Efficient One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones via a Three-Component Biginelli Reaction
Abstract
:1. Introduction
2. Results
2.1. Catalyst Screening and Condition Optimization
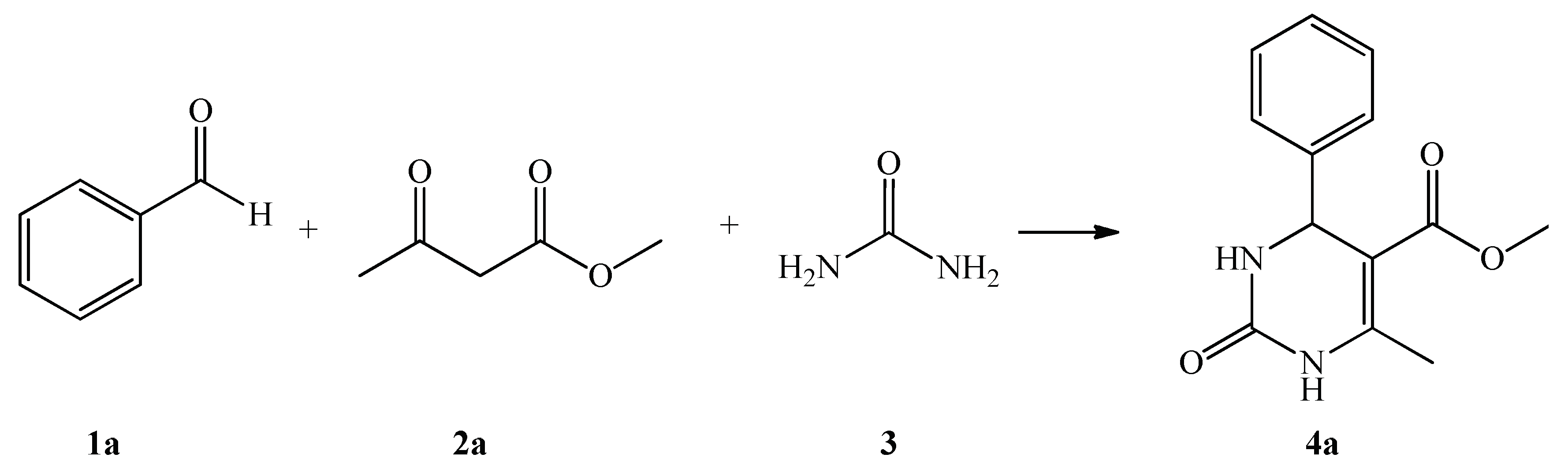
2.2. Substrate Screening
2.3. Hot Filtration Test
2.4. Catalyst Recycling Test
2.5. Green Metrics
3. Materials and Methods
3.1. General
3.2. Overall Method
3.3. Hot Filtration Test
3.4. Catalyst Recycling Test
3.5. Analytical Information
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sandhu, S.; Sandhu, J.S. Past, present and future of the Biginelli reaction: A critical perspective. ChemInform 2012, 10, 66–133. [Google Scholar]
- Mohammadi, B.; Behbahani, F.K. Recent Developments in the Synthesis and Applications of Dihydropyrimidin-2(1H)-ones and Thiones. Mol. Divers. 2018, 22, 405–446. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.L.H.; Masson, F.T.; Simeoni, L.A.; Homem-de-Mello, M. Biological activity of dihydropyrimidinone (DHPM) derivatives: A systematic review. Eur. J. Med. Chem. 2018, 143, 1779–1789. [Google Scholar]
- Anjaneyulu, B.; Dharma Rao, G.B. A Mini Review: Biginelli Reaction for the Synthesis of Dihydropyrimidinones. Int. J. Eng. Technol. Res. 2015, 3, 26–37. [Google Scholar]
- Alvim, H.G.O.; da Silva, J.E.N.; Neto, B.A.D. What do we know about multicomponent reactions? Mechanisms and trends for the Biginelli, Hantzsch, Mannich, Passerini and Ugi MCRs. RSC Adv. 2014, 4, 54282–54299. [Google Scholar] [CrossRef]
- Papeo, G.; Pulici, M. Italian chemists’ contributions to named reactions in organic synthesis: An historical perspective. Molecules 2013, 18, 10870–10900. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.M.; de Leon, P.; Tobio, A.Y.; dos Santos, M.R.; de Oliveira, H.C.B.; Gomes, A.F.; Gozzo, F.C.; de Oliveira, A.L.; Neto, B.A.D. Mechanistic Studies on Lewis Acid Catalyzed Biginelli Reactions in Ionic Liquids: Evidence for the Reactive Intermediates and the Role of the Reagents. J. Org. Chem. 2012, 77, 10184–10193. [Google Scholar] [CrossRef]
- Maskrey, T.S.; Frischling, M.C.; Rice, M.L.; Wipf, P. A Five-Component Biginelli-Diels-Alder Cascade Reaction. Front. Chem. 2018, 6, 376. [Google Scholar] [CrossRef]
- Phukan, M.; Kalita, M.K.; Borah, R. A new protocol for Biginelli (or like) reaction under solvent-free grinding method using Fe (NO3)3.9H2O as catalyst. Green Chem. Lett. Rev. 2010, 3, 329–334. [Google Scholar] [CrossRef]
- Rodríguez-Domínguez, J.C.; Bernardi, D.; Kirsch, G. ZrCl4 or ZrOCl2 under neat conditions: Optimized green alternatives for the Biginelli reaction. Tetrahedron Lett. 2007, 48, 5777–5780. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Homogeneous and heterogeneous catalysts for multicomponent reactions. RSC Adv. 2012, 2, 16–58. [Google Scholar] [CrossRef] [Green Version]
