Solid-State NMR Spectroscopy: A Key Tool to Unravel the Supramolecular Structure of Drug Delivery Systems
Abstract
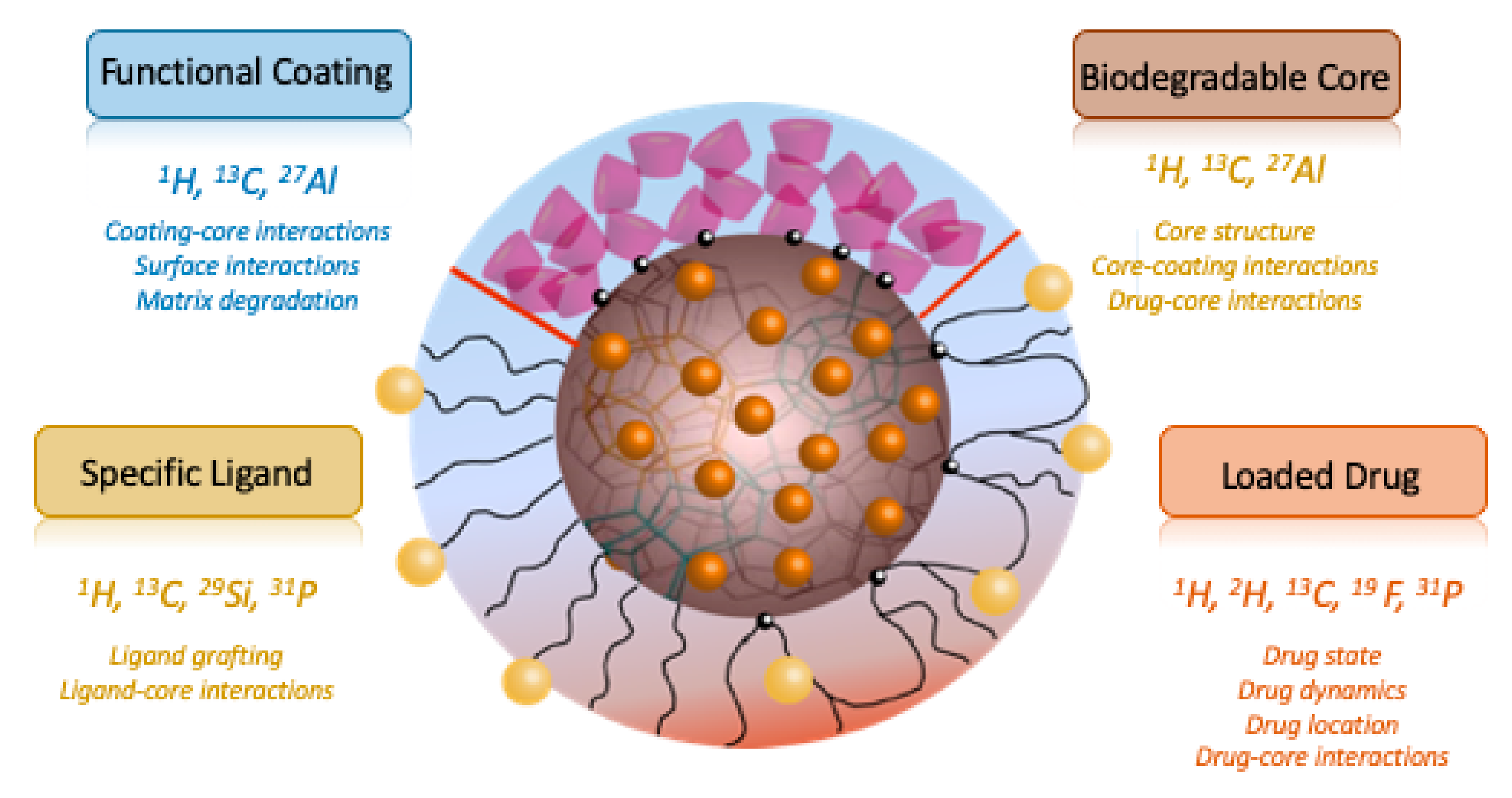
:1. Introduction
2. A Few Notes on ssNMR Spectroscopy
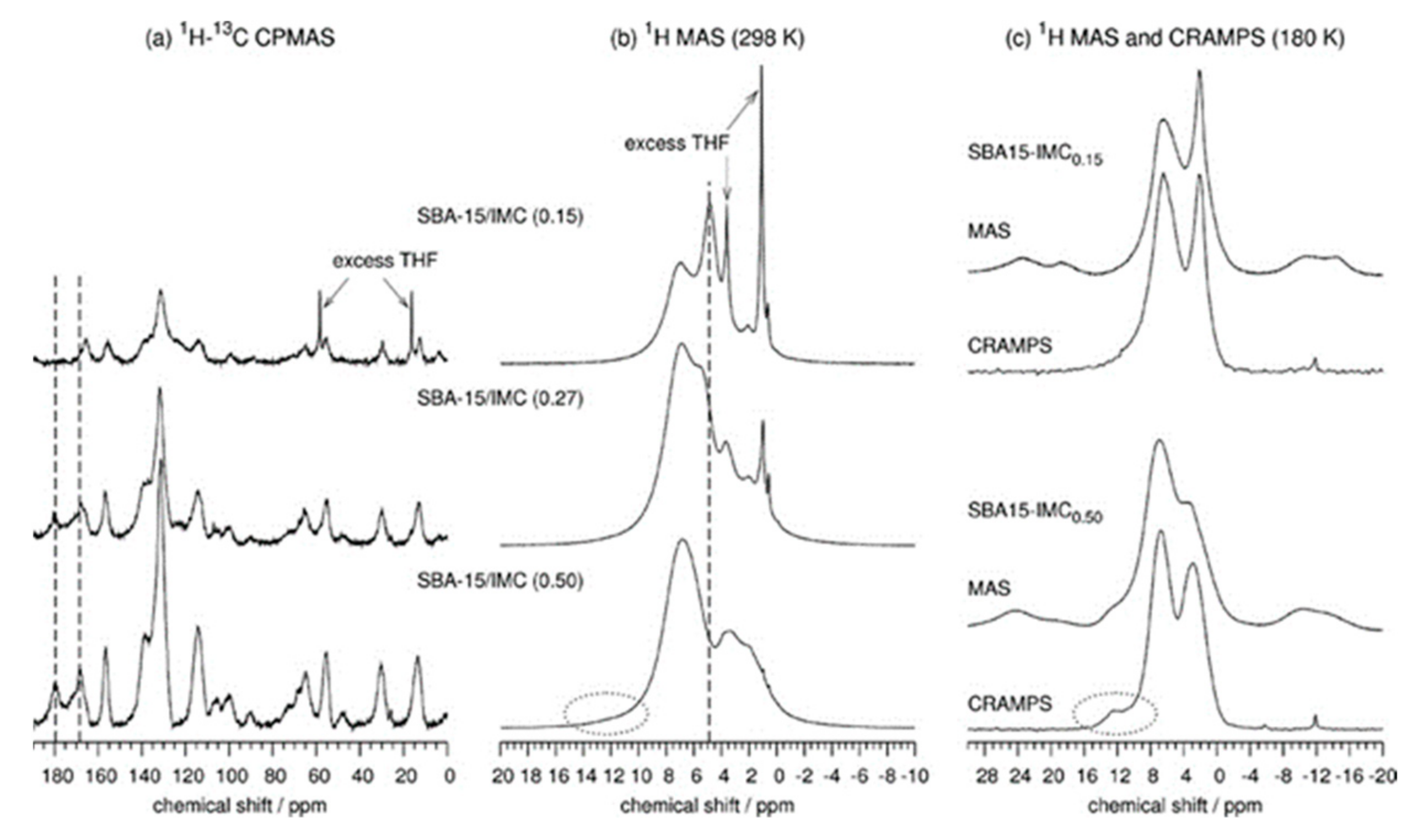
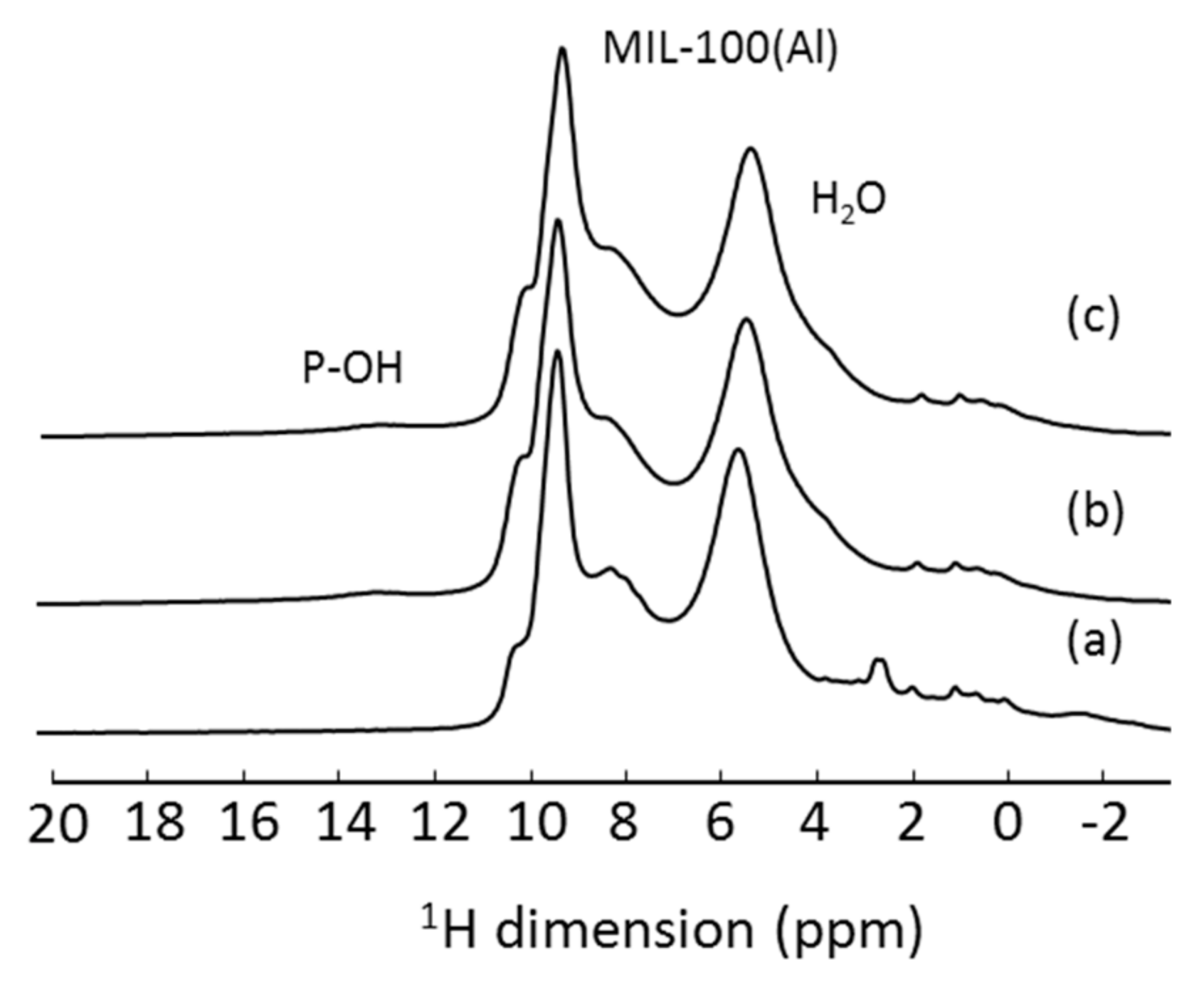
3. 1D 1H ssNMR Spectroscopy
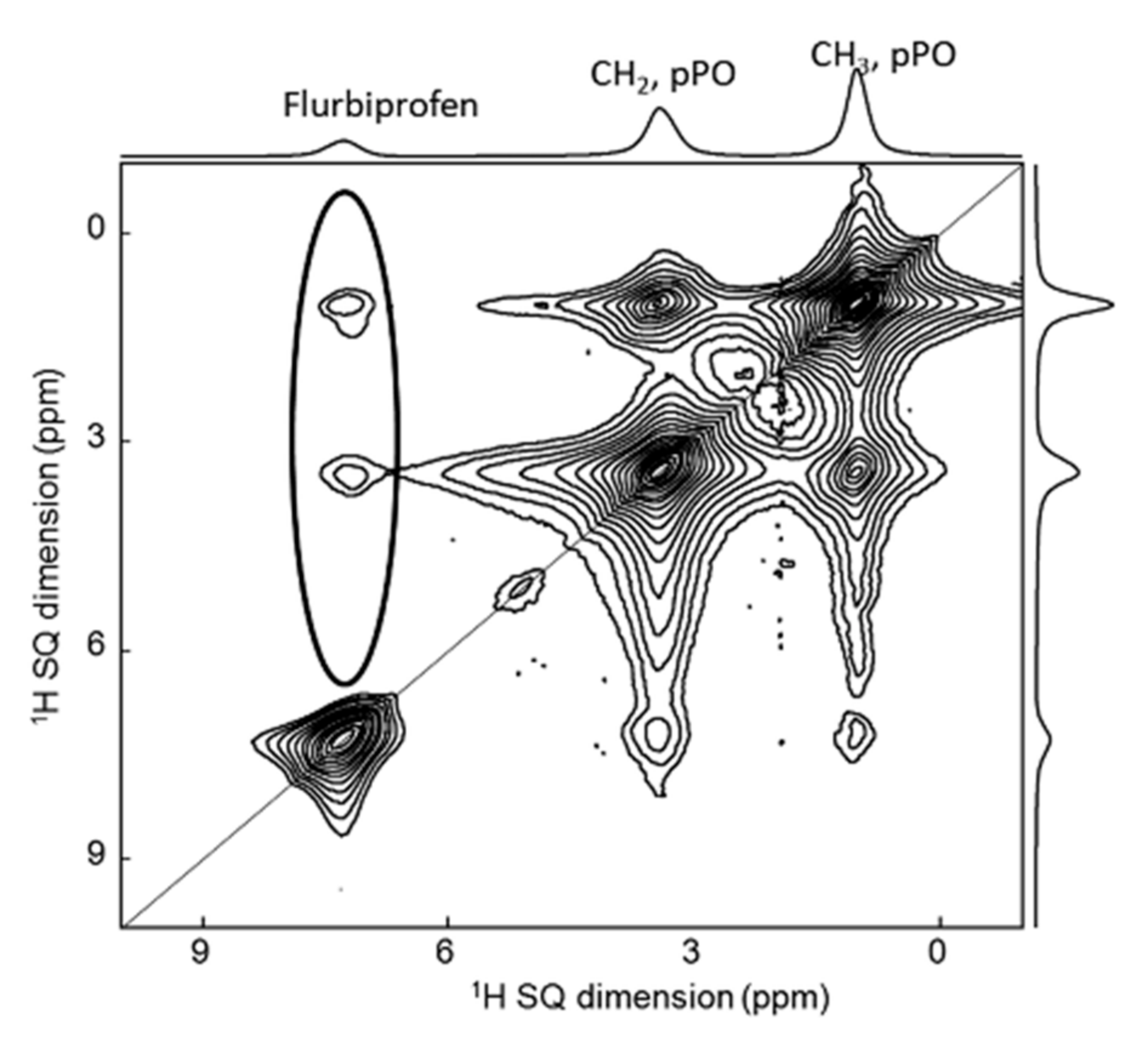
4. 1H-1H 2D NMR
5. 1H-X NMR
6. X-Y NMR
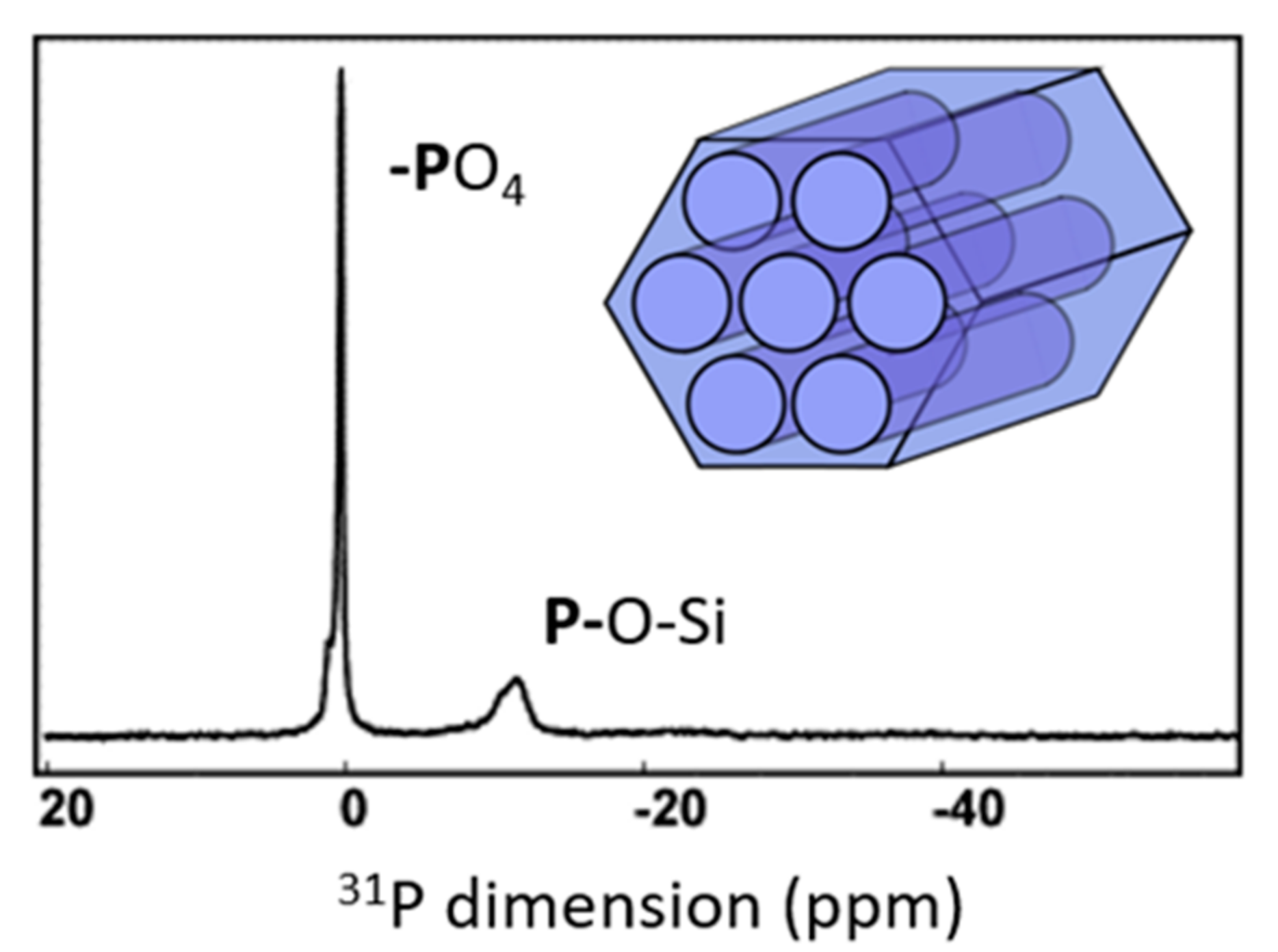
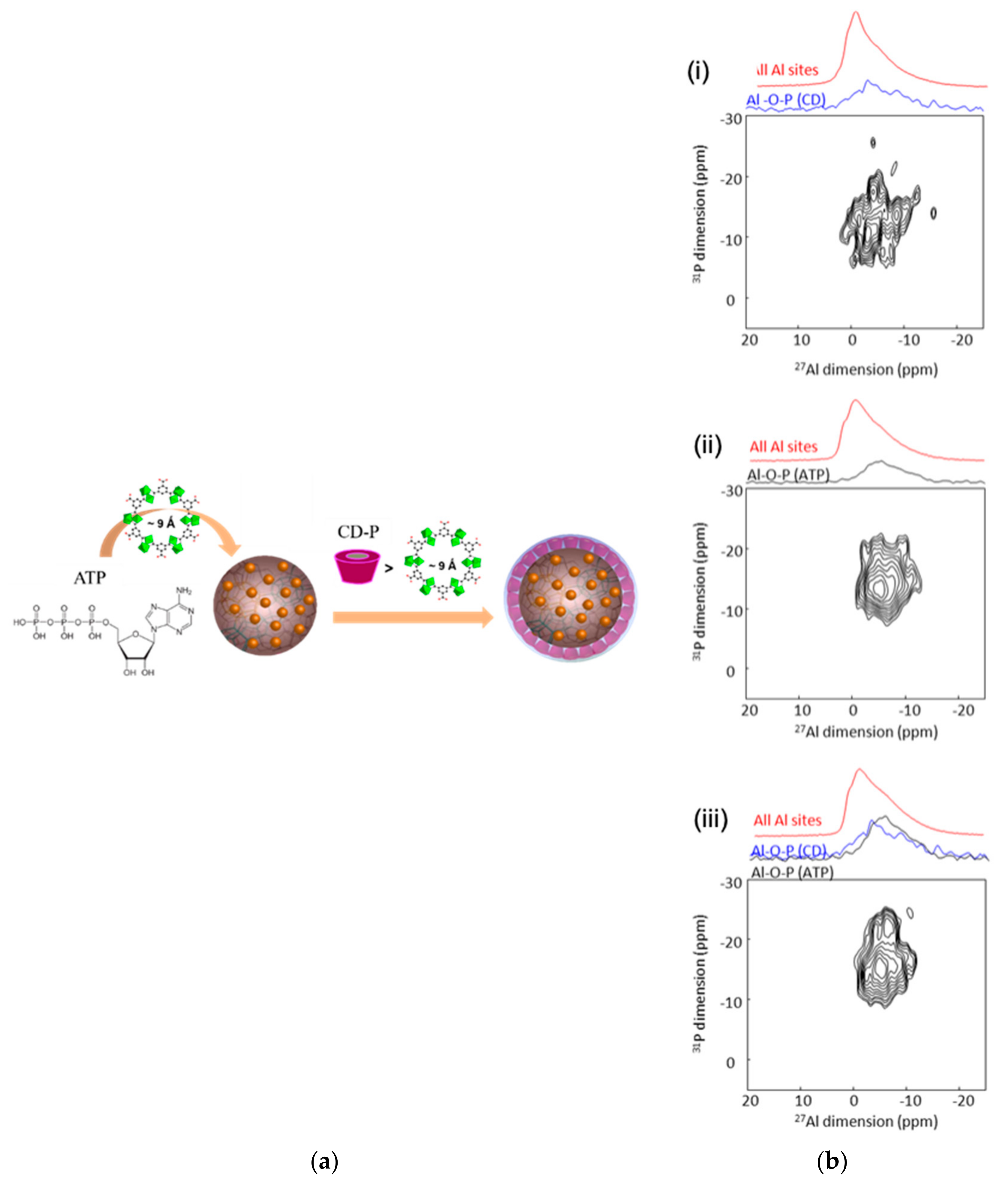
6.1. 27Al-31P
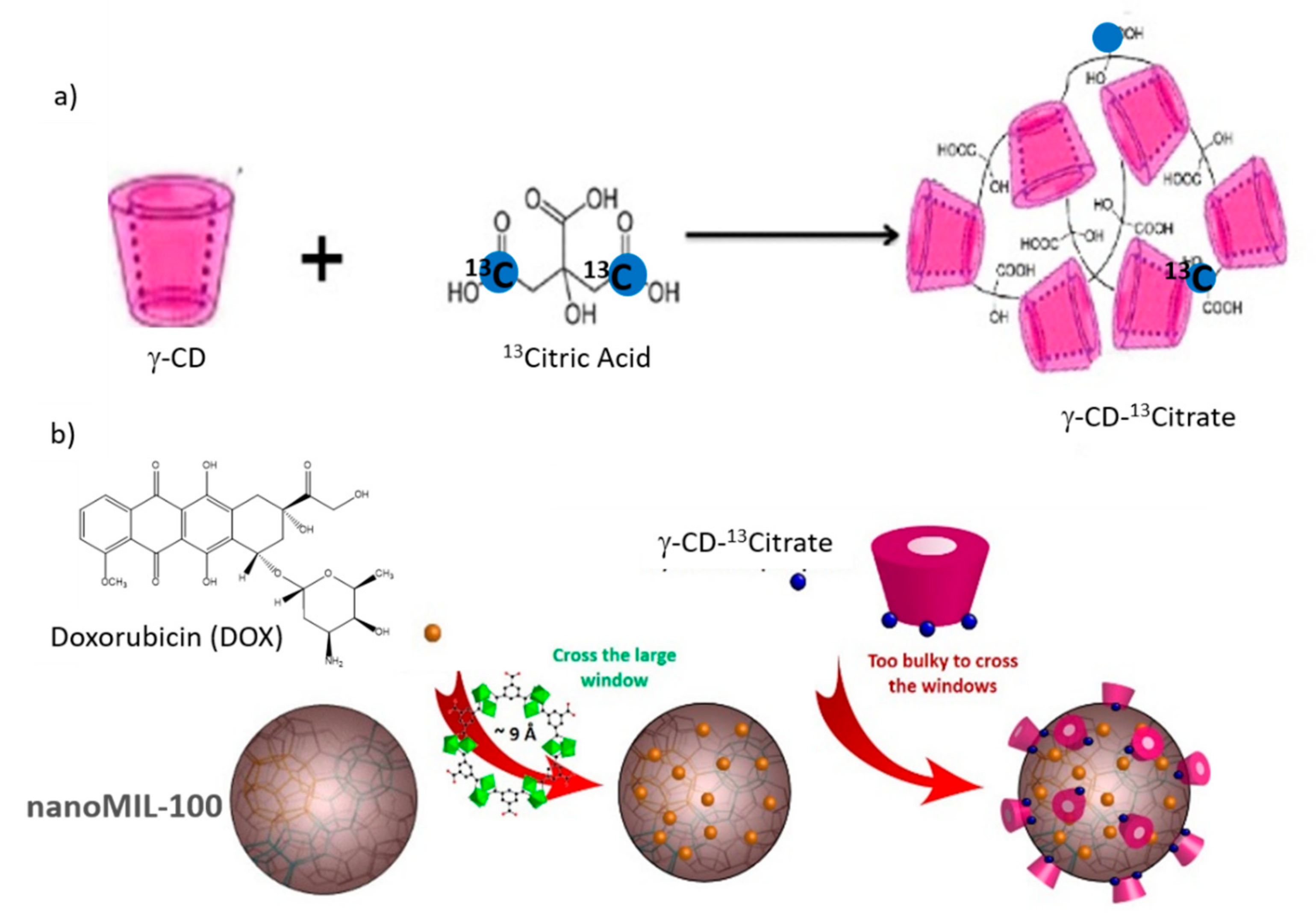
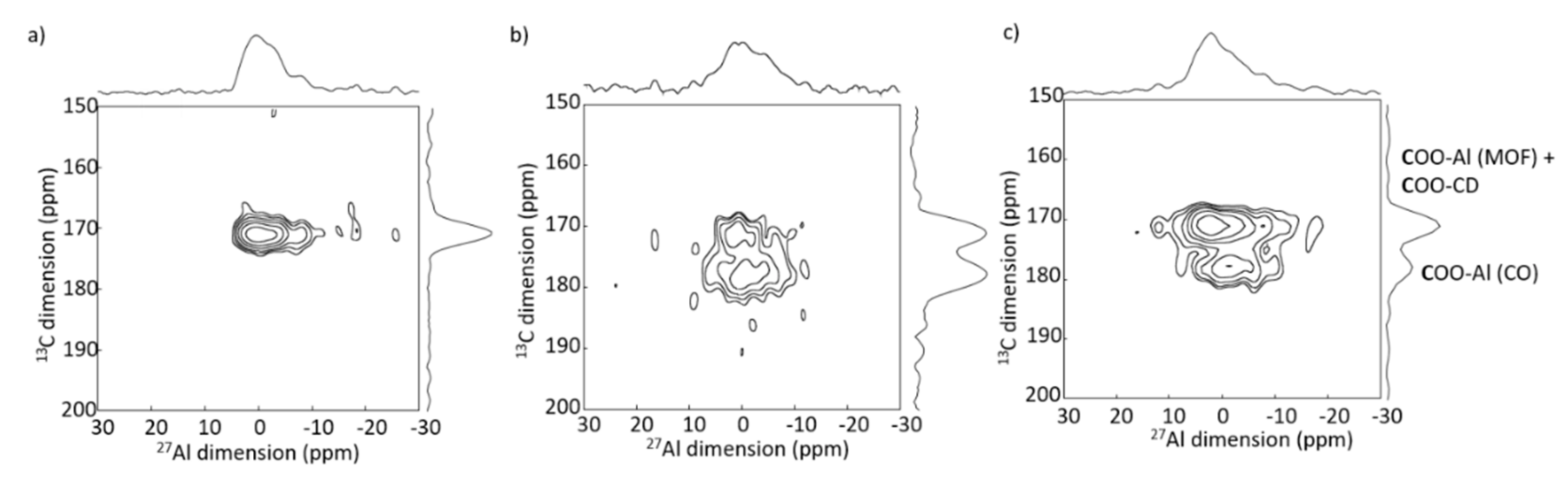
6.2. 27Al-13C
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, M.J.; Billinglsey, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Skorupska, E.; Jeziorna, A.; Kazmierski, S.; Potrzebowski, M.J. Recent progress in solid-state NMR studies of drugs confined within drug delivery systems. Solid State Nucl. Magn. Reson. 2014, 57–58, 2–16. [Google Scholar] [CrossRef]
- Bhowmik, D.; Gopinath, H.; Kumar, B.P.; Duraivel, S.; Kumar, K.P.S. Controlled released drug delivery systems. Pharma Innov. 2012, 1, 24–32. [Google Scholar]
