Mechanochemical Synthesis and Nitrogenation of the Nd1.1Fe10CoTi Alloy for Permanent Magnet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Powder Characterization
3. Result and Discussion
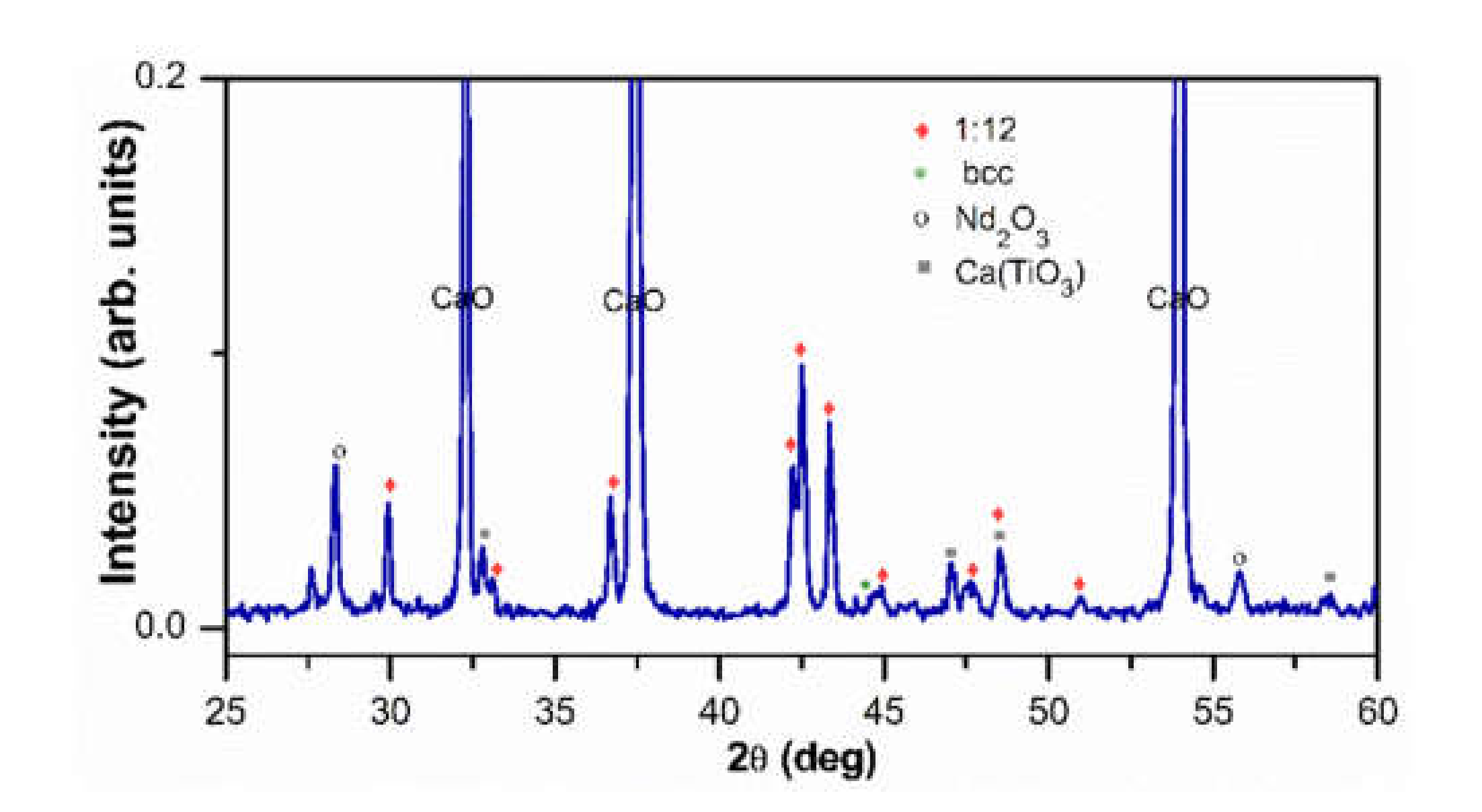
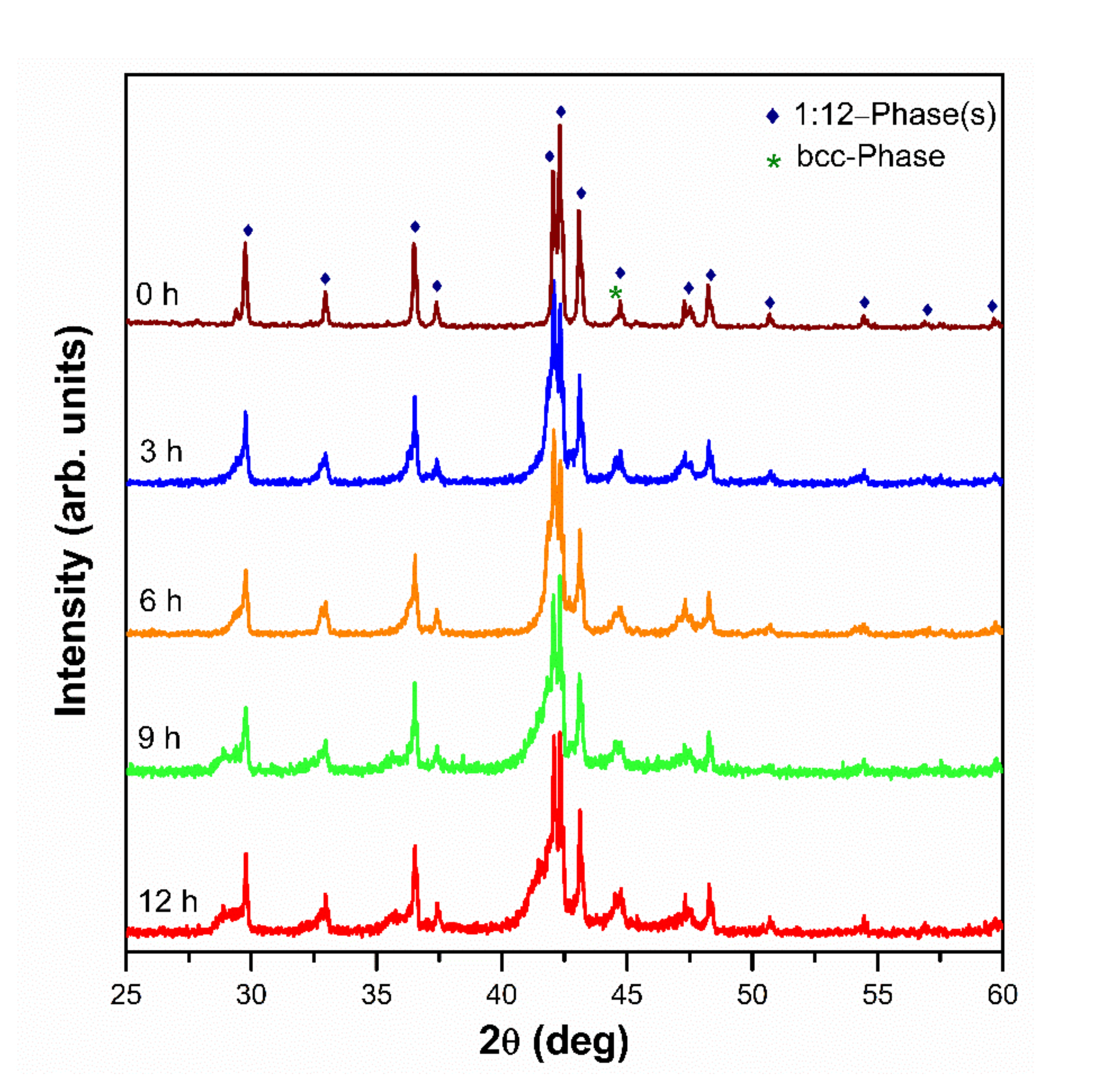
3.1. Structural and Magnetic Characterization before Washing
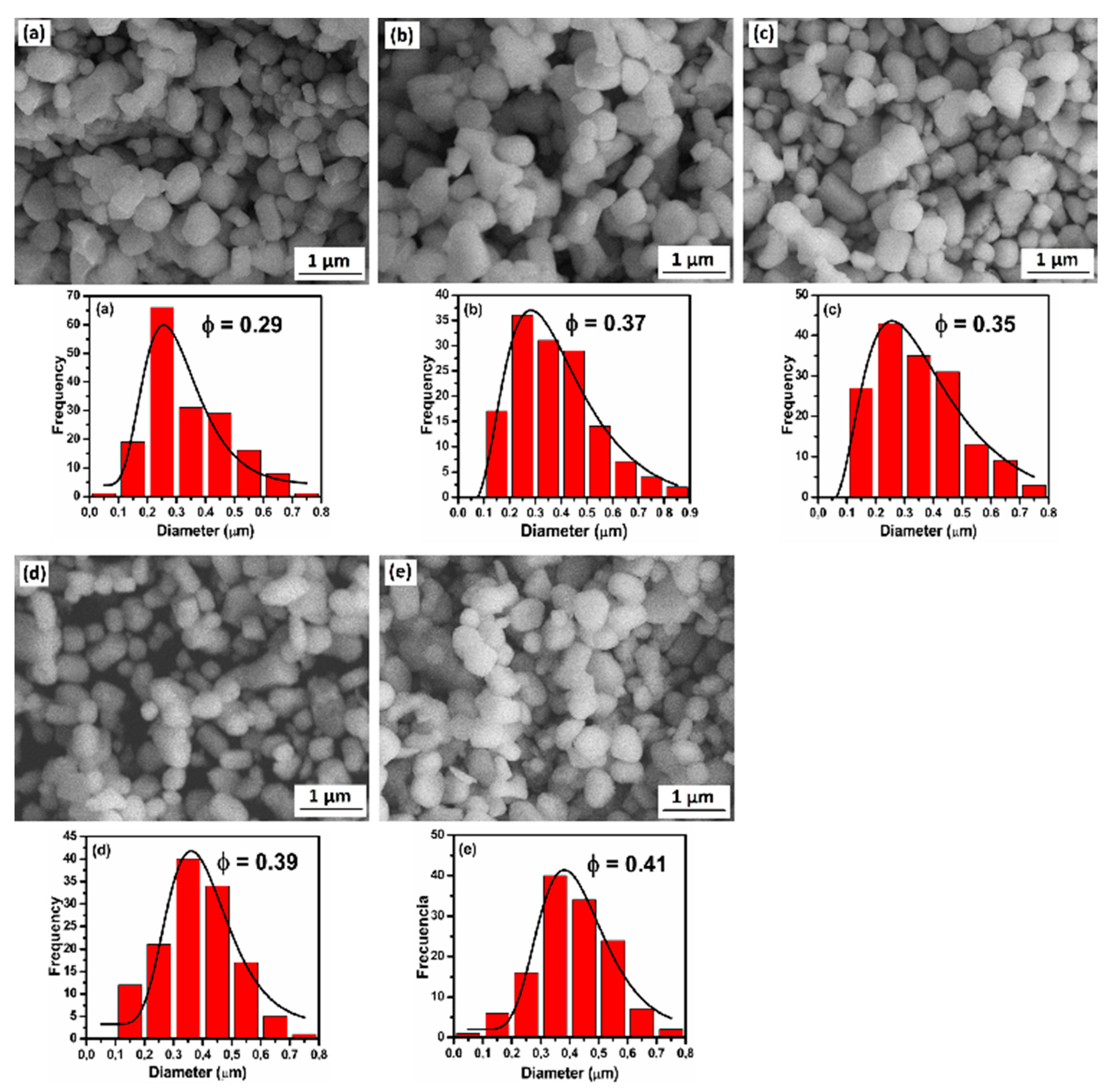
3.2. SEM Characterization
3.3. XRD after Washing
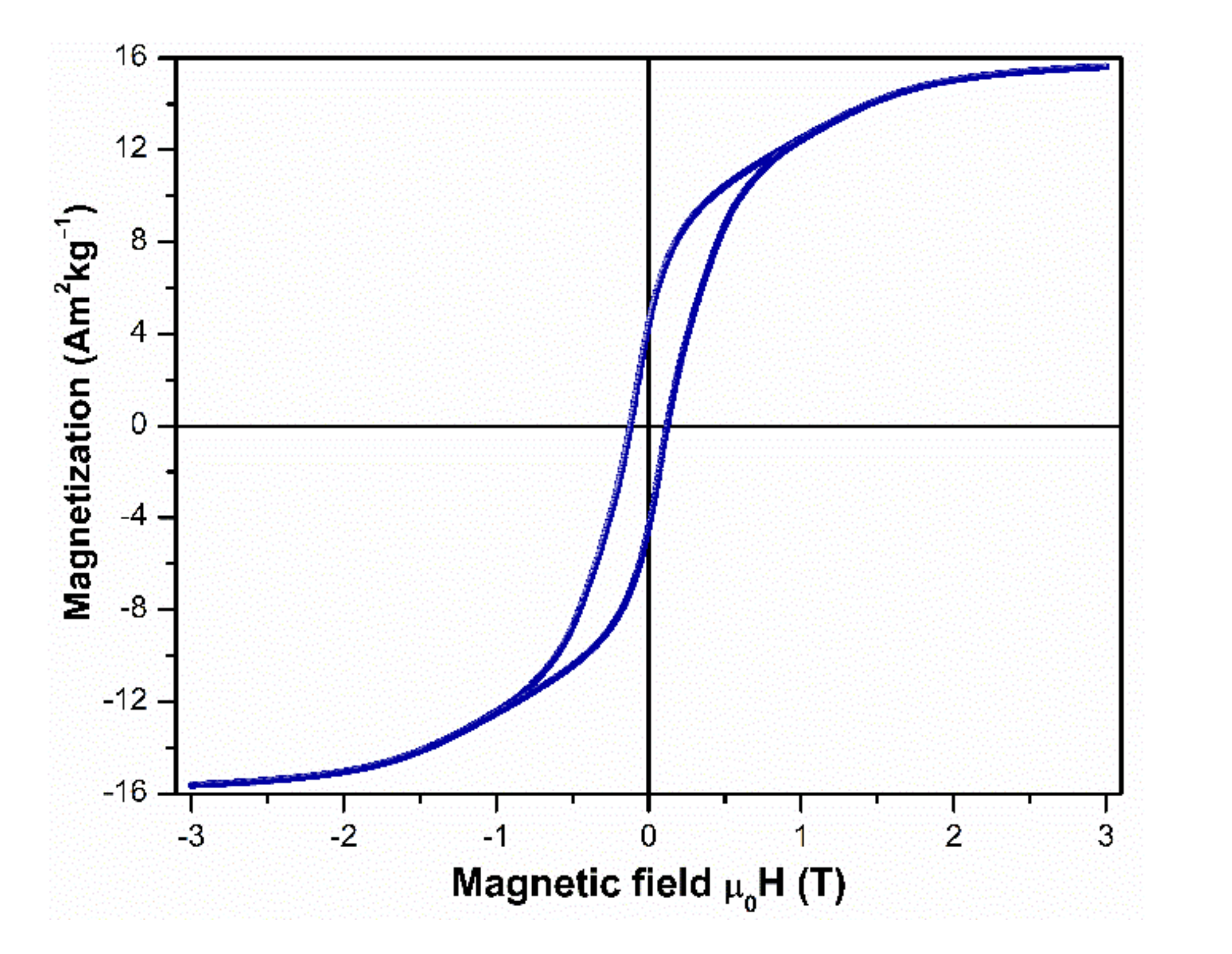
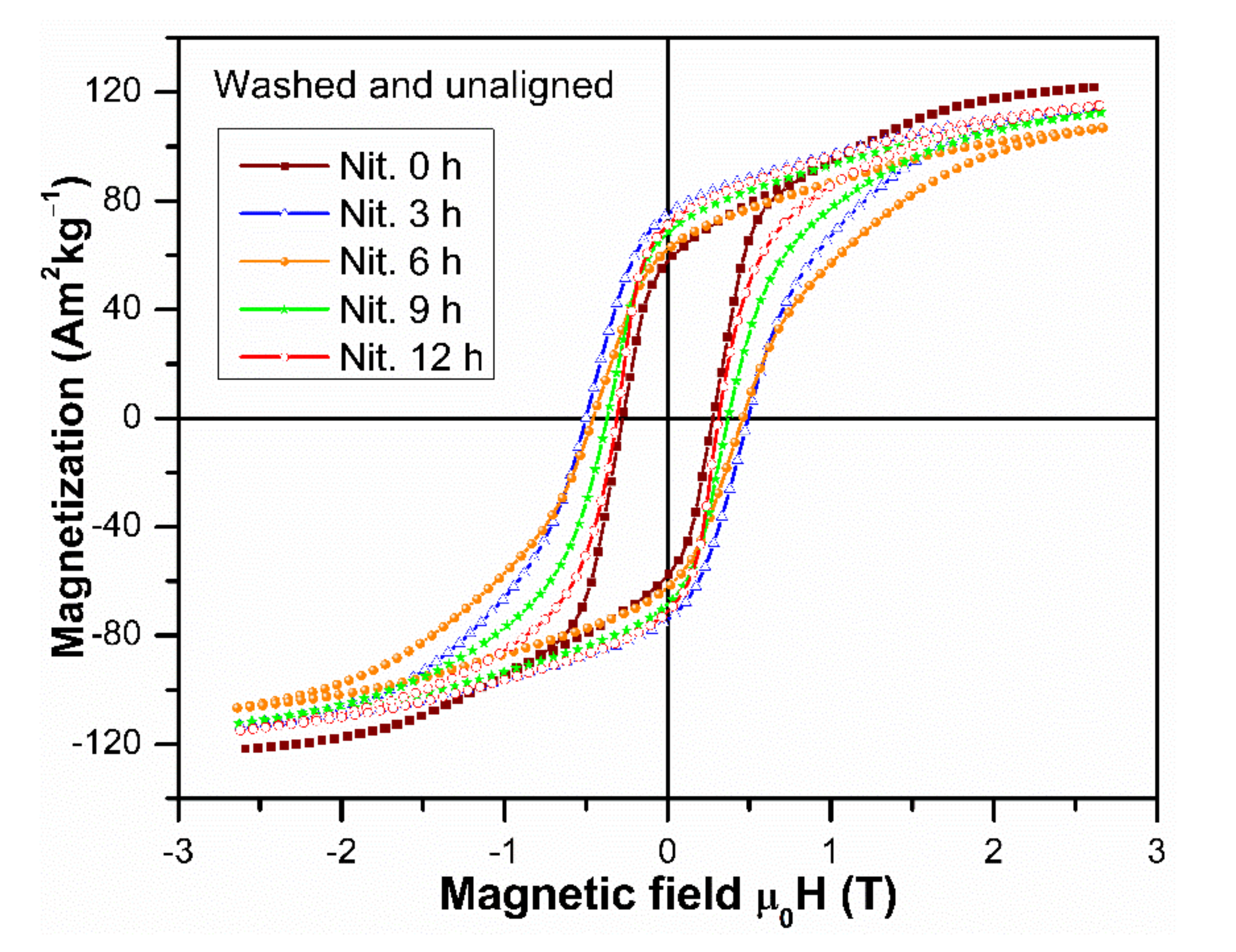
3.4. Magnetic Characterization of Aligned and Non-Aligned Powders
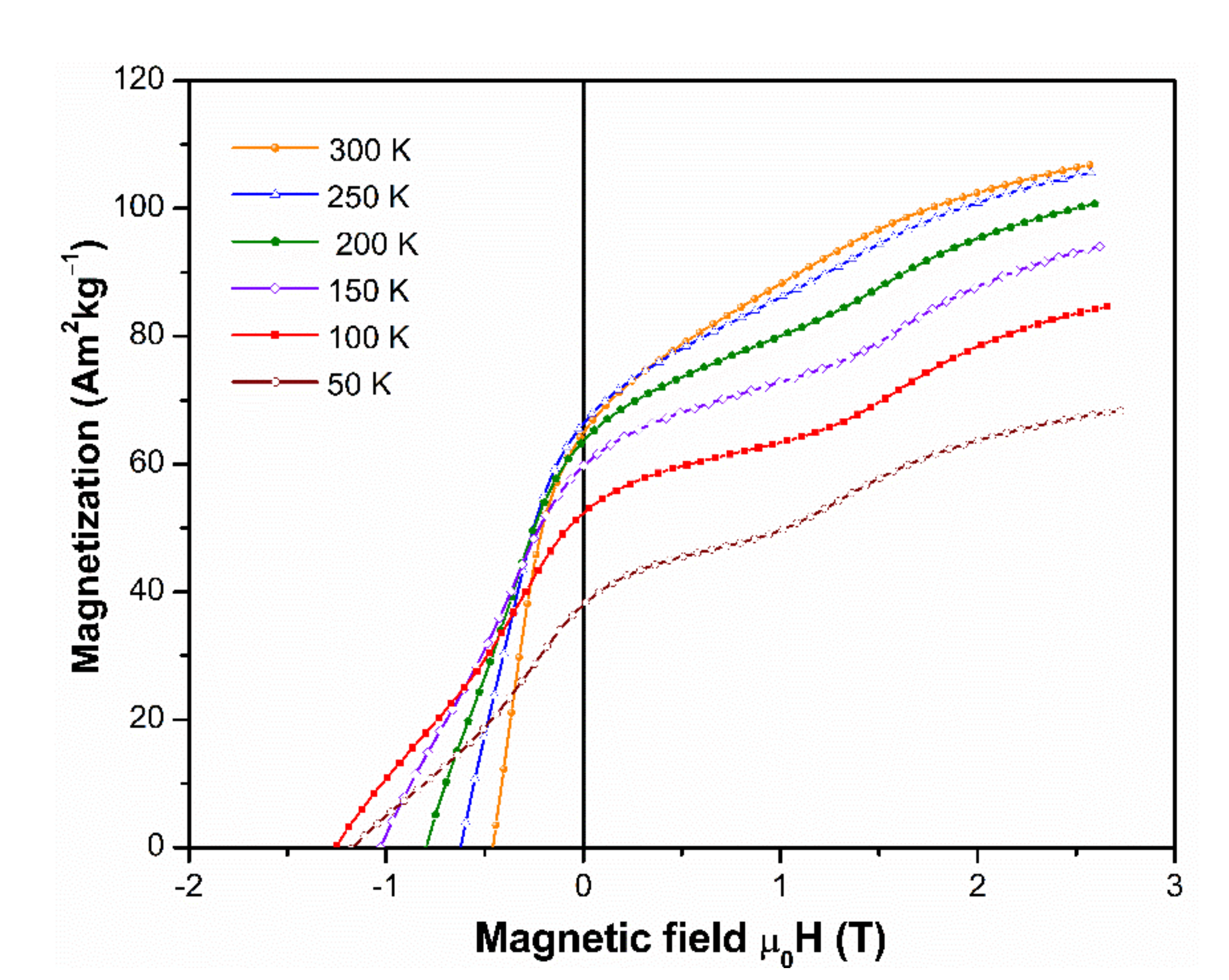
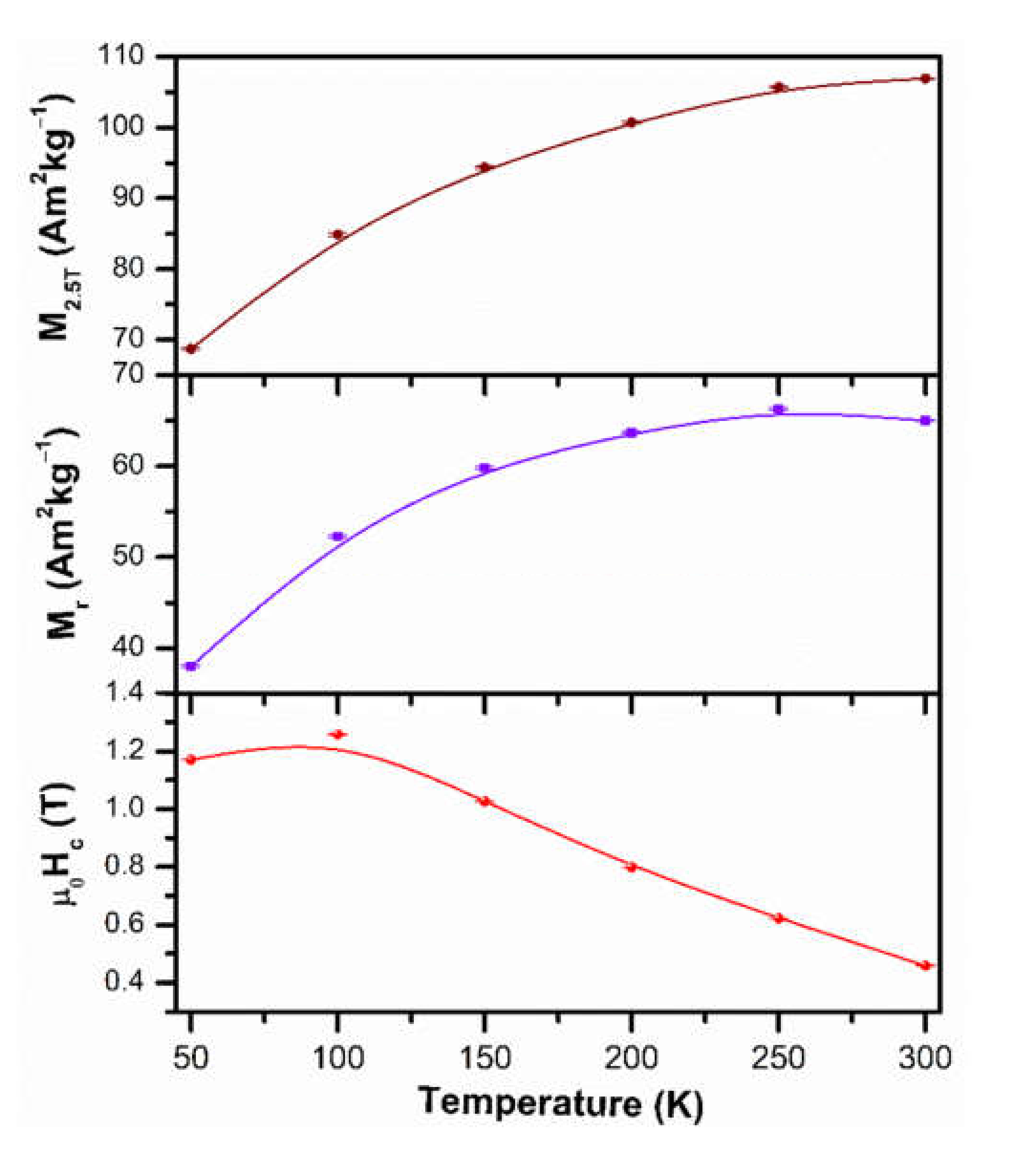
3.5. Magnetic Characterization at Low Temperatures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madugundo, R.; Rama Rao, N.V.; Schönhöbel, A.M.; Salazar, D.; El-Gendy, A.A. Recent Developments in Nanostructured Permanent Magnet Materials and Their Processing Methods. In Magnetic Nanostructured Materials: From Lab to Fab; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Tukker, A. Rare earth elements supply restrictions: Market failures, not scarcity, hamper their current use in high-tech applications. Environ. Sci. Technol. 2014, 48, 9973–9974. [Google Scholar] [CrossRef] [PubMed]