- Patel, H.A.; Sawant, A.M.; Rao, V.J.; Patel, A.L.; Bedekar, A.V. Polyaniline Supported FeCl3: An Effective Heterogeneous Catalyst for Biginelli Reaction. Catal. Lett. 2017, 147, 2306–2312. [Google Scholar] [CrossRef]
- Phukan, A.; Borah, S.J.; Bordoloi, P.; Sharma, K.; Borah, B.J.; Sarmah, P.P.; Dutta, D.K. An efficient and robust heterogeneous mesoporous montmorillonite clay catalyst for the Biginelli type reactions. Adv. Powder Technol. 2017, 28, 1585–1592. [Google Scholar] [CrossRef]
- Khiar, C.; Tassadit, M.; Bennini, L.; Halouane, M.; Benito, G.M.J.; Menad, S.; Tezkratt, S.; Rabia, C. Cobalt supported on alumina as green catalyst for Biginelli reaction in mild conditions: Effect of catalyst preparation method. Green Process. Synth. 2017, 6, 533–541. [Google Scholar] [CrossRef]
- Kheffache, O.; Lopez-Olmos, C.; Rodriguez-Ramos, I.; Cherifi, O. Clean 3,4-Dihydropyrimidones Synthesis via Biginelli Reaction over Supported Molybdenum: Structural and Textural Characteristic of αMoO3. Bull. Chem. React. Eng. Catal. 2020, 15, 698–713. [Google Scholar] [CrossRef]
- Clark, J.H.; Macquarrie, D.J.; Sherwood, J. The Combined Role of Catalysis and Solvent Effects on the Biginelli Reaction: Improving Efficiency and Sustainability. Chem. A Eur. J. 2013, 19, 5174–5182. [Google Scholar] [CrossRef] [PubMed]
- Bosica, G.; Demanuele, K.; Padrón, J.M.; Puerta, A. One-pot multicomponent green Hantzsch synthesis of 1,2-dihydropyridine derivatives with antiproliferative activity. Beilstein J. Org. Chem. 2020, 16, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S. Heterogeneous Catalysts for the One-Pot Synthesis of Chemicals and Fine Chemicals. Chem. Rev. 2011, 111, 1072–1133. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Bosica, G.; Abdilla, R. A regioselective one-pot aza-Friedel-Crafts reaction for primary, secondary and tertiary anilines using a heterogeneous catalyst. Green Chem. 2017, 19, 5683–5690. [Google Scholar] [CrossRef]
- Girard, C.; Onen, B.E.; Aufort, M.; Beauvière, S.; Samson, E.; Herscovici, J. Reusable Polymer-Supported Catalyst for the [3+2] Huisgen Cycloaddition in Automation Protocols. Org. Let. 2006, 8, 1689–1692. [Google Scholar] [CrossRef]
- Laszlo, P.; Mathy, A. Catalysis of Friedel-Crafts Alkylation by a Montmorillonite Doped with Transition-Metal Cations. Helv. Chim. Acta 1987, 70, 577–586. [Google Scholar] [CrossRef]