- Loganathan, S.; Valapa, R.B.; Mishra, R.K.; Pugazhenthi, G.; Thomas, S. Thermogravimetric Analysis for Characterization of Nanomaterials. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization, 1st ed.; Thomas, S., Thomas, R., Zachariah, A., Mishra, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 3, Chapter 4; pp. 67–108. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.V. Evaluation of the BET theory for the characterization of meso and microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef] [Green Version]
- Ukmar, T.; Čendak, T.; Mazaj, M.; Kaučič, V.; Mali, G. Structural and Dynamical Properties of Indomethacin Molecules Embedded within the Mesopores of SBA-15: A Solid-State NMR View. J. Phys. Chem. C 2012, 116, 2662–2671. [Google Scholar] [CrossRef]
- Wong, Y.T.A.; Martins, V.; Lucier, B.E.G.; Huang, Y. Solid-State NMR Spectroscopy: A Powerful Technique to Directly Study Small Gas Molecules Adsorbed in Metal–Organic Frameworks. Chem. Eur. J. 2019, 25, 1848–1853. [Google Scholar] [CrossRef]
- Levitt, M.H. Spin Dynamics; Wiley: Chichester, UK, 2008. [Google Scholar]
- Keeler, J. Understanding NMR Spectroscopy; Wiley: Chichester, UK, 2010. [Google Scholar]
- Struppe, J.; Quinn, C.M.; Sarkar, S.; Gronenborn, A.M.; Polenova, T. Ultrafast 1H MAS NMR Crystallography for Natural Abundance Pharmaceutical Compounds. Mol. Pharm. 2020, 17, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Nayak, U.; Raichur, A.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Shen, S.; Chow, P.S.; Kim, S.; Zhu, K.; Tan, R.B.H. Synthesis of carboxyl-modified rod-like SBA-15 by rapid co-condensation. J. Colloid Interface Sci. 2008, 321, 365–372. [Google Scholar] [CrossRef]
- Mellaerts, R.; Houthoofd, K.; Elen, K.; Chen, H.; Van Speybroeck, M.; Van Humbeeck, J.; Augustijns, P.; Mullens, J.; Van den Mooter, G.; Martens, J.A. Aging behavior of pharmaceutical formulations of itraconazole on SBA-15 ordered mesoporous silica carrier material. Microporous Mesoporous Mater. 2010, 130, 154–161. [Google Scholar] [CrossRef]