- Schönhöbel, A.M.; Madugundo, R.; Vekilova, O.Y.; Eriksson, O.; Herper, H.C.; Barandiarán, J.M.; Hadjipanayis, J.C. Intrinsic magnetic properties of SmFe12-xVx alloys with reduced V-concentration. J. Alloys Compd. 2019, 786, 969–974. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.P.; Li, H.S.; Gavigan, J.P.; Coey, J.M.D. Intrinsic magnetic properties of the iron-rich ThMn12-structure alloys R(Fe11Ti); R=Y, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm and Lu. J. Phys. Condens. Matter 1989, 1, 755–770. [Google Scholar] [CrossRef]
- Schultz, L.; Schnitzke, K.; Wecker, J. High coercivity in mechanically alloyed Sm-Fe-V magnets with a ThMn12 crystal structure. Appl. Phys. Lett. 1990, 56, 1376–1378. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y. Magnetic properties and interstitial atom effects in the R(Fe,M)12 compounds. In Handbook of Advanced Magnetic Materials; Springer: Boston, MA, USA, 2006; pp. 1414–1451. [Google Scholar]
- Yang, J.; Oleinek, P.; Müller, K.H. Hard magnetic properties of melt-spun R(Fe,M)12 nitrides (R= Nd or Pr; M= Mo or V). J. Appl. Phys. 2000, 88, 988–992. [Google Scholar] [CrossRef]
- Zhang, X.D.; Cheng, B.P.; Yang, Y.C. High coercivity in mechanically milled ThMn12-type Nd–Fe–Mo nitrides. Appl. Phys. Lett. 2000, 77, 4022–4024. [Google Scholar] [CrossRef]
