrossRef]
- Thakur, S.; Verma, A.; Sharma, B.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent developments in recycling of polystyrene based plastics. Curr. Opin. Green Sustain. Chem. 2018, 13, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A natural arsenal against bacterial pathogens. Antibiotics 2020, 9, 336. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Kang, K.-H.; Je, J.-Y.; Pham, H.N.-D.; Byun, H.-G.; Kim, S.-K. Biological effects of chitosan and its derivatives. Food Hydrocolloid 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Sharland, M.; Saroey, P.; Berezin, E.N. The global threat of antimicrobial resistance—The need for standardized surveillance tools to define burden and develop interventions. J. Pediatr. 2015, 91, 410–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertesteanu, S.; Chifiriuc, M.C.; Grumezescu, A.M.; Printza, A.G.; Marie-Paule, T.; Grumezescu, V.; Mihaela, V.; Lazar, V.; Grigore, R. Biomedical applications of synthetic, biodegradable polymers for the development of anti-infective strategies. Curr. Med. Chem. 2014, 21, 3383–3390. [Google Scholar] [CrossRef]
- O’Rourke, A.; Beyhan, S.; Choi, Y.; Morales, P.; Chan, A.P.; Espinoza, J.L.; Dupont, C.L.; Meyer, K.J.; Spoering, A.; Lewis, K.; et al. Mechanism-of-action classification of antibiotics by global transcriptome profiling. Antimicrob. Agents Chemother. 2020, 64, e01207-19. [Google Scholar] [CrossRef] [Green Version]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.F. Antimicrobial polymers: The potential replacement of existing antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Alarćon-Suesca, C.; González-Delgado, A.D. Exergetic sensibility analysis and environmental evaluation of chitosan production from shrimp exoskeleton in Colombia. J. Clean. Prod. 2020, 248, 119285. [Google Scholar] [CrossRef]
- Batista, M.K.S.; Pinto, L.F.; Gomes, C.A.R.; Gomes, P. Novel highly-soluble peptide-chitosan polymers: Chemical synthesis and spectral characterization. Carbohyd. Polym. 2006, 64, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Carraher, C.E., Jr.; Seymour, R.B. Polymer Chemistry, 7th ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 41. [Google Scholar]
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Reporting degree of deacetylation values of chitosan: The influence of analytical methods. J. Pharm. Pharm. Sci. 2002, 5, 205–212. [Google Scholar]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; Jie, P.; Zhu, J.; Cheng, F. Preparation of chitosan/lignosulfonate for effectively removing Pb(II) in water. Polymer 2021, 228, 123878. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Enhanced antifungal activity of novel cationic chitosan derivative bearing triphenylphosphonium salt via azide-alkyne click reaction. Int. J. Biol. Macromol. 2020, 165, 1765–1772. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Fan, L.; Wu, P.; Zhang, J.; Gao, S.; Wang, L.; Li, M.; Sha, M.; Xie, W.; Nie, M. Synthesis and anticoagulant activity of the quaternary ammonium chitosan sulfates. Int. J. Biol. Macromol. 2012, 50, 31–37. [Google Scholar] [CrossRef]
