Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preformulation Investigation
2.2.1. Solubility Study
2.2.2. Emulsification Efficiency
2.3. Preparation of Thymoquinone Loaded Nanoemulsion through Ultrasonication
2.4. Characterization of Thymoquinone Loaded Nanoemulsion
2.4.1. Thermodynamic Stability Study
2.4.2. Analysis of Droplet Size, Polydispersity Index, and Zeta Potential
2.4.3. Determination of Viscosity
2.4.4. Analysis of Drug Content
2.5. In-Vitro Drug Release
2.6. Preparation and Characterization of TMQ Nano-Emulgel
2.7. Ex-Vivo Skin Permeability
2.8. In-Vivo Animal Study
2.8.1. Experimental Protocol
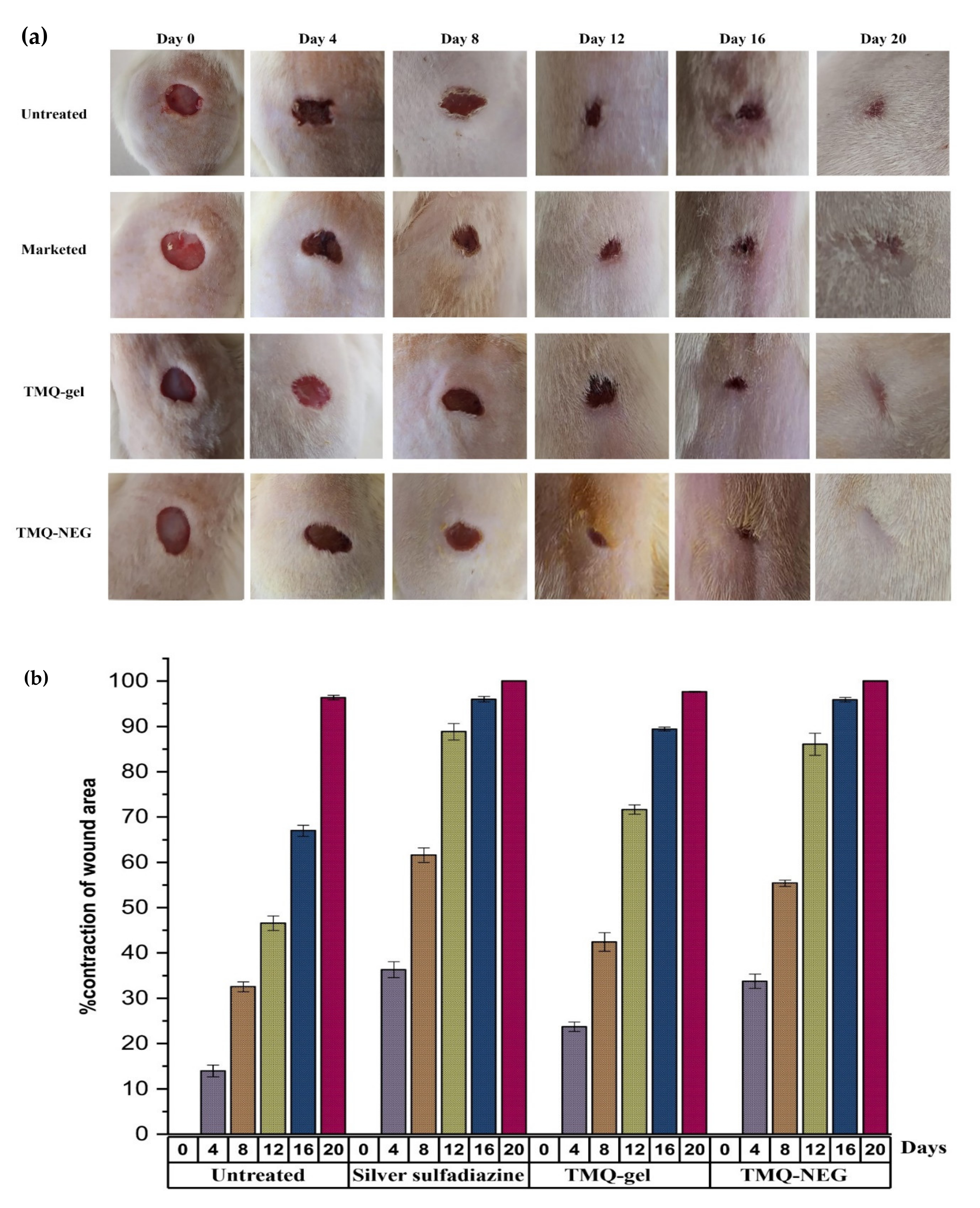
2.8.2. Assessment of Wound Healing Area
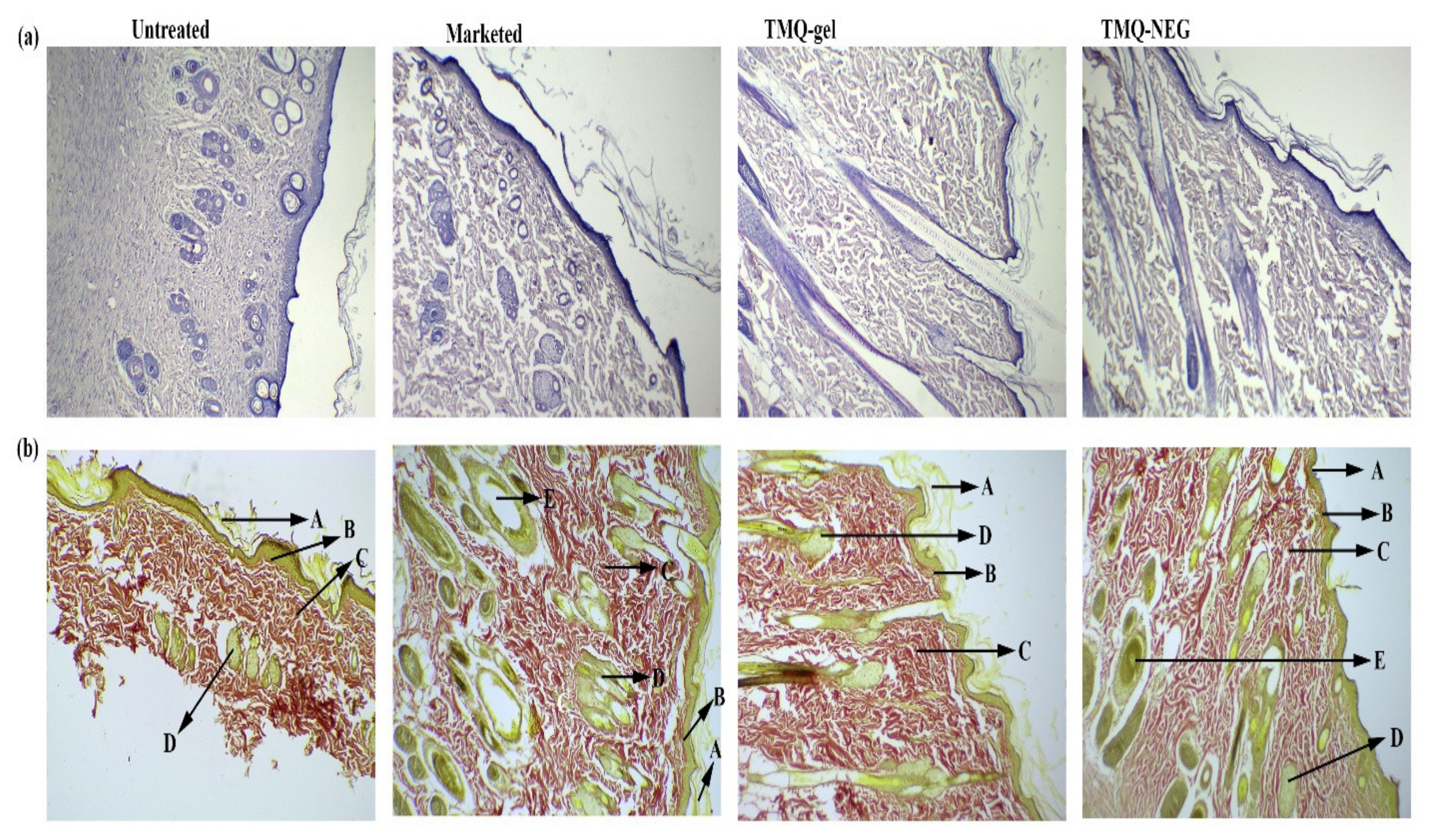
2.8.3. Histopathology
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preformulation Investigation
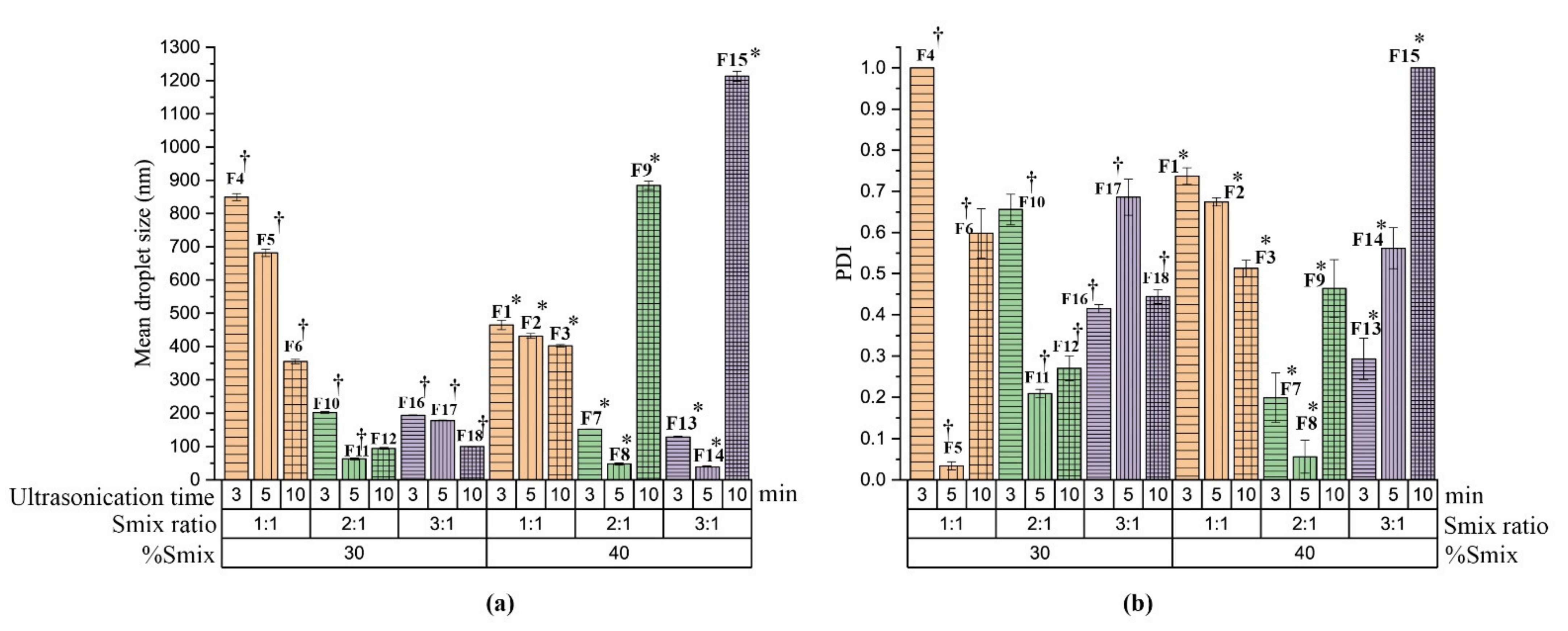
3.2. Preparation of Nanoemulsion
3.3. Characterization of Nanoemulsion
3.3.1. Thermodynamic Stability Study
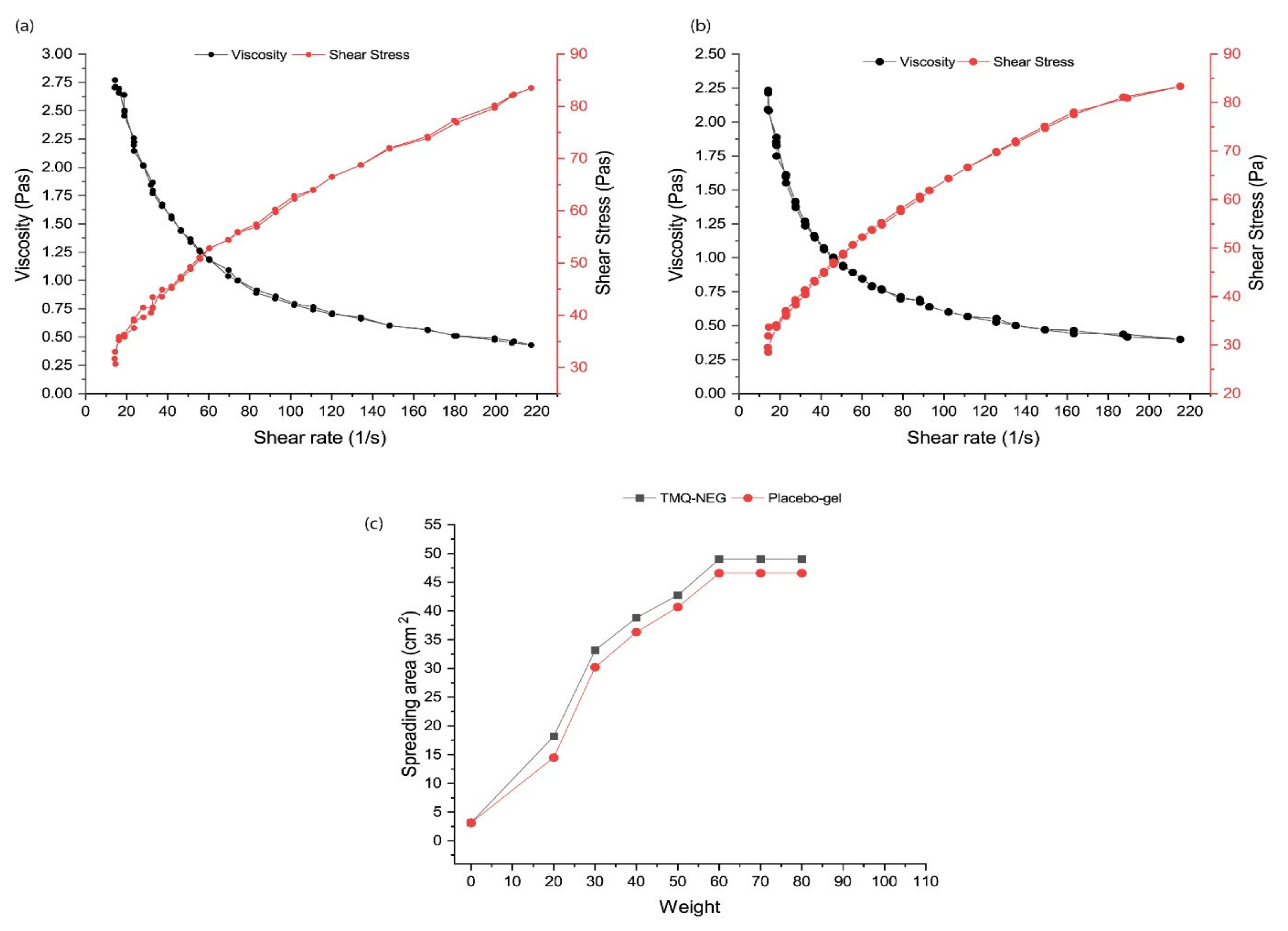
3.3.2. Viscosity
3.3.3. Analysis of Drug Content
3.4. In-Vitro Drug Release
3.5. Preparation and Characterization of Nano-Emulgel
3.6. Ex-Vivo Skin Permeability
3.7. In-Vivo Wound Healing Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, M.; Govindarajan, R.; Nath, V.; Rawat, A.K.; Mehrotra, S. Antimicrobial, wound healing and antioxidant activity of Plagiochasma appendiculatum Lehm. et Lind. J. Ethnopharmacol. 2006, 107, 67–72. [Google Scholar] [CrossRef]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic wound repair and healing in older adults: Current status and future research. J. Am. Geriatr. Soc. 2015, 63, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing--A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef] [Green Version]
