Lipidomic Profiling of Ipsilateral Brain and Plasma after Celastrol Post-Treatment in Transient Middle Cerebral Artery Occlusion Mice Model
Abstract
1. Introduction
2. Results
2.1. Cerebral Infarction Evaluation and TUNEL Staining
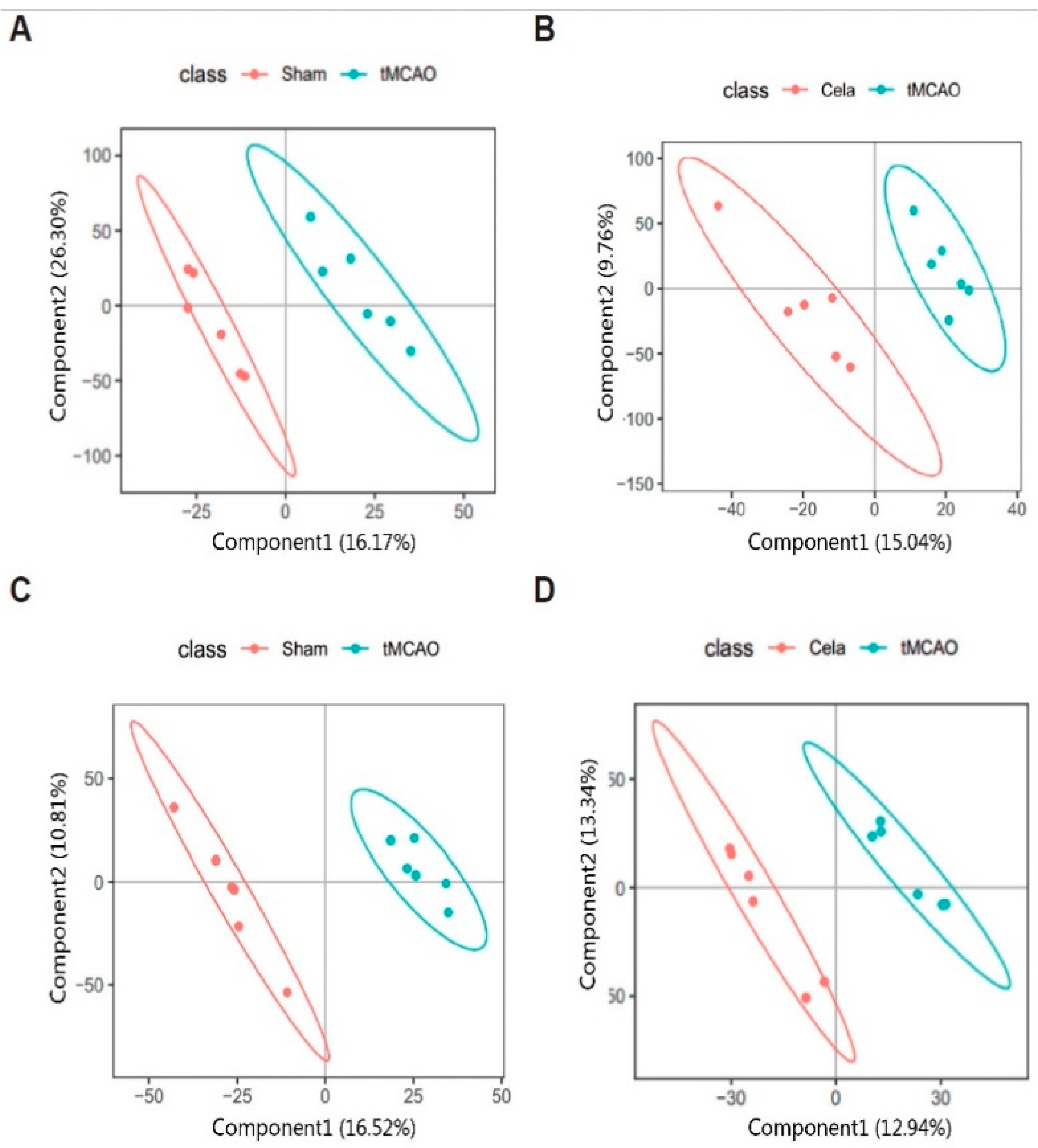
2.2. Multivariate Data Analysis for Plasma and Brain Samples
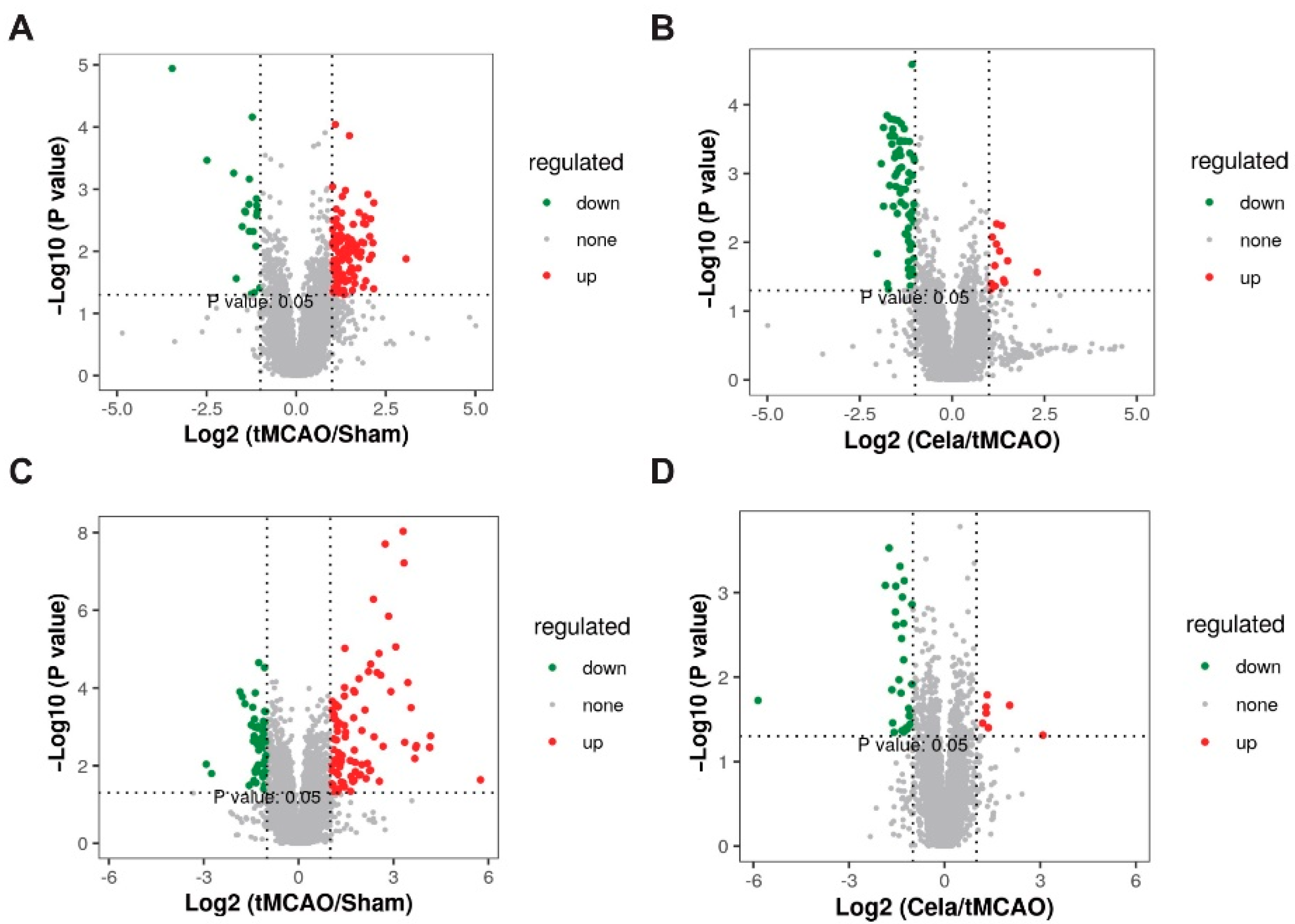
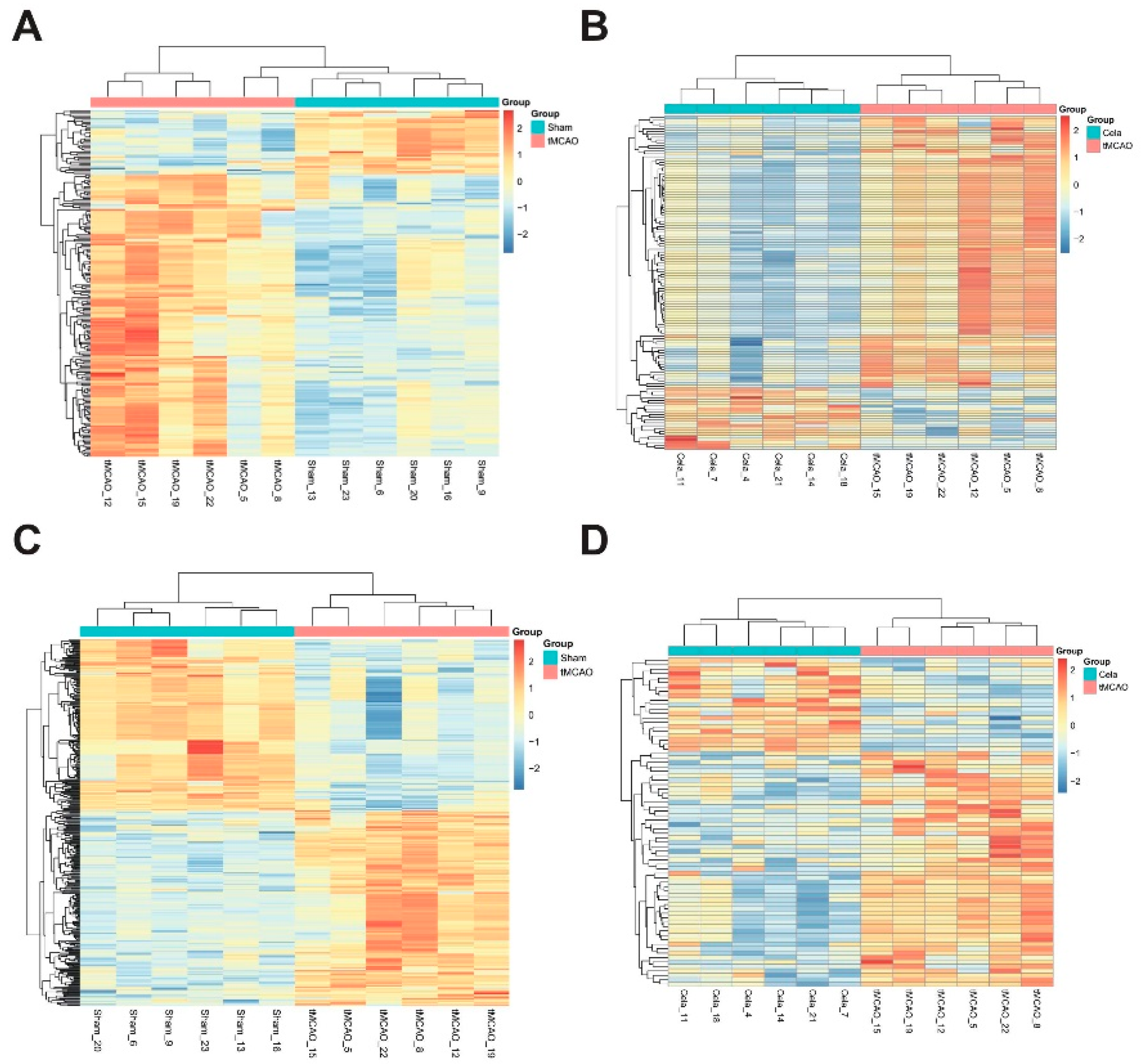
2.3. Differentially Expressed Lipids (DELs) Identification
2.4. Pathway Analysis and Possible Underlying Pathways
3. Discussion
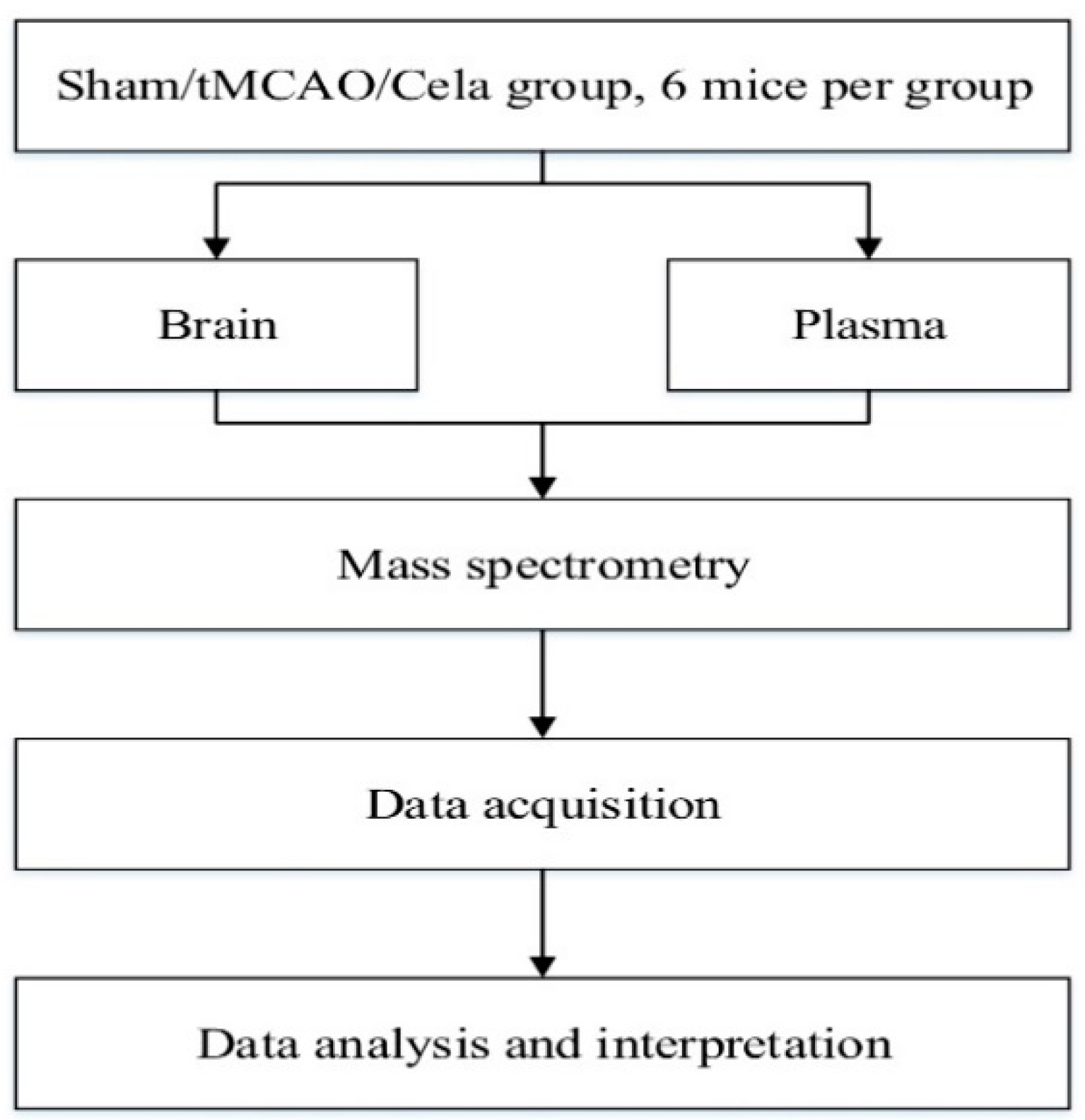
4. Materials and Methods
4.1. Animals
4.2. Transient Middle Cerebral Artery Occlusion (tMCAO) Model
4.3. Cerebral Infarct Volume Measurement
4.4. TUNEL Staining
4.5. Sample Collection and Preparation
4.6. Chromatography Analysis
4.7. Mass Spectrometry Analysis
4.8. Data Processing and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Patel, U.K.; Dave, M.; Lekshminarayanan, A.; Malik, P.; DeMasi, M.; Chandramohan, S.; Pillai, S.; Tirupathi, R.; Shah, S.; Jani, V.B.; et al. Risk Factors and Incidence of Acute Ischemic Stroke: A Comparative Study Between Young Adults and Older Adults. Cureus 2021, 13, e14670. [Google Scholar] [CrossRef]
- Tejada Meza, H.; Artal Roy, J.; Pérez Lázaro, C.; Bestué Cardiel, M.; Alberti González, O.; Tejero Juste, C.; Hernando Quintana, N.; Jarauta Lahoz, L.; Giménez Muñoz, A.; Campello Morer, I.; et al. Epidemiology and characteristics of ischaemic stroke in young adults in Aragon. Neurologia 2021. In Press. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Konstas, A.A.; Bateman, B.; Ortolano, G.A.; Pile-Spellman, J. Reperfusion injury following cerebral ischemia: Pathophysiology, MR imaging, and potential therapies. Neuroradiology 2007, 49, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Fan, J.; Han, J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm. Sin. B 2015, 5, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Luo, Q.; Alitongbieke, G.; Chong, S.; Xu, C.; Xie, L.; Chen, X.; Zhang, D.; Zhou, Y.; Wang, Z.; et al. Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol. Cell 2017, 66, 141–153. [Google Scholar] [CrossRef]