- Aiello, D.; Folliet, N.; Laurent, G.; Testa, F.; Gervais, C.; Babonneau, F.; Azaïs, T. Solid state NMR characterization of phenylphosphonic acid encapsulated in SBA-15 and aminopropyl-modified SBA-15. Microporous Mesoporous Mater. 2013, 166, 109. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Žižak, Z.; Sierra, I.; Prashar, S.; del Hierro, I.; Fajardo, M.; Juranić, Z.D.; Kaluđerović, G.N. A new generation of anticancer drugs: Mesoporous materials modified with titanocene complexes. Chem. Eur. J. 2009, 15, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.P. Applications of high-resolution 1H solid-state NMR. Solid State Nucl. Magn. Reson. 2012, 41, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, S.; Saïdi, F.; Vandervoort, N.; Van den Mooter, G.; Martineau, C.; Taulelle, F.; Martens, J.A. Silica Capsules Enclosing P123 Triblock Copolymer Micelles for Flurbiprofen Storage and Release. J. Mater. Chem. B 2015, 3, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Mali, G. Looking into Metal-Organic Frameworks with Solid-State NMR Spectroscopy; InTech: London, UK, 2016. [Google Scholar]
- Devautour-Vinot, S.; Martineau, C.; Diaby, S.; Ben-Yahia, M.; Miller, S.; Serre, C.; Horcajada, P.; Cunha, D.; Taulelle, F.; Maurin, G. Caffeine Confinement into a Series of Functionalized Porous Zirconium MOFs: A Joint Experimental/Modelling Exploration. J. Phys. Chem. C 2013, 117, 11694–11704. [Google Scholar] [CrossRef]
- Pham, T.N.; Watson, S.A.; Edwards, A.J.; Chavda, M.; Clawson, J.S.; Strohmeier, M.; Vogt, F.G. Analysis of amorphous solid dispersions using 2D solid-state NMR and (1)H T(1) relaxation measurements. Mol. Pharm. 2010, 7, 1667–1691. [Google Scholar] [CrossRef]
- Ito, A.; Watanabe, T.; Yada, S.; Hamaura, T.; Nakagami, H.; Higashib, K.; Moribe, K.; Yamamoto, K. Prediction of recrystallization behavior of troglitazone/polyvinylpyrrolidone solid dispersion by solid-state NMR. Int. J. Pharm. 2010, 383, 18–23. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Su, Y.; Kun, E.; Zhang, F. Clay-Polymer Nanocomposites Prepared by Reactive Melt Extrusion for Sustained Drug Release. Pharmaceutics 2020, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Lin, X.; Xu, K.; He, J.; Yang, H.; Li, H. Co-grinding Effect on Crystalline Zaltoprofen with β-cyclodextrin/Cucurbit[7]uril in Tablet Formulation. Sci. Rep. 2017, 7, 45984. [Google Scholar] [CrossRef] [Green Version]
- Chierentin, L.; Garnero, C.; Chattah, A.K.; Delvadia, P.; Karnes, T.; Longhi, M.R.; Salgado, H.R. Influence of β-cyclodextrin on the Properties of Norfloxacin Form A. AAPS PharmSciTech 2014, 16, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Garnero, C.; Chattah, A.K.; Longhi, M. Improving furosemide polymorphs properties through supramolecular complexes of β-cyclodextrin. J. Pharm. Biomed. Anal. 2014, 95, 139–154. [Google Scholar] [CrossRef]
- McCoy, C.F.; Apperley, D.C.; Malcolm, R.; Variano, B.; Sussman, H.; Louven, D.; Boyd, P.; Malcolm, R.K. Solid state 13C NMR spectroscopy provides direct evidence for reaction between ethinyl estradiol and a silicone elastomer vaginal ring drug delivery system. Int. J. Pharm. 2018, 548, 689. [Google Scholar] [CrossRef] [Green Version]