- Zhong, Z.; Ji, X.; Xing, R.; Liu, S.; Guo, Z.; Chen, X.; Li, P. The preparation and antioxidant activity of the sulfanilamide derivatives of chitosan and chitosan sulfates. Bioorg. Med. Chem. 2007, 15, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, Y.; Xie, W.; Chen, L.; Zheng, H.; Fan, L. Preparation and anticoagulant activity of N-succinyl chitosan sulfates. Int. J. Biol. Macromol. 2012, 51, 808–814. [Google Scholar] [CrossRef]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S. Synthesis, characteristic and antibacterial activity of N,N,N-trimethyl chitosan and its carboxymethyl derivatives. Carbohyd. Polym. 2010, 81, 931–936. [Google Scholar] [CrossRef]
- Benediktsdóttir, B.E.; Gaware, V.S.; Rúnarsson, Ö.V.; Jόnsdόttir, S.; Jensen, K.J.; Másson, M. Synthesis of N,N,N-trimethyl chitosan homopolymer and highly substituted N-alkyl-N,N-dimethyl chitosan derivatives with the aid of di-tert-butyldimethylsilyl chitosan. Carbohyd. Polym. 2011, 86, 1451–1460. [Google Scholar] [CrossRef]
- Geisberger, G.; Gyenge, E.B.; Maake, C.; Patzke, G.T. Trimethyl and carboxymethyl chitosan carriers for bio-active polymer–inorganic nanocomposites. Carbohyd. Polym. 2013, 91, 58–67. [Google Scholar] [CrossRef]
- Anitha, A.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Development of mucoadhesive thiolated chitosan nanoparticles for biomedical applications. Carbohyd. Polym. 2011, 83, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Hombach, J.; Hoyer, H.; Bernkop-Schnürch, A. Thiolated chitosans: Development and in vitro evaluation of an oral tobramycin sulphate delivery system. Eur. J. Pharm. Sci. 2008, 33, 1–8. [Google Scholar] [CrossRef]
- Schuerer, N.; Stein, E.; Inic-Kanada, A.; Ghasemian, E.; Stojanovic, M.; Montanaro, J.; Bintner, N.; Hohenadl, C.; Sachsenhofer, R.; Barisani-Asenbauer, T. Effects of chitosan and chitosan N-acetylcysteine solutions on conjunctival epithelial cells. J. EuCornea 2018, 1, 12–18. [Google Scholar] [CrossRef]
- Peng, Y.; Han, B.; Liu, W.; Xu, X. Preparation and antimicrobial activity of hydroxypropyl chitosan. Carbohyd. Res. 2005, 340, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-C.; Chou, C.-C.; Li, C.-F. Antibacterial activity of N-alkylated disaccharide chitosan derivatives. Int. J. Food Microbiol. 2005, 97, 237–245. [Google Scholar] [CrossRef]
- An, N.T.; Dung, P.L.; Thien, D.T.; Dong, N.T.; Nhi, T.T.Y. An improved method for synthesizing N,N′-dicarboxymethylchitosan. Carbohyd. Polym. 2008, 73, 261–264. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nagahama, H.; Furuike, T.; Tamura, H. Synthesis of phosphorylated chitosan by novel method and its characterization. Int. J. Biol. Macromol. 2008, 42, 335–339. [Google Scholar] [CrossRef]
- Li, B.; Huang, L.; Wang, X.; Ma, J.; Xie, F. Biodegradation and compressive strength of phosphorylated chitosan/chitosan/ hydroxyapatite bio-composites. Mater. Des. 2011, 32, 4543–4547. [Google Scholar] [CrossRef]