- Shi, K.; Chen, X.; Xie, B.; Yang, S.S.; Liu, D.; Dai, G.; Chen, Q. Celastrol Alleviates Chronic Obstructive Pulmonary Disease by Inhibiting Cellular Inflammation Induced by Cigarette Smoke via the Ednrb/Kng1 Signaling Pathway. Front. Pharmacol. 2018, 9, 1276. [Google Scholar] [CrossRef]
- Han, L.P.; Sun, B.; Li, C.J.; Xie, Y.; Chen, L.M. Effect of celastrol on tolllike receptor 4mediated inflammatory response in free fatty acidinduced HepG2 cells. Int. J. Mol. Med. 2018, 42, 2053–2061. [Google Scholar] [CrossRef]
- Trott, A.; West, J.D.; Klaic, L.; Westerheide, S.D.; Silverman, R.B.; Morimoto, R.I.; Morano, K.A. Activation of heat shock and antioxidant responses by the natural product celastrol: Transcriptional signatures of a thiol-targeted molecule. Mol. Biol. Cell 2008, 19, 1104–1112. [Google Scholar] [CrossRef]
- Han, X.B.; Tan, Y.; Fang, Y.Q.; Li, F. Protective effects of celastrol against gamma irradiation-induced oxidative stress in human umbilical vein endothelial cells. Exp. Ther. Med. 2018, 16, 685–694. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Yang, X.; Yang, M.; Sun, H.; Wang, C. Celastrol suppresses obesity process via increasing antioxidant capacity and improving lipid metabolism. Eur. J. Pharmacol. 2014, 744, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Q.; Xiao, X.; Yang, R.; Hu, D.; Zhu, X.; Gonzalez, F.J.; Li, F. Modulation of Lipid Metabolism by Celastrol. J. Proteome Res. 2019, 18, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gu, X.; Dai, W.; Ye, J.; Lu, F.; Chai, Y.; Fan, G.; Gonzalez, F.J.; Duan, G.; Qi, Y. A lipidomics investigation into the intervention of celastrol in experimental colitis. Mol. Biosyst. 2016, 12, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, D.; Zhang, X.; Liu, Z.; Zhang, X.; Dong, L.; Xing, Y.; Wang, C.; Qiao, H.; Zhu, C.; et al. Protective effect of celastrol in rat cerebral ischemia model: Down-regulating p-JNK, p-c-Jun and NF-kappaB. Brain Res. 2012, 1464, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, X.; Zhang, D.; Wang, Y.; Hu, X.; Xu, F.; Jin, M.; Cao, F.; Xu, L. Celastrol treatment protects against acute ischemic stroke-induced brain injury by promoting an IL-33/ST2 axis-mediated microglia/macrophage M2 polarization. J. Neuroinflamm. 