- Wulff, M.; Aldén, M.; Tegenfeldt, J. Solid-State NMR Investigation of Indomethacin−Cyclodextrin Complexes in PEG 6000 Carrier. Bioconjug. Chem. 2002, 13, 240–248. [Google Scholar] [CrossRef] [PubMed]
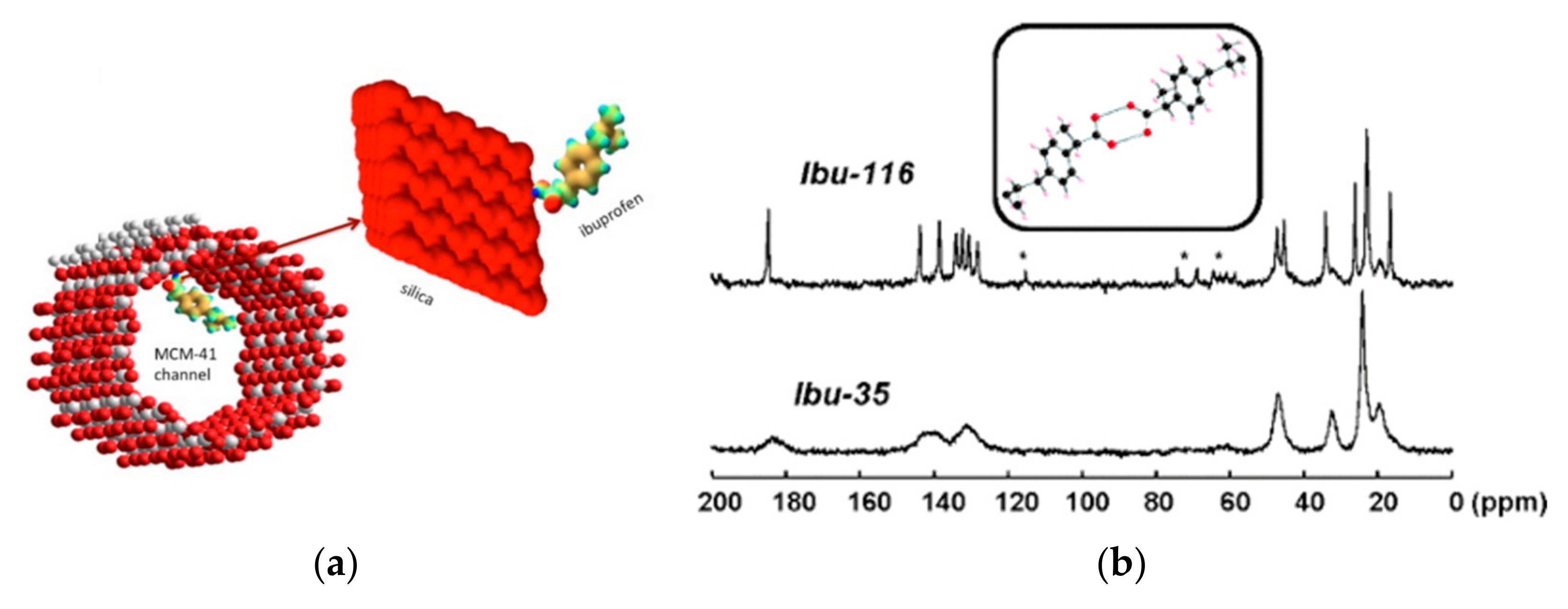
- Azaïs, T.; Tourné-Péteilh, C.; Aussenac, F.; Baccile, N.; Coelho, C.; Devoisselle, J.-M.; Babonneau, F. Solid-State NMR Study of Ibuprofen Confined in MCM-41 Material. Chem. Mater. 2006, 18, 6382–6390. [Google Scholar] [CrossRef]
- Čendak, T.; Žunkovič, E.; Godec, T.U.; Mazaj, M.; Logar, N.Z.; Mali, G. Indomethacin Embedded into MIL-101 Frameworks: A Solid-State NMR Study. J. Phys. Chem. C 2014, 118, 6140–6150. [Google Scholar] [CrossRef]
- Kirk, K.L. Fluorine in medicinal chemistry: Recent therapeutic applications of fluorinated small molecules. J. Fluor. Chem. 2006, 127, 1013–1029. [Google Scholar] [CrossRef]
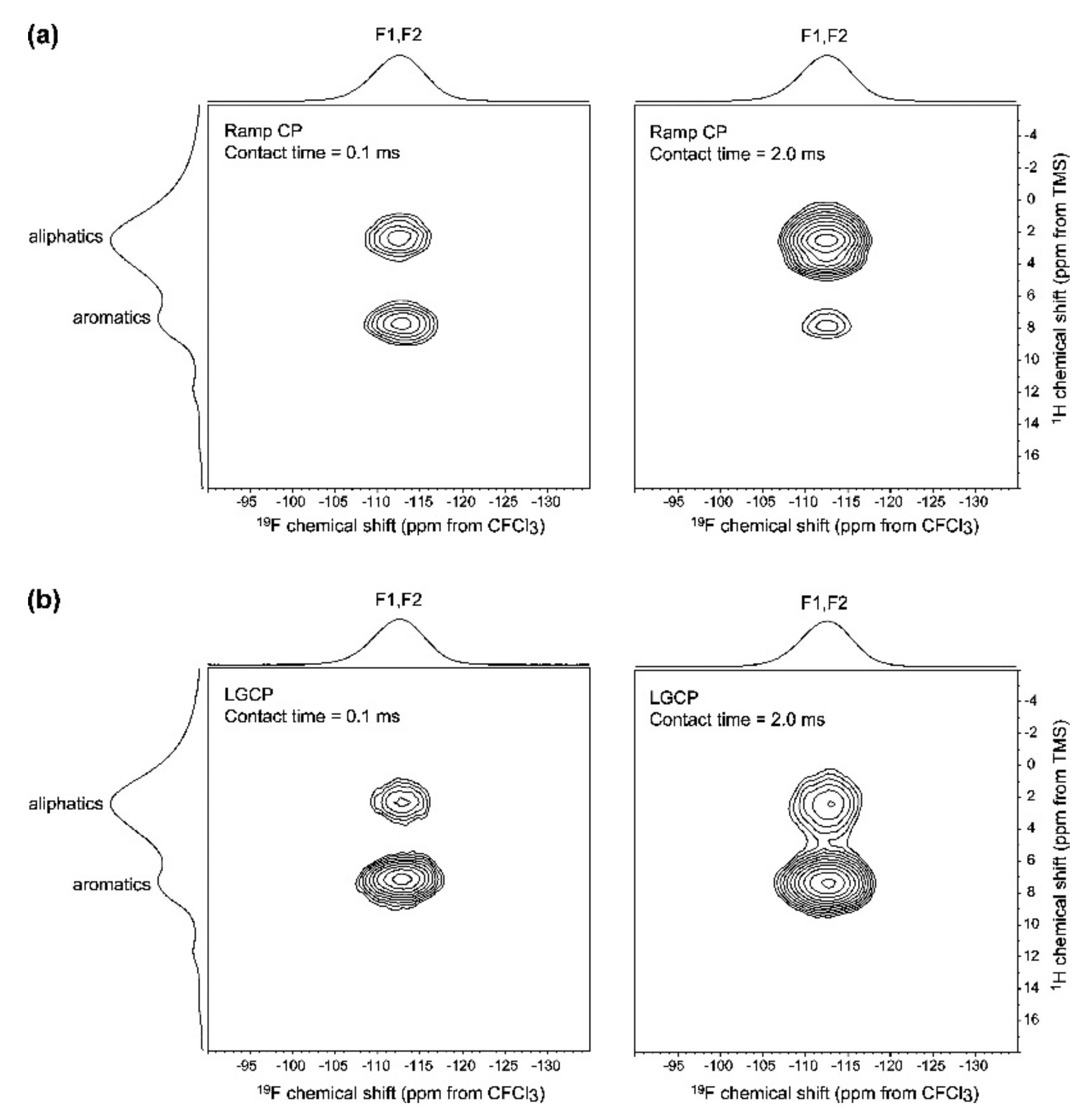