- Lang, G. Chitosan Derivatives—Preparation and Potential Uses. In Chitin and Chitosan: The Versatile Environmentally Friendly Modern Materials; Collection of Working Papers 28; Zakaria, M.B., Wan Muda, W.M., Abdullah, M.P., Eds.; Penrbit University Kebangsaan, Anpang Press Adn. Bhd.: Bangi, Malaysia, 1995; pp. 109–118. [Google Scholar]
- Yang, J.M.; Yang, S.J.; Lin, H.T.; Wu, T.-H.; Chen, H.-J. Chitosan containing PU/Poly(NIPAAm) thermosensitive membrane for wound dressing. Mat. Sci. Eng. C Mater. 2008, 28, 150–156. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Kaprou, G.D.; Bergšpica, I.; Alexa, E.A.; Alvarez-Ordóñez, A.; Prieto, M. Rapid methods for antimicrobial resistance diagnostics. Antibiotics 2021, 10, 209. [Google Scholar] [CrossRef]
- Bassetti, M.; Carnelutti, A.; Peghin, M. Patient specific risk stratification for antimicrobial resistance and possible treatment strategies in gram-negative bacterial infections. Expert Rev. Anti-Inf. 2017, 15, 55–65. [Google Scholar] [CrossRef]
- Guitor, A.K.; Raphenya, A.R.; Klunk, J.; Kuch, M.; Alcock, B.; Surette, M.G.; McArthur, A.G.; Poinar, H.N.; Wright, G.D. Capturing the resistome: A targeted capture method to reveal antibiotic resistance determinants in metagenomes. Antimicrob. Agents Chemother. 2020, 64, e01324-19. [Google Scholar] [CrossRef] [Green Version]
- Almasaudi, S.B. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi J. Biol. Sci. 2018, 25, 586–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Aldisio, E.; Marchese, A.E.; Mattaliano, A.R.; Tsakris, A. Antibiotic trends of Klebsiella pneumoniae and Acinetobacter baumannii resistance indicators in an intensive care unit of Southern Italy, 2008–2013. Antimicrob. Resist. Infect. Control 2015, 4, 43–51. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Dimopoulos, G.; Poulakou, G.; Akova, M.; Cisneros, J.M.; de Waele, J.; Petrosillo, N.; Seifert, H.; Timsit, J.F.; Vila, J.; et al. Task force on managament and prevention of Acinetobacter baumannii infections in the ICU. Intens. Care Med. 2015, 41, 2057–2075. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Mantravadi, P.K.; Kalesh, K.A.; Dobson, R.C.J.; Hudson, A.O.; Parthasarathy, A. The quest for novel antimicrobial compounds: Emerging trends in research, development, and technologies. Antibiotics 2019, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, X.; Chen, Q. Synthesis and characterization of water-soluble chitosan derivate and its antibacterial activity. Carbohyd. Polym. 2007, 69, 142–147. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kennedy, J.F.; Nie, J. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohyd. Polym. 2008, 74, 121–126. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.L.; Wu, J.C.G.; Lin, J.G. Relationship between antibacterial activity of chitosans and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Raafat, D.; Bargen, K.V.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microb. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.-C.; Chen, C.-Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Malcata, F.X. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef]