- Liu, S.; Han, J.; Du, H.; Wang, C.; Yang, Y.; Yang, J.; Chang, H. High energy product in mechanically alloyed ThMn12-type compound with exchange coupling effect. J. Magn. Magn. Mater. 2007, 312, 449–452. [Google Scholar] [CrossRef]
- Lin, Z.; Han, J.; Liu, S.; Xing, M.; Yang, Y.; Yang, J.; Wang, C.; Dua, H.; Yang, Y. Phase composition, microstructures and magnetic properties of melt-spun Nd1.2Fe10.5Mo1.5 ribbons and their nitrides. J. Magn. Magn. Mater. 2012, 324, 196–199. [Google Scholar] [CrossRef]
- Tang, S.L.; Wu, C.H.; Jin, X.M.; Wang, B.W.; Li, G.S.; Ding, B.Z.; Chuang, Y.C. Phase formation and magnetic properties of annealed mechanical alloying Nd-Fe-V-Ti alloys and their nitrides; J. Alloys Compd. 1998, 264, 240–243. [Google Scholar] [CrossRef]
- Yang, J.; Mao, W.; Yang, Y.; Ge, S.; Zhao, Z.; Li, F. Nitrogenation of the magnetic compound R(Fe,M)12. J. Appl. Phys. 1998, 83, 1983–1987. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, X.; Ji, C.; Chang, H.; Cheng, B.; Yang, Y.; Du, H.; Xue, Y.; Zhang, B.; Wang, L.; et al. Structural and magnetic properties of PrFe12-xVx and their nitrides. Acta Mater 2001, 49, 721–728. [Google Scholar] [CrossRef]