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother. Res. 2019, 33, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Mouwakeh, A.; Telbisz, Á.; Spengler, G.; Mohácsi-Farkas, C.; Kiskó, G. Antibacterial and Resistance Modifying Activities of Nigella sativa Essential Oil and its Active Compounds Against Listeria monocytogenes. In Vivo 2018, 32, 737–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalili, C.; Salahshoor, M.R.; Hoseini, M.; Roshankhah, S.; Sohrabi, M.; Shabanizadeh, A. Protective Effect of Thymoquinone against Morphine Injuries to Kidneys of Mice. Iran. J. Kidney Dis. 2017, 11, 142–150. [Google Scholar] [PubMed]
- Aslam, H.; Shahzad, M.; Shabbir, A.; Irshad, S. Immunomodulatory effect of thymoquinone on atopic dermatitis. Mol. Immunol. 2018, 101, 276–283. [Google Scholar] [CrossRef]
- Noorbakhsh, M.F.; Hayati, F.; Samarghandian, S.; Shaterzadeh-Yazdi, H.; Farkhondeh, T. An Overview of Hepatoprotective Effects of Thymoquinone. Recent Pat. Food Nutr. Agric. 2018, 9, 14–22. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M.; Fu, J. Epigenetic role of thymoquinone: Impact on cellular mechanism and cancer therapeutics. Drug Discov. Today 2019, 24, 2315–2322. [Google Scholar] [CrossRef]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kim, K.H.; Kumar, S. Improvement of antihyperglycemic activity of nano-thymoquinone in rat model of type-2 diabetes. Chem. Biol. Interact. 2018, 295, 119–132. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Havakhah, S.; Boskabady, M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J. Ethnopharmacol. 2016, 190, 372–386. [Google Scholar] [CrossRef]