- Ortega-Ortiz, H.; Gutierrez-Rodriguez, B.; Cadenas-Pliego, G.; Ibarra-Jimenez, L. Antibacterial Activity of Chitosan and the Interpolyelectrolyte Complexes of Poly(acrylic acid)-Chitosan. Braz. Arch. Biol. Technol. 2010, 53, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M.M. Insights into chitosan antibiofilm activity against methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2016, 122, 1547–1557. [Google Scholar] [CrossRef]
- Park, S.-C.; Nam, J.-P.; Kim, J.-H.; Kim, Y.-M.; Nah, J.-W.; Jang, M.-K. Antimicrobial action of water-soluble β-chitosan against clinical multi-drug resistant bacteria. Int. J. Mol. Sci. 2015, 16, 7995–8007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, M.; Chen, X.G.; Liu, C.S.; Liu, C.G.; Meng, X.H.; Yu, L.J. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloid. Surf. B Biointerfaces 2008, 65, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated by the deacetylation degree. Biochem. Eng. J. 2008, 40, 485–491. [Google Scholar] [CrossRef]
- Lim, S.-H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohyd. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef]
- Supotngarmkul, A.; Panichuttra, A.; Ratisoontorn, C.; Nawachinda, M.; Matangkasombut, O. Antibacterial property of chitosan against E. faecalis standard strain and clinical isolates. Dent. Mater. J. 2020, 39, 456–463. [Google Scholar] [CrossRef]
- Saito, H.; Sakakibara, Y.; Sakata, A.; Kurashige, R.; Murakami, D.; Kageshima, H.; Saito, A.; Miyazaki, Y. Antibacterial activity of lysozyme-chitosan oligosaccharide conjugates (LYZOX) against Pseudomonas aeruginosa, Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus. PLoS ONE 2019, 14, e0217504. [Google Scholar] [CrossRef]
- Shahini Shams Abadi, M.; Mirzaei, E.; Bazargani, A.; Gholipour, A.; Heidari, H.; Hadi, N. Antibacterial activity and mechanism of action of chitosan nanofibers against toxigenic Clostridioides (Clostridium) difficile isolates. Ann. Ig. 2020, 32, 72–80. [Google Scholar] [CrossRef]
- Zhang, F.; Ramachandran, G.; Mothana, R.A.; Noman, O.M.; Alobaid, W.A.; Rajivgandhi, G.; Manoharan, N. Anti-bacterial activity of chitosan loaded plant essential oil against multi drug resistant K. pneumoniae. Saudi J. Biol. Sci. 2020, 27, 3449–3455. [Google Scholar] [CrossRef]
- Jamil, B.; Habib, H.; Abbasi, S.A.; Ihsan, A.; Nasir, H.; Imran, M. Development of cefotaxime impregnated chitosan as nano-antibiotics: De novo strategy to combat biofilm forming multi-drug resistant pathogens. Front. Microbiol. 2016, 7, 330. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.S.; Oshiro-Junior, J.A.; Sato, M.R.; Conceição, M.M.; Medeiros, A.C.D. Polymeric nanoparticle associated with ceftriaxone and extract of Schinopsis Brasiliensis Engler against Multiresistant Enterobacteria. Pharmaceutics 2020, 12, 695. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Elberry, M.H.; Farroh, K.Y.; Mohamed, H.T.; Mohamed, A.A.; Mohamed, E.B.; Faraag, A.H.I.; Mousa, S.A. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. Int. J. Nanomed. 2020, 15, 2699–2715. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Sun, Y.; Cong, H.; Hu, H.; Xu, F.-J. An overview of chitosan and its application in infectious diseases. Drug Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef]