- Han, J.; Liu, S.; Xing, M.; Lin, Z.; Kong, X.; Yang, J.; Wang, C.; Du, H.; Yang, Y. Preparation of anisotropic Nd(Fe,Mo)12N1.0 magnetic materials by strip casting technique and direct nitrogenation for the strips. J. Appl. Phys. 2011, 109, 07A738. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Han, J.; Wang, C.; Liu, S.; Du, H. Research and development of interstitial compounds. IEEE Trans. Magn. 2015, 51, 2103806. [Google Scholar] [CrossRef]
- Fu, J.B.; Yu, X.; Qi, Z.Q.; Yang, W.Y.; Liu, S.Q.; Wang, C.S.; Du, H.L.; Han, J.Z.; Yang, Y.C.; Yang, J.B. Magnetic properties of Nd(Fe1-xCox)10.5M1.5 (M = Mo and V) and their nitrides. AIP Adv. 2017, 7, 056202. [Google Scholar] [CrossRef] [Green Version]
- Akayama, M.; Fujii, H.; Yamamoto, K.; Tatami, K. Physical properties of nitrogenated RFe11Ti intermetallic compounds (R = Ce, Pr and Nd) with ThMn12-type structure. J. Magn. Magn. Mater. 1994, 130, 99–107. [Google Scholar] [CrossRef]
- Gabay, A.M.; Hadjipanayis, G.C. Mechanochemical synthesis of magnetically hard anisotropic RFe10Si2 powders with R representing combination of Sm, Ce and Zr. J. Magn. Magn. Mater. 2017, 422, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Gabay, A.M.; Martín-Cid, A.; Barandiaran, J.M.; Salazar, D.; Hadjipanayis, G.C. Low-cost Ce1-xSmx(Fe,Co,Ti)12 alloys for permanent magnets. AIP Adv. 2016, 6, 056015. [Google Scholar] [CrossRef] [Green Version]