- Rajabian, A.; Hosseinzadeh, H. Dermatological effects of nigella sativa and its constituent, thymoquinone: A review. In Nuts and Seeds in Health and Disease Prevention; Academic Press: New York, NY, USA, 2020; pp. 329–355. [Google Scholar]
- Hosseinzadeh, H.; Parvardeh, S.; Asl, M.N.; Sadeghnia, H.R.; Ziaee, T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine 2007, 14, 621–627. [Google Scholar] [CrossRef]
- Selçuk, C.T.; Durgun, M.; Tekin, R.; Yolbas, L.; Bozkurt, M.; Akçay, C.; Alabalk, U.; Basarali, M.K. Evaluation of the effect of thymoquinone treatment on wound healing in a rat burn model. J. Burn Care Res. 2013, 34, e274–e281. [Google Scholar] [CrossRef]
- Negi, P.; Sharma, G.; Verma, C.; Garg, P.; Rathore, C.; Kulshrestha, S.; Lal, U.R.; Gupta, B.; Pathania, D. Novel thymoquinone loaded chitosan-lecithin micelles for effective wound healing: Development, characterization, and preclinical evaluation. Carbohydr. Polym. 2020, 230, 115659. [Google Scholar] [CrossRef]
- Kausar, H.; Mujeeb, M.; Ahad, A.; Moolakkadath, T.; Aqil, M.; Ahmad, A.; Akhter, M.H. Optimization of ethosomes for topical thymoquinone delivery for the treatment of skin acne. J. Drug Deliv. Sci. Technol. 2019, 49, 177–187. [Google Scholar] [CrossRef]
- Al-Qubaisi, M.S.; Rasedee, A.; Flaifel, M.H.; Eid, E.E.M.; Hussein-Al-Ali, S.; Alhassan, F.H.; Salih, A.M.; Hussein, M.Z.; Zainal, Z.; Sani, D.; et al. Characterization of thymoquinone/hydroxypropyl-β-cyclodextrin inclusion complex: Application to anti-allergy properties. Eur. J. Pharm. Sci. 2019, 133, 167–182. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-delivery of imiquimod and curcumin by nanoemugel for improved topical delivery and reduced psoriasis-like skin lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for Topical delivery of curcumin in Psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Alam, M.S.; Algahtani, M.S.; Ahmad, J.; Kohli, K.; Shafiq-Un-Nabi, S.; Warsi, M.H.; Ahmad, M.Z. Formulation design and evaluation of aceclofenac nanogel for topical application. Ther. Deliv. 2020, 11, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Veseli, A.; Žakelj, S.; Kristl, A. A review of methods for solubility determination in biopharmaceutical drug characterization. Drug Dev. Ind. Pharm. 2019, 45, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Chang, Q.; Chan, C.K.; Meng, Z.Y.; Wang, G.N.; Sun, J.B.; Wang, Y.T.; Tong, H.H.; Zheng, Y. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech 2009, 10, 172–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, K.; Kumar, R.; Goel, S.; Uppal, S.; Bhatia, A.; Mehta, S.K. Physiochemical and cytotoxicity study of TPGS stabilized nanoemulsion designed by ultrasonication method. Ultrason. Sonochem. 2017, 34, 173–182. [Google Scholar] [CrossRef]
- Sugumar, S.; Nirmala, J.; Ghosh, V.; Anjali, H.; Mukherjee, A.; Chandrasekaran, N. Bio-based nanoemulsion formulation, characterization and antibacterial activity against food-borne pathogens. J. Basic Microbiol. 2013, 53, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Chiang, B.H. Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason. Sonochem. 2012, 19, 192–197. [Google Scholar] [CrossRef]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef]