2018, 15, 78. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Q.; Chen, X.; Wu, X.; Sha, R.; Song, G.; Zhang, C.; Chen, X. Neuroprotective Effects of Celastrol on Transient Global Cerebral Ischemia Rats via Regulating HMGB1/NF-kappaB Signaling Pathway. Front. Neurosci. 2020, 14, 847. [Google Scholar] [CrossRef] [PubMed]
- Zullig, T.; Trotzmuller, M.; Kofeler, H.C. Lipidomics from sample preparation to data analysis: A primer. Anal. Bioanal. Chem. 2020, 412, 2191–2209. [Google Scholar] [CrossRef]
- Au, A. Metabolomics and Lipidomics of Ischemic Stroke. Adv. Clin. Chem. 2018, 85, 31–69. [Google Scholar] [CrossRef]
- Ryu, W.S.; Schellingerhout, D.; Jeong, S.W.; Nahrendorf, M.; Kim, D.E. Association between Serum Lipid Profiles and Early Neurological Deterioration in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2024–2030. [Google Scholar] [CrossRef]
- Tziomalos, K.; Giampatzis, V.; Bouziana, S.D.; Spanou, M.; Kostaki, S.; Papadopoulou, M.; Angelopoulou, S.M.; Tsopozidi, M.; Savopoulos, C.; Hatzitolios, A.I. Prognostic significance of major lipids in patients with acute ischemic stroke. Metab. Brain Dis. 2017, 32, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Kloska, A.; Malinowska, M.; Gabig-Ciminska, M.; Jakobkiewicz-Banecka, J. Lipids and Lipid Mediators Associated with the Risk and Pathology of Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 3618. [Google Scholar] [CrossRef]
- Abe, T.; Niizuma, K.; Kanoke, A.; Saigusa, D.; Saito, R.; Uruno, A.; Fujimura, M.; Yamamoto, M.; Tominaga, T. Metabolomic Analysis of Mouse Brain after a Transient Middle Cerebral Artery Occlusion by Mass Spectrometry Imaging. Neurol. Med. Chir. 2018, 58, 384–392. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Wang, H.; Xiao, H.; Jing, F.; Tang, L.; Li, D.; Zhang, Y.; Wu, H.; Yang, H. Metabolomics study on the effects of Buchang Naoxintong capsules for treating cerebral ischemia in rats using UPLC-Q/TOF-MS. J. Ethnopharmacol. 2016, 180, 1–11. [Google Scholar] [CrossRef]
- Duan, W.; Wang, L.; Lv, J.; Gao, K.; Lu, Y.; Qin, S.; Ma, X.; Li, J.; Ge, X. Metabolomics Study on the Effects of Salvianolic Acid B and Borneol for Treating Cerebral Ischemia in Rats by Ultra-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. Rejuvenation Res. 2019, 22, 313–324. [Google Scholar] [CrossRef]