- Dirba, I.; Li, J.; Sepehri-Amin, H.; Ohkubo, T.; Schrefl, T.; Hono, K. Anisotropic, Single-crystalline SmFe12-based microparticles with high roundness fabricated by jet-milling. J. Alloys Compd. 2019, 804, 155–162. [Google Scholar] [CrossRef]
- Su, M.Z.; Liu, S.F.; Qian, X.L.; Lin, J.H. An alternative approach to the finely crystalline powder of rare earth-transition metal alloys: J. Alloys Compd. 1997, 249, 229–233. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Aubert, A.; Madugundo, R.; Schönhöbel, A.M.; Salazar, D.; Garitaonandia, J.S.; Barandiaran, J.M.; Hadjipanayis, G. Structural and magnetic properties of Nd-Fe-Mo-(N) melt-spun ribbons with ThMn12 structure. Acta Mater. 2020, 195, 519–526. [Google Scholar] [CrossRef]
- Kubo, T.; Ohtani, T.; Kojima, S.; Kato, N.; Kojima, K.; Sakamoto, Y.; Tsukahara, M. Machinable Anisotropic Permanent Magnets of Mn-Al-C Alloys. U.S. Patent 3, 976, 519, 24 August 1976. [Google Scholar]
- Yang, J.; Yang, W.; Shao, Z.; Liang, D.; Zhao, H.; Xia, Y.; Yang, Y. Mn-based permanent magnets. Chin. Phys. B. 2018, 27, 117503. [Google Scholar] [CrossRef]
- Boltich, E.B.; Ma, B.M.; Zhang, L.Y.; Pourarian, F.; Malik, S.K.; Sankar, S.G.; Wallace, W.E. Spin reorientations in RTiFe11 systems (R = Tb, Dy and Ho). J. Magn. Magn. Mater. 1989, 78, 364–370. [Google Scholar] [CrossRef]