- Akhter, S.; Anwar, M.; Siddiqui, M.A.; Ahmad, I.; Ahmad, J.; Ahmad, M.Z.; Bhatnagar, A.; Ahmad, F.J. Improving the topical ocular pharmacokinetics of an immunosuppressant agent with mucoadhesive nanoemulsions: Formulation development, in-vitro and in-vivo studies. Colloids Surf. B Biointerfaces 2016, 148, 19–29. [Google Scholar] [CrossRef]
- Ahmad, J.; Gautam, A.; Komath, S.; Bano, M.; Garg, A.; Jain, K. Topical Nano-emulgel for Skin Disorders: Formulation Approach and Characterization. Recent Pat. Anti-Infect. Drug Discov. 2019, 14, 36–48. [Google Scholar] [CrossRef]
- Barkat, M.A.; Ahmad, J.; Ali, R.; Rahman, M.A.; Kaleem, S.; Singh, S.P.; Ahmad, F.J. Formulation design of micronized silver sulfadiazine containing Aloe vera gel for wound healing. Curr. Bioact. Compd. 2016, 12, 63–68. [Google Scholar] [CrossRef]
- Nava, G.; Piñón, E.; Mendoza, L.; Mendoza, N.; Quintanar, D.; Ganem, A. Formulation and in Vitro, ex Vivo and in Vivo Evaluation of Elastic Liposomes for Transdermal Delivery of Ketorolac Tromethamine. Pharmaceutics 2011, 3, 954–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntimenou, V.; Fahr, A.; Antimisiaris, S.G. Elastic vesicles for transdermal drug delivery of hydrophilic drugs: A comparison of important physicochemical characteristics of different vesicle types. J. Biomed. Nanotechnol. 2012, 8, 613–623. [Google Scholar] [CrossRef] [PubMed]
- El-Hadidy, G.N.; Ibrahim, H.K.; Mohamed, M.I.; El-Milligi, M.F. Microemulsions as vehicles for topical administration of voriconazole: Formulation and in vitro evaluation. Drug Dev. Ind. Pharm. 2012, 38, 64–72. [Google Scholar] [CrossRef]
- Manconi, M.; Sinico, C.; Valenti, D.; Lai, F.; Fadda, A.M. Niosomes as carriers for tretinoin. III. A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int. J. Pharm. 2006, 311, 11–19. [Google Scholar] [CrossRef]