- Liu, W.; Mu, F.; Liu, T.; Xu, H.; Chen, J.; Jia, N.; Zhang, Y.; Dou, F.; Shi, L.; Li, Y.; et al. Ultra Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry-Based Metabonomics Reveal Protective Effect of Terminalia chebula Extract on Ischemic Stroke Rats. Rejuvenation Res. 2018, 21, 541–552. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Q.; Zhang, H.; Mo, C.; Yao, J.; Huang, T.; Zhou, T.; Tan, W. Lipidomics study of the protective effects of isosteviol sodium on stroke rats using ultra high-performance supercritical fluid chromatography coupling with ion-trap and time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 157, 145–155. [Google Scholar] [CrossRef]
- Rabiei, Z.; Bigdeli, M.R.; Rasoulian, B.; Ghassempour, A.; Mirzajani, F. The neuroprotection effect of pretreatment with olive leaf extract on brain lipidomics in rat stroke model. Phytomedicine 2012, 19, 940–946. [Google Scholar] [CrossRef]
- Sun, N.; Keep, R.F.; Hua, Y.; Xi, G. Critical Role of the Sphingolipid Pathway in Stroke: A Review of Current Utility and Potential Therapeutic Targets. Transl. Stroke Res. 2016, 7, 420–438. [Google Scholar] [CrossRef]
- Di Pardo, A.; Maglione, V. Sphingolipid Metabolism: A New Therapeutic Opportunity for Brain Degenerative Disorders. Front. Neurosci. 2018, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Prinetti, A. The role of sphingolipids in neuronal plasticity of the brain. J. Neurochem. 2016, 137, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Chao, H.C.; Lee, T.H.; Chiang, C.S.; Yang, S.Y.; Kuo, C.H.; Tang, S.C. Sphingolipidomics Investigation of the Temporal Dynamics after Ischemic Brain Injury. J. Proteome Res. 2019, 18, 3470–3478. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M. The role and metabolism of sulfatide in the nervous system. Mol. Neurobiol. 2008, 37, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Suzuki, T. Role of sulfatide in normal and pathological cells and tissues. J. Lipid Res. 2012, 53, 1437–1450. [Google Scholar] [CrossRef]