- Piquer, C.; Grandjean, F. A magnetic and Mössbauer spectral study of the spin reorientation in NdFe11Ti and NdFe11TiH. J. Appl. Phys. 2004, 95, 6308–6316. [Google Scholar] [CrossRef]
- Guslienko, K.Y.; Kou, X.C.; Grössinger, R. Magnetic anisotropy and spin-reorientation transitions in RFe11Ti (R= Nd, Tb, Dy, Er) rare-earth intermetallics, J. Magn. Magn. Mater. 1995, 150, 383–392. [Google Scholar] [CrossRef]
- Luong, N.H.; Thuy, N.P.; Tal, L.T.; Hoang, N.V. Effect of Y substitution of the spin reorientation in NdFe11Ti compound. Phys. Status Solidi 1990, 121, 607–610. [Google Scholar] [CrossRef]
- Pateti, L.; Paoluzi, A.; Albertini, F. Magnetic anisotropy and magnetization processes in 3:29 and 1:12 Nd(FeTi)-based compounds. J. Appl. Phys. 1994, 76, 7473–7477. [Google Scholar] [CrossRef]
- Kolchugina, N.B.; Zheleznyi, M.V.; Savchenko, A.G.; Menushenkov, V.P.; Burkhanov, G.S.; Koshkid’ko, Y.S.; Ćwik, J.; Dormidontov, N.A.; Skotnicova, K.; Kursa, M.; et al. Simulating the hysteretic characteristics of hard magnetc materials based on Nd2Fe14B and Ce2Fe14B intermetallics. Crystals 2020, 10, 518. [Google Scholar] [CrossRef]

| Nitrogenation Time (h) | Phase | a (Å) | c (Å) | V (Å3) | Fraction (vol. %) | ρ (g/cm3) |