- Chollet, J.L.; Jozwiakowski, M.J.; Phares, K.R.; Reiter, M.J.; Roddy, P.J.; Schultz, H.J.; Ta, Q.V.; Tomai, M.A. Development of a topically active imiquimod formulation. Pharm. Dev. Technol. 1999, 4, 35–43. [Google Scholar] [CrossRef]
- Nagar, H.K.; Srivastava, A.K.; Srivastava, R.; Kurmi, M.L.; Chandel, H.S.; Ranawat, M.S. Pharmacological Investigation of the Wound Healing Activity of Cestrum nocturnum (L.) Ointment in Wistar Albino Rats. J. Pharm. 2016, 2016, 9249040. [Google Scholar] [CrossRef] [Green Version]
- Castro Souza Junior Neto, J.; Estevão, L.R.; Baratella-Evêncio, L.; Vieira, M.G.; Simões, R.S.; Florencio-Silva, R.; Evêncio-Luz, L.; Evêncio-Neto, J. Mast cell concentration and skin wound contraction in rats treated with Ximenia americana L. Acta Cir. Bras. 2017, 32, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdi Jafari, S.; He, Y.; Bhandari, B. Nano-emulsion production by sonication and microfluidization—A comparison. Int. J. Food Prop. 2006, 9, 475–485. [Google Scholar] [CrossRef]
- Tang, S.Y.; Manickam, S.; Wei, T.K.; Nashiru, B. Formulation development and optimization of a novel Cremophore EL-based nanoemulsion using ultrasound cavitation. Ultrason. Sonochem. 2012, 19, 330–345. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Kotta, S.; Ahmad, J.; Akhter, S.; Shoaib Alam, M.; Khan, M.A.; Awan, Z.; Sivakumar, P.M. Improved Analgesic and Anti-Inflammatory Effect of Diclofenac Sodium by Topical Nanoemulgel: Formulation Development—In Vitro and In Vivo Studies. J. Chem. 2020, 2020, 4071818. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, K. Importance of surface energy in nanoemulsion. In Nanoemulsions: Properties, Fabrications and Applications; Kai Seng, K., Voon Loong, W., Eds.; Intech Open: London, UK, 2019; pp. 1–20. [Google Scholar]