- Agalave, S.G.; Pharande, S.G.; Gade, S.M.; Pore, V.S. Alumina-Supported Copper Iodide: An Efficient and Recyclable Catalyst for Microwave-Assisted Synthesis of 1,4-Disubstituted 1,2,3-Triazoles via Three-Component Reaction in Water. Asian J. Org. Chem. 2015, 4, 943–951. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, Y.; Gao, X.; Mo, T.; Zhu, Y.; Li, Y. Production of bioadditives from glycerol esterification over zirconia supported heteropolyacids. Bioresour. Technol. 2013, 130, 45–51. [Google Scholar] [CrossRef]
- Engin, A.; Haluk, H.; Gurkan, K. Production of lactic acid esters catalyzed by heteropoly acid supported over ion-exchange resins. Green Chem. 2003, 5, 460–466. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Mekki, S.; Krabia, L.; Saleh, M.S.M.; Saidi-Besbes, S. Synthesis of 3,4-Dihydropyrimidin-2(1H)-one Derivatives Using Activated Montmorillonite as Catalyst. Lett. Org. Chem. 2019, 16, 59–65. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Ranjbar, Y.; Masuodi, A.; Rezaee, P.; Tajbakhsh, M.; Fallah, Z. Ultrasonic-Assisted Surface- Modification of Nanosilica Chloride and its Use for Synthesis of 3,4- Dihydropyrimidinones. Lett. Org. Chem. 2014, 11, 361–367. [Google Scholar] [CrossRef]
- Ali, F.; Khan, K.M.; Salar, U.; Iqbal, S.; Taha, M.; Ismail, N.H.; Perveen, S.; Wadood, A.; Ghufran, M.; Ali, B. Dihydropyrimidones: As novel class of β-glucuronidase inhibitors. Bioorganic Med. Chem. 2016, 24, 3624–3635. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.B.D.; Acharya, B.N.; Verma, S.K.; Kaushik, M.P. N,N′-Dichlorobis(2,4,6-trichlorophenyl)urea (CC-2) as a new reagent for the synthesis of pyrimidone and pyrimidine derivatives via Biginelli reaction. Tetrahedron Lett. 2011, 52, 809–812. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Lashkari, M. Three-component reaction of β-ketoesters, aromatic aldehydes and urea/thiourea promoted by caffeine, a green and natural, biodegradable catalyst for eco-safe Biginelli synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones derivatives under solvent-free conditions. J. Serb. Chem. Soc. 2018, 83, 673–684. [Google Scholar]
- Bigdeli, M.A.; Gholami, G.; Sheikhhosseini, E. P-Dodecylbenzenesulfonic acid (DBSA), a Brønsted acid-surfactant catalyst for Biginelli reaction in water and under solvent free conditions. Chin. Chem. Lett. 2011, 22, 903–906. [Google Scholar] [CrossRef]
- Singh, K.; Singh, S.; Mahajan, A. Metalation of Biginelli Compounds. A General Unprecedented Route to C-6 Functionalized 4-Aryl-3,4-dihydropyrimidinones. J. Org. Chem. 2005, 70, 6114–6117. [Google Scholar] [CrossRef] [PubMed]
- Safari, J.; Gandomi-Ravandi, S. Titanium dioxide supported on MWCNTs as an eco-friendly catalyst in the synthesis of 3,4-dihydropyrimidin-2(1H)-ones accelerated under microwave irradiation. New J. Chem. 2014, 38, 3514–3521. [Google Scholar] [CrossRef]