|---|---|---|---|---|---|---|
| 0 | 1:12 | 8.589 | 4.808 | 354.7 | 95.7 | 7.579 |
| bcc | 2.870 | 4.3 | 7.848 | |||
| 3 | 1:12 (a) | 8.585 | 4.804 | 354.1 | 43.7 | 7.593 |
| 1:12(b) | 8.631 | 4.843 | 360.8 | 17.7 | 7.452 | |
| 1:12(c) | 8.642 | 4.953 | 369.9 | 34.2 | 7.268 | |
| bcc | 2.870 | 4.4 | 7.843 | |||
| 6 | 1:12(a) | 8.583 | 4.805 | 354.0 | 42.3 | 7.595 |
| 1:12(b) | 8.616 | 4.849 | 360.0 | 22.4 | 7.469 | |
| 1:12(c) | 8.638 | 4.971 | 370.9 | 29.5 | 7.249 | |
| bcc | 2.871 | 5.8 | 7.840 | |||
| 9 | 1:12(a) | 8.585 | 4.804 | 354.1 | 34.3 | 7.593 |
| 1:12(b) | 8.631 | 4.843 | 360.8 | 10.0 | 7.452 | |
| 1:12(c) | 8.700 | 4.977 | 376.7 | 48.9 | 7.136 | |
| bcc | 2.873 | 6.8 | 7.820 | |||
| 12 | 1:12(a) | 8.584 | 4.802 | 353.8 | 36.5 | 7.597 |
| 1:12(b) | 8.642 | 4.841 | 361.5 | 9.5 | 7.436 | |
| 1:12(c) | 8.741 | 4.950 | 378.2 | 45.5 | 7.107 | |
| bcc | 2.871 | 8.5 | 7.840 |
| Nitrogenation Time (h) | M3T (Am2kg−1) | Mr (Am2kg−1) | μ0Hc (T) | (BH)max (kJ·m−3) |
|---|---|---|---|---|
| 0 | 122.83 (121.92) | 108.21 (57.79) | 0.294 (0.276) | 81.07 (30.44) |
| 3 | 128.80 (114.17) | 116.34 (75.27) | 0.504 (0.500) | 113.74 (58.38) |
| 6 | 128.97 (106.90) | 116.14 (62.16) | 0.518 (0.457) | 111.41 (39.89) |
| 9 | 126.38 (112.45) | 113.24 (68.28) | 0.395 (0.371) | 96.40 (44.02) |