- Palavicini, J.P.; Wang, C.; Chen, L.; Ahmar, S.; Higuera, J.D.; Dupree, J.L.; Han, X. Novel molecular insights into the critical role of sulfatide in myelin maintenance/function. J. Neurochem. 2016, 139, 40–54. [Google Scholar] [CrossRef]
- Kanter, J.L.; Narayana, S.; Ho, P.P.; Catz, I.; Warren, K.G.; Sobel, R.A.; Steinman, L.; Robinson, W.H. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat. Med. 2006, 12, 138–143. [Google Scholar] [CrossRef]
- Halder, R.C.; Jahng, A.; Maricic, I.; Kumar, V. Mini review: Immune response to myelin-derived sulfatide and CNS-demyelination. Neurochem. Res. 2007, 32, 257–262. [Google Scholar] [CrossRef]
- Jeon, S.B.; Yoon, H.J.; Park, S.H.; Kim, I.H.; Park, E.J. Sulfatide, a major lipid component of myelin sheath, activates inflammatory responses as an endogenous stimulator in brain-resident immune cells. J. Immunol. 2008, 181, 8077–8087. [Google Scholar] [CrossRef]
- Zeng, Y.; Cheng, H.; Jiang, X.; Han, X. Endosomes and lysosomes play distinct roles in sulfatide-induced neuroblastoma apoptosis: Potential mechanisms contributing to abnormal sulfatide metabolism in related neuronal diseases. Biochem. J. 2008, 410, 81–92. [Google Scholar] [CrossRef]
- Okuno, S.; Saito, A.; Hayashi, T.; Chan, P.H. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J. Neurosci. 2004, 24, 7879–7887. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Q.; Yang, S.; Huang, J.; Feng, X.; Peng, J.; Lin, Z.; Liu, W.; Tao, J.; Chen, L. Electroacupuncture Inhibits Apoptosis of Peri-Ischemic Regions via Modulating p38, Extracellular Signal-Regulated Kinase (ERK1/2), and c-Jun N Terminal Kinases (JNK) in Cerebral Ischemia-Reperfusion-Injured Rats. Med. Sci. Monit. 2018, 24, 4395–4404. [Google Scholar] [CrossRef]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859 Pt B, 1558–1572. [Google Scholar] [CrossRef]
- Mendes-Frias, A.; Santos-Lima, B.; Furtado, D.Z.S.; Ruperez, F.J.; Assuncao, N.A.; Matias, M.J.; Gomes, V.; Gaifem, J.; Barbas, C.; Castro, A.G.; et al. Dysregulation of glycerophospholipid metabolism during Behcet’s disease contributes to a pro-inflammatory phenotype of circulating monocytes. J. Transl. Autoimmun. 2020, 3, 100056. [Google Scholar] [CrossRef]