- Artan, M.; Karadeniz, F.; Karagozlu, M.Z.; Kim, M.-M.; Kim, S.-K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohyd. Res. 2010, 345, 656–662. [Google Scholar] [CrossRef]
- Zheng, M.; Qu, D.; Wang, H.; Sun, Z.; Liu, X.; Chen, J.; Li, C.; Li, X.; Chen, Z. Intranasal administration of chitosan against Influenza A (H7N9) virus infection in a mouse model. Sci. Rep. 2016, 6, 28729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Modak, C.; Singh, P.K.; Kumar, R.; Khatri, D.; Singh, S.B. Underscoring the immense potential of chitosan in fighting a wide spectrum of viruses: A plausible molecule against SARS-CoV-2? Int. J. Biol. Macromol. 2021, 179, 33–44. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Sadeghi, S.; Azizi, M.; Rastegari-Pouyani, M.; Pouriran, R.; Hoseini, M.H.M. Chitin and chitosan as tools to combat COVID-19: A triple approach. Int J Biol Macromol. 2021, 183, 235–244. [Google Scholar] [CrossRef]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloid. Surface B 2014, 118, 117–125. [Google Scholar] [CrossRef]
- Kubbinga, M.; Nguyen, M.A.; Staubach, P.; Teerenstra, S.; Langguth, P. The influence of chitosan on the oral bioavailability of acyclovir—a comparative bioavailability study in humans. Pharm. Res. 2015, 32, 2241–2249. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Balducci, A.G.; Zironi, E.; Colombo, G.; Bortolotti, F.; Lorenzini, L.; Galligioni, V.; Pagliuca, G.; Scagliarini, A.; Calzà, L.; et al. In vivo nose-to-brain delivery of the hydrophilic antiviral ribavirin by microparticle agglomerates. Drug Deliv. 2018, 25, 376–387. [Google Scholar] [CrossRef] [Green Version]
- Shital, L.; Bowen, J.; Badhan, R. Development and evaluation of a novel intranasal spray for the delivery of amantadine. J. Pharm. Sci. 2016, 105, 1209–1220. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Guarner, J. Emerging and reemerging fungal infections. Semin. Diagn. Pathol. 2019, 36, 177–181. [Google Scholar] [CrossRef]
- Pagano, L.; Akova, M.; Dimopoulos, G.; Herbrecht, R.; Drgona, L.; Blijlevens, N. Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. J. Antimicrob. Chemoth. 2011, 66, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbee, D.D.; Pierce, S.S.; Manning, J. Opportunistic fungal infections in critical care units. Crit. Care Nurs. Clin. 2017, 29, 67–79. [Google Scholar] [CrossRef]
- Silva, R.F.e. Fungal infections in immunocompromised patients. J. Bras. Pneumol. 2010, 36, 142–147. [Google Scholar] [CrossRef]
- Schmiedel, Y.; Zimmerli, S. Common invasive fungal diseases: An overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med. Wkly. 2016, 146, w14281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramythiotou, E.; Frantzeskaki, F.; Flevari, A.; Armaganidis, A.; Dimopoulos, G. Invasive fungal infections in the ICU: How to approach, how to treat. Molecules 2014, 19, 1085–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug. Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Jensen, R.H. Resistance in human pathogenic yeasts and filamentous fungi: Prevalence, underlying molecular mechanism and link to the use of antifungals in humans and the environment. Dan. Med. J. 2016, 63, B5288. [Google Scholar]