- Ahmad, J.; Mir, S.R.; Kohli, K.; Amin, S. Effect of oil and co-surfactant on the formation of Solutol HS 15 based colloidal drug carrier by Box–Behnken statistical design. Colloids Surf. A Physicochem. Eng. Asp. 2014, 453, 68–77. [Google Scholar] [CrossRef]
- Arora, R.; Aggarwal, G.; Harikumar, S.L.; Kaur, K. Nanoemulsion based hydrogel for enhanced transdermal delivery of ketoprofen. Adv. Pharm. 2014, 2014. [Google Scholar] [CrossRef]
- Sinko, P.J.; Singh, Y. Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences; Walter Kluer: Alphen aan den Rijn, The Netherlands, 2011. [Google Scholar]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Dragicevic, N., Maibach, H.I., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Negi, P.; Sharma, I.; Hemrajani, C.; Rathore, C.; Bisht, A.; Raza, K.; Katare, O.P. Thymoquinone-loaded lipid vesicles: A promising nanomedicine for psoriasis. BMC Complement. Altern. Med. 2019, 19, 334. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Sahu, K.; Singh, S.P.; Jain, B. Wound healing activity of curcumin conjugated to hyaluronic acid: In vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]


| Formulation Code | Thermodynamic Stability | Mean Droplet Size (nm) | PdI | Zeta Potential (mV) | Drug Content (%) | Viscosity (mPas) | ||
|---|---|---|---|---|---|---|---|---|
| Heating Cooling Cycle | Centrifugation Study | Freeze-Thaw Cycle | ||||||
| F8 | √ | √ | √ | 48.45 ± 0.74 | 0.052 ± 0.004 | −29.5 ± 0.30 | 99.32 ± 0.119 | 77.81 ± 1.55 |
| F11 | √ | √ | √ | 64.22 ± 0.94 | 0.203 ± 0.01 | −30.6 ± 0.40 | 99.14 ± 0.112 | 74.91 ± 1.74 |