- Dehlaghi Jadid, K.; Davidsson, J.; Lidin, E.; Hanell, A.; Angeria, M.; Mathiesen, T.; Risling, M.; Gunther, M. COX-2 Inhibition by Diclofenac Is Associated With Decreased Apoptosis and Lesion Area After Experimental Focal Penetrating Traumatic Brain Injury in Rats. Front. Neurol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Joshi, V.; Venkatesha, S.H.; Ramakrishnan, C.; Nanjaraj Urs, A.N.; Hiremath, V.; Moudgil, K.D.; Velmurugan, D.; Vishwanath, B.S. Celastrol modulates inflammation through inhibition of the catalytic activity of mediators of arachidonic acid pathway: Secretory phospholipase A2 group IIA, 5-lipoxygenase and cyclooxygenase-2. Pharmacol. Res. 2016, 113 Pt A, 265–275. [Google Scholar] [CrossRef]
- Ansari, S.; Azari, H.; McConnell, D.J.; Afzal, A.; Mocco, J. Intraluminal middle cerebral artery occlusion (MCAO) model for ischemic stroke with laser doppler flowmetry guidance in mice. J. Vis. Exp. 2011, 51, 2879. [Google Scholar] [CrossRef] [PubMed]
- Rousselet, E.; Kriz, J.; Seidah, N.G. Mouse model of intraluminal MCAO: Cerebral infarct evaluation by cresyl violet staining. J. Vis. Exp. 2012, 69, 4038. [Google Scholar] [CrossRef]



| Pathway Name | p-Value | Metabolite | Impact |
|---|---|---|---|
| Sphingolipid metabolism | 0.00005 | Sphingomyelin; Digalactosylceramide; Glucosylceramide; Sulfatide | 0.07708 |
| Arachidonic acid metabolism | 0.00568 | 5,6-EET; Phosphatidylcholine; 14,15-DHET | 0.02120 |
| Linoleic acid metabolism | 0.05207 | Phosphatidylcholine | 0.00000 |
| Glycerophospholipid metabolism | 0.05404 | Phosphatidylethanolamine; Phosphatidylcholine | 0.19895 |
| Valine, leucine and isoleucine biosynthesis | 0.08208 | L-Isoleucine | 0.00000 |
| alpha-Linolenic acid metabolism | 0.13013 | Phosphatidylcholine | 0.00000 |
| Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 0.13945 | Phosphatidylethanolamine | 0.00399 |
| Terpenoid backbone biosynthesis | 0.17581 | Dimethylallyl diphosphate | 0.00000 |
| Biosynthesis of unsaturated fatty acids | 0.32232 | (4Z,7Z,10Z,13Z,16Z,19Z)-Docosahexaenoic acid | 0.00000 |
| Valine, leucine and isoleucine degradation | 0.35138 | L-Isoleucine | 0.00000 |
| Steroid biosynthesis | 0.36546 | Cholesterol ester | 0.00000 |
| Pathway Name | p-Value | Metabolite | Impact |
|---|---|---|---|