- Lo, W.-H.; Deng, F.-S.; Chang, C.-J.; Lin, C.-H. Synergistic antifungal activity of chitosan with fluconazole against Candida albicans, Candida tropicalis, and fluconazole-resistant strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef]
- Parente-Rocha, J.A.; Bailão, A.M.; Amaral, A.C.; Taborda, C.P.; Paccez, J.D.; Borges, C.L.; Pereira, M. Antifungal resistance, metabolic routes as drug targets, and new antifungal agents: An overview about endemic dimorphic fungi. Mediat. Inflamm. 2017, 2017, 9870679. [Google Scholar] [CrossRef]
- Fuentefria, A.M.; Pippi, B.; Dalla Lana, D.F.; Donato, K.K.; Andrade, S.F. Antifungals discovery: An insight into new strategies to combat antifungal resistance. Lett. Appl. Microbiol. 2017, 66, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alburquenque, C.; Bucarey, S.A.; Andrónico, N.C.; Urzúa, B.; Hermosilla, G.; Tapia, C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010, 48, 1018–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña, A.; Sánchez, N.S.; Calahorra, M. Effects of chitosan on Candida albicans: Conditions for its antifungal activity. BioMed Res. Int. 2013, 2013, 527549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganan, M.; Lorentzen, S.B.; Aam, B.B.; Eijsink, V.G.H.; Gaustad, P.; Sørlie, M. Antibiotic saving effect of combination therapy through synergistic interactions between well-characterized chito-oligosaccharides and commercial antifungals against medically relevant yeasts. PLoS ONE 2019, 14, e0227098. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, K.; Du, L.; Cheng, Z.; Zhang, T.; Ding, J.; Li, W.; Xu, B.; Zhu, M. Construction of chitosan scaffolds with controllable microchannel for tissue engineering and regenerative medicine. Mater. Sci. Eng. C 2021, 126, 112178. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Gallaher, C.M.; Munion, J.; Hesslink, R., Jr.; Wise, J.; Gallaher, D.D. Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J. Nutr. 2000, 130, 2753–2759. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Xie, C.; Wang, C.; Huang, J.; Yin, Z.; Heng, B.C.; Chen, X.; Shen, W. Promoting musculoskeletal system soft tissue regeneration by biomaterial-mediated modulation of macrophage polarization. Bioact. Mater. 2021, 6, 4096–4109. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.A.; Rey-Rico, A.; Oliveira, R.A.; Marceneiro, S.; Alvarez-Lorenzo, C.; Concheiro, A.; Júnior, R.N.C.; Braga, M.E.M.; de Sousa, H.C. Wound dressings loaded with an anti-inflammatory jucá (Libidibia ferrea) extract using supercritical carbon dioxide technology. J. Supercrit. Fluids 2013, 74, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Panzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef]