| F12 | √ | √ | √ | 94.67 ± 0.71 | 0.26 ± 0.03 | −30.5 ± 0.30 | 98.74 ± 0.445 | 71.04 ± 1.02 |
| F14 | √ | √ | √ | 40.02 ± 0.83 | 0.542 ± 0.05 | −26.7 ± 0.26 | 99.09 ± 0.49 | 88.82 ± 1.27 |
| F18 | √ | √ | √ | 99.66 ± 1.43 | 0.428 ± 0.017 | −28.9 ± 0.25 | 99.04 ± 0.258 | 85.38 ± 2.25 |
| Formulation | The Cumulative Amount of Drug Permeated (µg/cm2) | Drug Deposited in the Skin (µg/cm2) | Lag Time (h) | Flux (µg/cm2·h) | Permeability Coefficient (K × 10−3) | Local Accumulation Efficiency (LAE) |
|---|---|---|---|---|---|---|
| TMQ-NEG | 549.16 ± 3.10 | 965.65 ± 12.84 | 0.89 ± 0.01 | 23.14 ± 0.22 | 9.26 ± 0.09 | 1.76 ± 0.015 |
| TMQ-gel | 120.75 ± 2.43 | 150.93 ± 1.80 | 2.09 ± 0.04 | 4.78 ± 0.08 | 1.91 ± 0.03 | 1.25 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algahtani, M.S.; Ahmad, M.Z.; Shaikh, I.A.; Abdel-Wahab, B.A.; Nourein, I.H.; Ahmad, J. Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation. Molecules 2021, 26, 3863. https://doi.org/10.3390/molecules26133863
Algahtani MS, Ahmad MZ, Shaikh IA, Abdel-Wahab BA, Nourein IH, Ahmad J. Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation. Molecules. 2021; 26(13):3863. https://doi.org/10.3390/molecules26133863
Chicago/Turabian StyleAlgahtani, Mohammed S., Mohammad Zaki Ahmad, Ibrahim Ahmed Shaikh, Basel A. Abdel-Wahab, Ihab Hamed Nourein, and Javed Ahmad. 2021. "Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation" Molecules 26, no. 13: 3863. https://doi.org/10.3390/molecules26133863
APA StyleAlgahtani, M. S., Ahmad, M. Z., Shaikh, I. A., Abdel-Wahab, B. A., Nourein, I. H., & Ahmad, J. (2021). Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation. Molecules, 26(13), 3863. https://doi.org/10.3390/molecules26133863