| Glycerophospholipid metabolism | 0.00180 | Phosphatidylethanolamine; Phosphatidylcholine; Phosphatidylserine | 0.24596 |
| Linoleic acid metabolism | 0.03000 | Phosphatidylcholine | 0.00000 |
| alpha-Linolenic acid metabolism | 0.09125 | Phosphatidylcholine | 0.00000 |
| Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 0.09795 | Phosphatidylethanolamine | 0.00399 |
| Sphingolipid metabolism | 0.14357 | Sulfatide | 0.00000 |
| Arachidonic acid metabolism | 0.23436 | Phosphatidylcholine | 0.00000 |
| Tryptophan metabolism | 0.26262 | 5-Hydroxykynurenamine | 0.01390 |
| Pathway Name | p-Value | Metabolite | Impact |
|---|---|---|---|
| Sphingolipid metabolism | 0.00200 | Sphingosine; N-Acylsphingosine; Lactosylceramide; Sulfatide | 0.31440 |
| alpha-Linolenic acid metabolism | 0.04800 | Phosphatidylcholine; (9Z,12Z,15Z)-Octadecatrienoic acid | 0.33333 |
| Linoleic acid metabolism | 0.13204 | Phosphatidylcholine | 0.00000 |
| Glycosaminoglycan biosynthesis-chondroitin sulfate/dermatan sulfate | 0.24701 | Chondroitin | 0.00000 |
| Metabolism of xenobiotics by cytochrome P450 | 0.26300 | 2-Bromoacetaldehyde | 0.01418 |
| Arachidonic acid metabolism | 0.26558 | S-(1,2-Dichlorovinyl)glutathione | 0.07980 |
| Glycerophospholipid metabolism | 0.26558 | (1aalpha,2beta,3alpha,11calpha)-1a,2,3,11c-Tetrahydro-6,11-dimethylbenzo[6,7]phenanthro[3,4-b]oxirene-2,3-diol | 0.19895 |
| Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 0.32816 | Phosphatidylethanolamine | 0.00399 |
| Arginine biosynthesis | 0.32816 | L-Arginine | 0.07614 |
| Terpenoid backbone biosynthesis | 0.40074 | Dimethylallyl diphosphate | 0.00000 |
| Propanoate metabolism | 0.48077 | 2-Hydroxybutanoic acid | 0.00000 |
| Drug metabolism-cytochrome P450 | 0.53720 | Alcophosphamide | 0.00000 |
| Alanine, aspartate and glutamate metabolism | 0.55034 | N-Acetyl-L-aspartate | 0.08654 |
| Purine metabolism | 0.55802 | Allantoate;(S)-Ureidoglycolate | 0.00012 |
| Biosynthesis of unsaturated fatty acids | 0.64315 | (9Z,12Z,15Z)-Octadecatrienoic acid | 0.00000 |
| Arginine and proline metabolism | 0.66326 | L-Arginine | 0.05786 |
| Fatty acid degradation | 0.67289 | L-Palmitoylcarnitine | 0.00000 |