| 12 | 126.97 (115.12) | 112.87 (71.15) | 0.316 (0.312) | 87.85 (45.74) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Sánchez, H.; Hadjipanayis, G.; Pérez Alcázar, G.A.; Zamora Alfonso, L.E.; Trujillo Hernández, J.S. Mechanochemical Synthesis and Nitrogenation of the Nd1.1Fe10CoTi Alloy for Permanent Magnet. Molecules 2021, 26, 3854. https://doi.org/10.3390/molecules26133854
Martínez Sánchez H, Hadjipanayis G, Pérez Alcázar GA, Zamora Alfonso LE, Trujillo Hernández JS. Mechanochemical Synthesis and Nitrogenation of the Nd1.1Fe10CoTi Alloy for Permanent Magnet. Molecules. 2021; 26(13):3854. https://doi.org/10.3390/molecules26133854
Chicago/Turabian StyleMartínez Sánchez, Hugo, George Hadjipanayis, Germán Antonio Pérez Alcázar, Ligia Edith Zamora Alfonso, and Juan Sebastián Trujillo Hernández. 2021. "Mechanochemical Synthesis and Nitrogenation of the Nd1.1Fe10CoTi Alloy for Permanent Magnet" Molecules 26, no. 13: 3854. https://doi.org/10.3390/molecules26133854
APA StyleMartínez Sánchez, H., Hadjipanayis, G., Pérez Alcázar, G. A., Zamora Alfonso, L. E., & Trujillo Hernández, J. S. (2021). Mechanochemical Synthesis and Nitrogenation of the Nd1.1Fe10CoTi Alloy for Permanent Magnet. Molecules, 26(13), 3854. https://doi.org/10.3390/molecules26133854