- Valeru, A.; Luo, Z.; Khan, I.; Liu, B.; Sngepu, B.; Godumagadda, N.R.; Xu, Y.; Xie, J. Multicomponent synthesis and anticancer activity studies of novel 6-(Trifluoromethyl)-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylate derivatives. Synth. Commun. 2018, 48, 2226–2231. [Google Scholar] [CrossRef]
- Cepanec, I.; Litvić, M.; Bartolinčić, A.; Lovrić, M. Ferric chloride/tetraethyl orthosilicate as an efficient system for synthesis of dihydropyrimidinones by Biginelli reaction. Tetrahedron 2005, 61, 4275–4280. [Google Scholar] [CrossRef]
- do Nascimento, L.G.; Dias, I.M.; de Souza, G.B.M.; Dancini-Pontes, I.; Fernandes, N.R.C.; de Souza, P.S.; de Oliveira, G.R.; Alonso, C.G. Niobium Oxides as Heterogeneous Catalysts for Biginelli Multicomponent Reaction. J. Org. Chem. 2020, 85, 11170–11180. [Google Scholar] [CrossRef]
- Murata, H.; Ishitani, H.; Iwamoto, M. Synthesis of Biginelli dihydropyrimidinone derivatives with various substituents on aluminium-planted mesoporous silica catalyst. Org. Biomol. Chem. 2010, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Gomes, R.; Mondal, R.; Dey, S.K.; Dasgupta, P.; Poddar, A.; Reddy, V.R.; Bhaumik, A.; Kumar, S. Stable room temperature magnetic ordering and excellent catalytic activity of mechanically activated high surface area nanosized Ni0.45Zn0.55Fe2O4. RSC Adv. 2015, 5, 78508–78518. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, S.; Peng, M.; Wang, C. Isolation of polyphenol compounds from olive waste and inhibition of their derivatives for α-glucosidase and α-amylase. Nat. Prod. Res. 2020, 34, 2398–2402. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.; Campos, G.; Santos, A.O.; Falcão, A.; Silvestre, S.; Alves, G. Potential antitumoral 3,4-dihydropyrimidin-2(1H)-ones: Synthesis, in vitro biological evaluation and QSAR studies. RSC Adv. 2016, 6, 84943–84958. [Google Scholar] [CrossRef]


| Entry a | Catalyst | Yield (%) b | Reaction Time (hours) |
|---|---|---|---|
| 1 | Montmorillonite K30 | 62 | 14 |
| 2 | CuI–Amberlyst 21 c (1.59 mmol/g) | No yield | 20 |
| 3 | Fe(II) Montmorillonite K10 d | 16 | 20 |
| 4 | Zn(II) Montmorillonite K10 d | 26 | 20 |
| 5 | CuI–Alumina e (1.39 mmol/g) | 60 | 20 |
| 6 | 40% PW/Al2O3 f | 30 | 11 |
| 7 | 40% PW/SiO2 f | 68 | 20 |
| 8 | 40% WSi/Al2O3 f | 66 | 20 |
| 9 | 30%WSi/A15 g | 70 | 20 |
| Entry | % w/w WSi on A15 | Reactant Ratio a | Mass of Catalyst (g/mmol of Benzaldehyde) [WSi Molar Percentage] (mmol %) | Yield (%) b [Time (hrs)] |
|---|---|---|---|---|
| 1 c 2 c 3 c 4 d 5 e 6 e 7 e 8 e 9 e | 30% | 1:1:1.2 1:1:1.2 | 0.05 [0.52] 0.035 [0.36] | 58 [4.5] 45 [4] |