- Öztürk, E.; Agalar, C.; Keçeci, K.; Denkbas, E.B. Preparation and characterization of ciprofloxacin-loaded alginate/chitosan sponge as wound dressing material. J. Appl. Polym. Sci. 2006, 101, 1602–1609. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Lupascu, F.; Panzariu, A.; Dubruel, P.; Lupascu, D.; Tuchilus, C.; Vasile, C.; Profire, L. Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing. Int. J. Mol. Sci. 2015, 16, 29843–29855. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ma, L.; Mao, Z.; Gao, C. Chitosan-Based Biomaterials for Tissue Repair And Regeneration. In Chitosan for Biomaterials II; Jayakumar, R., Prabaharan, M., Muzzarelli, R.A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 244, pp. 81–127. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yano, R.; Miyatake, K.; Tomohiro, I.; Shigemasa, Y.; Minami, S. Effects of chitin and chitosan on blood coagulation. Carbohyd. Polym. 2003, 53, 337–342. [Google Scholar] [CrossRef]
- Gouthamchandra, K.; Mahmood, R.; Manjunatha, H. Free radical scavenging, antioxidant enzymes and wound healing activities of leaves extracts from Clerodendrum infortunatum L. Environ. Toxicol. Pharmacol. 2010, 30, 11–18. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Vinsova, J.; Vavrikova, E. Recent advances in drugs and prodrugs design of chitosan. Curr. Pharm. Des. 2008, 14, 1311–1326. [Google Scholar] [CrossRef] [Green Version]
- Tao, F.; Ma, S.; Tao, H.; Jin, L.; Luo, Y.; Zheng, J.; Xiang, W.; Deng, H. Chitosan-based drug delivery systems: From synthesis strategy to osteomyelitis treatment—A review. Carbohyd. Polym. 2021, 251, 117063. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Mohan, J.C.; Sreeja, V.; Tamura, H.; Patzke, G.R.; Hussain, F.; Weyeneth, S.; Nair, S.V.; Jayakumar, R. Novel carboxymethyl chitin nanoparticles for cancer drug delivery applications. Carbohyd. Polym. 2010, 79, 1073–1079. [Google Scholar] [CrossRef]
- Lupascu, F.; Dash, M.; Samal, S.K.; Dubruel, P.; Lupusoru, C.E.; Lupusoru, R.V.; Dragostin, O.; Profire, L. Development, optimization and biological evaluation of chitosan scaffold formulations of new xanthine derivatives for treatment of type-2 diabetes mellitus. Eur. J. Pharm. Sci. 2015, 77, 122–134. [Google Scholar] [CrossRef]

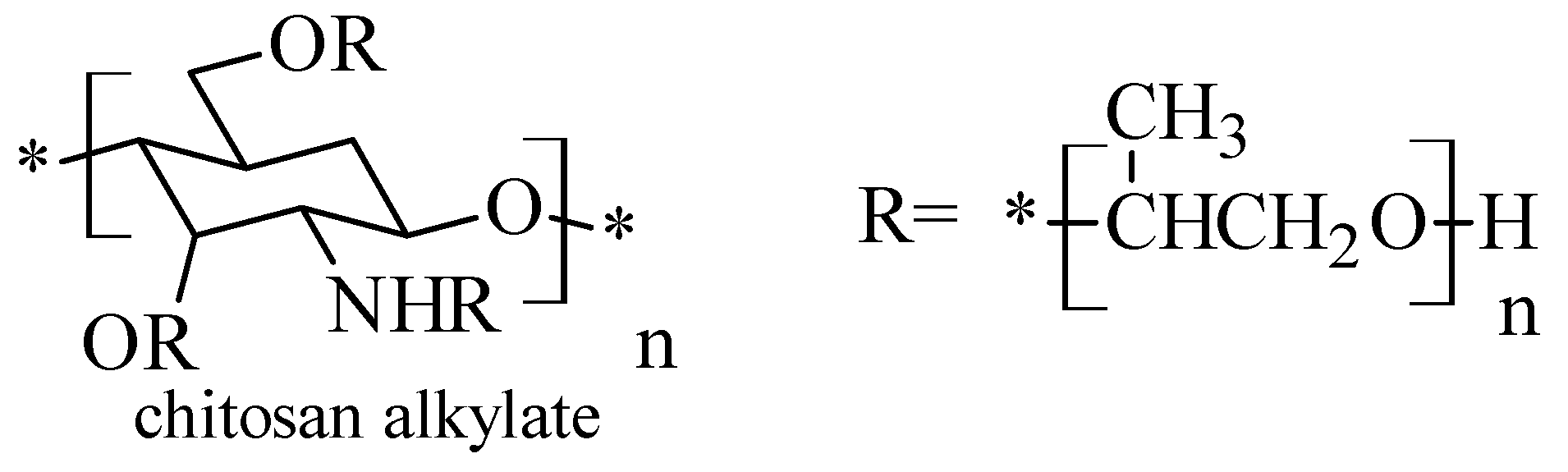
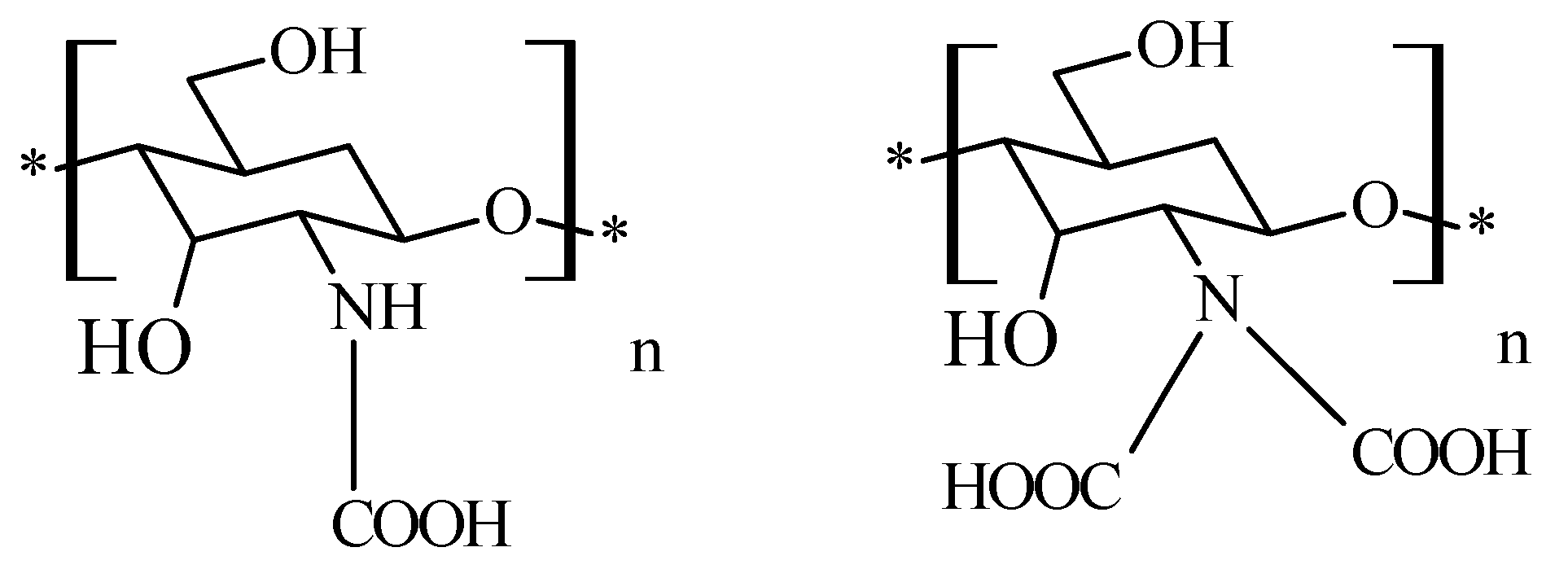
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. https://doi.org/10.3390/molecules26123694
Confederat LG, Tuchilus CG, Dragan M, Sha’at M, Dragostin OM. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules. 2021; 26(12):3694. https://doi.org/10.3390/molecules26123694
Chicago/Turabian StyleConfederat, Luminita Georgeta, Cristina Gabriela Tuchilus, Maria Dragan, Mousa Sha’at, and Oana Maria Dragostin. 2021. "Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review" Molecules 26, no. 12: 3694. https://doi.org/10.3390/molecules26123694
APA StyleConfederat, L. G., Tuchilus, C. G., Dragan, M., Sha’at, M., & Dragostin, O. M. (2021). Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules, 26(12), 3694. https://doi.org/10.3390/molecules26123694