| Steroid biosynthesis | 0.70021 | Cholesterol ester | 0.00000 |
| Aminoacyl-tRNA biosynthesis | 0.74833 | L-Arginine | 0.00000 |
| Steroid hormone biosynthesis | 0.89308 | Pregnenolone | 0.03776 |
| Pathway Name | p-Value | Metabolite | Impact |
|---|---|---|---|
| Sphingolipid metabolism | 0.01000 | N-Acylsphingosine; Sulfatide | 0.26978 |
| Propanoate metabolism | 0.18199 | 2-Hydroxybutanoic acid | 0.00000 |
| Alanine, aspartate and glutamate metabolism | 0.21727 | N-Acetyl-L-aspartate | 0.08654 |
| Glycerophospholipid metabolism | 0.27080 | 1-Acyl-sn-glycero-3-phosphocholine | 0.01736 |
| Steroid biosynthesis | 0.30871 | Cholesterol ester | 0.00000 |
| Metabolism of xenobiotics by cytochrome P450 | 0.43269 | (1aalpha,2beta,3alpha,11calpha)-1a,2,3,11c-Tetrahydro-6,11-dimethylbenzo[6,7]phenanthro[3,4-b]oxirene-2,3-diol | 0.00000 |
| Steroid hormone biosynthesis | 0.49595 | 11-Deoxycortisol | 0.01317 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Chen, M.; Luo, Y.; Wang, H.; Huang, H.; Peng, Z.; Li, M.; Fei, H.; Luo, W.; Yang, J. Lipidomic Profiling of Ipsilateral Brain and Plasma after Celastrol Post-Treatment in Transient Middle Cerebral Artery Occlusion Mice Model. Molecules 2021, 26, 4124. https://doi.org/10.3390/molecules26144124
Liu M, Chen M, Luo Y, Wang H, Huang H, Peng Z, Li M, Fei H, Luo W, Yang J. Lipidomic Profiling of Ipsilateral Brain and Plasma after Celastrol Post-Treatment in Transient Middle Cerebral Artery Occlusion Mice Model. Molecules. 2021; 26(14):4124. https://doi.org/10.3390/molecules26144124
Chicago/Turabian StyleLiu, Maozhu, Mengyuan Chen, Ying Luo, Hong Wang, Haifeng Huang, Zhe Peng, Miaomiao Li, Huizhi Fei, Wen Luo, and Junqing Yang. 2021. "Lipidomic Profiling of Ipsilateral Brain and Plasma after Celastrol Post-Treatment in Transient Middle Cerebral Artery Occlusion Mice Model" Molecules 26, no. 14: 4124. https://doi.org/10.3390/molecules26144124
APA StyleLiu, M., Chen, M., Luo, Y., Wang, H., Huang, H., Peng, Z., Li, M., Fei, H., Luo, W., & Yang, J. (2021). Lipidomic Profiling of Ipsilateral Brain and Plasma after Celastrol Post-Treatment in Transient Middle Cerebral Artery Occlusion Mice Model. Molecules, 26(14), 4124. https://doi.org/10.3390/molecules26144124