| 1:1:1 | 0.05 [0.52] | 60 [4.5] | ||
| 1:1:1.2 1:1:1.2 1:1:1.2 1:1:1.2 1:1:1 1:1:1.2 | 0.05 [0.52] 0.05 [0.52] 0.05 [0.52] 0.035 [0.36] 0.05 [0.52] 0.05 [0.52] | 52 [4.5] 76 [4.5] 69 [1.5] 61 [1.5] 62 [1.5] 79 [20] | ||
| 10 e 11 e | 38% | 1:1:1.2 | 0.068 [0.9] | 75 [4.5] |
| 1:1:1.2 | 0.095 [1.25] | 78 [3.5] | ||
| 12 e 13 e 14 e | 40% | 1:1:1.2 1:1:1.2 | 0.05 [0.69] 0.05 [0.69] | 82 [4.5] 65 [2.5] |
| 1:1:1.2 | 0.05 [0.69] | 66 [3.5] | ||
| 15 f | Tungstosilicic acid hydrate | 1:1:1 1:1:1 | 0.012 [0.4] 0.012 [0.4] 0.0012 [0.04] 0.010 [0.35] 0.012 [0.4] | 47 [6] 64 [20] 13 [6] 36 [6] 78 [12] |
| 16 g | - | 1:1:1.2 | - - | 0 [24] |
| 17 h | A15 | 1:1:1.2 1:1:1 | 0.05 - | 54 [6] 63 [16] |
 | |||||
| Product a | R1 | R2 | R3 | Yield (%)b | Reaction Time (h) |
| 4a |  1a | CH3 | CH3 | 82 | 5 |
| 2a | |||||
| 4b |  1a | CH3CH2 | CH3 | 88 c | 4.5 |
| 2b | |||||
| 4c |  1b | CH3 | CH3 | 72 | 6 |
| 2a | |||||
| 4d |  1c | CH3 | CH3 | 98 | 4 |
| 2a | |||||
| 4e |  1d | CH3 | CH3 | 92 | 22 |
| 2a | |||||
| 4f |  1e | CH3 | CH3 | 50 | 5 |
| 2a | |||||
| 4g |  1a | C6H5CH2 | CH3 | 77 | 6 |
| 2c | |||||
| 4h |  1c | C6H5CH2 | CH3 | 65 | 6 |
| 2c | |||||
| 4i |  1f | CH3 | CH3 | 38 | 5 |
| 2a | |||||
| 4j |  1a | CH3CH2 | CF3 | 37 | 11 |
| 2d | |||||
| 4k |  1a | (CH3)2CHCH2 | CH3 | 41 | 8 |
| 2e | |||||
| 4l |  1g | CH3 | CH3 | 66 | 6 |
| 2a | |||||
| 4m |  1g | CH3CH2 | CH3 | 79 | 7 |
| 2b | |||||
| 4n |  1h | CH3 | CH3 | 65 c | 6 |
| 2a | |||||
| 4o |  1i | CH3CH2 | CH3 | 86 c | 23 |
| 2b | |||||
| 4p |  1j | CH3 | CH3 | 82 | 8 |
| 2a | |||||
| 4q |  1k | CH3 | CH3 | 75 | 4.5 |
| 2a | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosica, G.; Cachia, F.; De Nittis, R.; Mariotti, N. Efficient One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones via a Three-Component Biginelli Reaction. Molecules 2021, 26, 3753. https://doi.org/10.3390/molecules26123753
Bosica G, Cachia F, De Nittis R, Mariotti N. Efficient One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones via a Three-Component Biginelli Reaction. Molecules. 2021; 26(12):3753. https://doi.org/10.3390/molecules26123753
Chicago/Turabian StyleBosica, Giovanna, Fiona Cachia, Riccardo De Nittis, and Nicole Mariotti. 2021. "Efficient One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones via a Three-Component Biginelli Reaction" Molecules 26, no. 12: 3753. https://doi.org/10.3390/molecules26123753
APA StyleBosica, G., Cachia, F., De Nittis, R., & Mariotti, N. (2021). Efficient One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones via a Three-Component Biginelli Reaction. Molecules, 26(12), 3753. https://doi.org/10.3390/molecules26123753