- Lau, S.; Stanhope, N.; Griffin, J.; Hughes, E.; Middleton, D.A. Drug orientations within statin-loaded lipoprotein nanoparticles by 19F solid-state NMR. Chem. Commun. 2019, 55, 13287–13290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechmann, M.; Hain, K.; Marichal, C.; Sebald, A. X-[1H, 19F] triple resonance with a X-[1H] CP MAS probe and characterisation of a 29Si-19F spin pair. Solid State Nucl. Magn. Reson. 2003, 23, 50–61. [Google Scholar] [CrossRef]
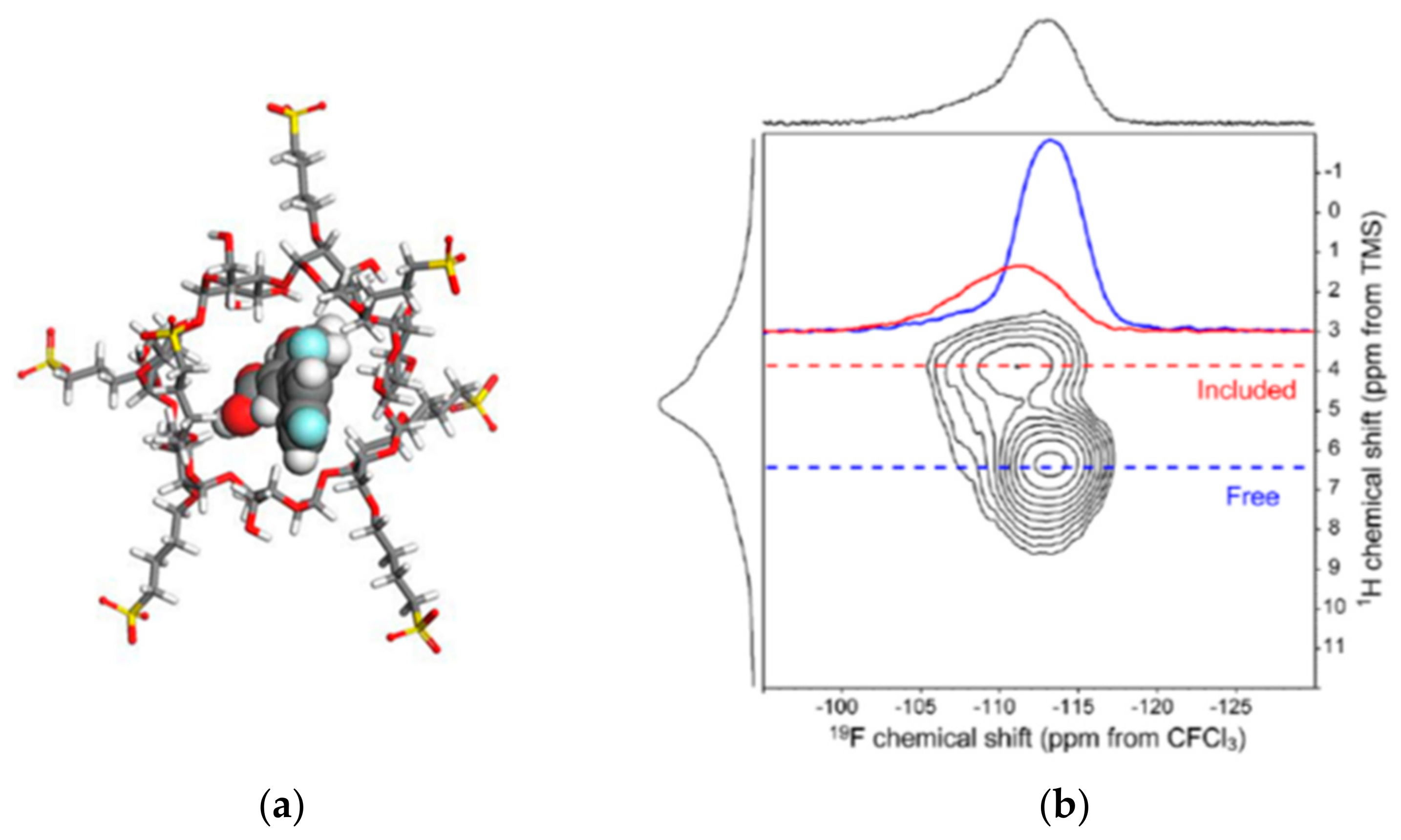
- Vogt, F.G.; Strohmeier, M. 2D Solid-State NMR Analysis of Inclusion in Drug—Cyclodextrin Complexes. Mol. Pharm. 2012, 9, 3357–3374. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Porcino, M.; Martineau-Corcos, C.; Guo, T.; Xiong, T.; Zhu, W.; Patriarche, G.; Péchoux, C.; Perronne, B.; Hassan, A.; et al. Efficient incorporation and protection of lansoprazole in cyclodextrin metal-organic frameworks. Int. J. Pharm. 2020, 585, 119442. [Google Scholar] [CrossRef] [PubMed]
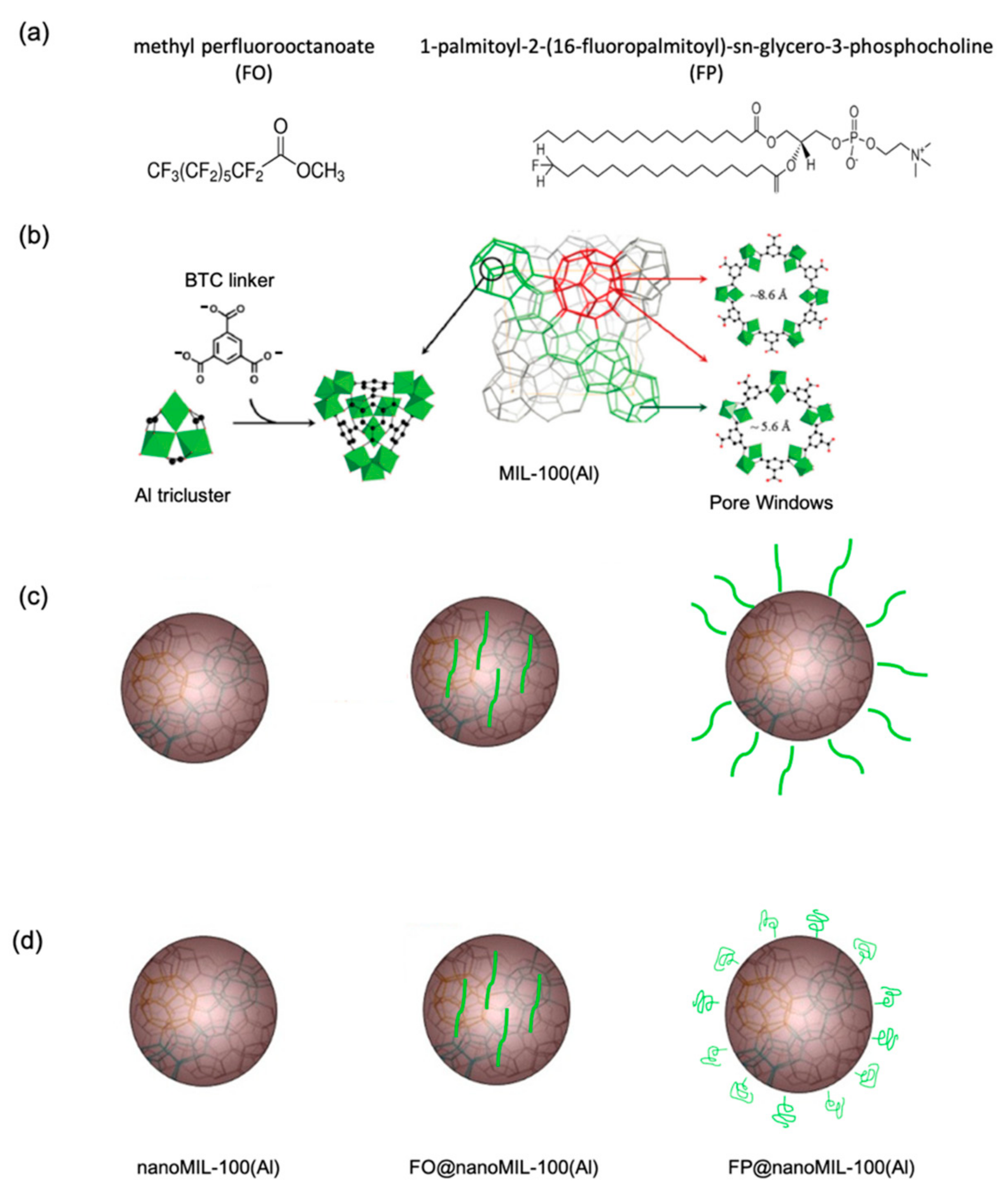
- Porcino, M.; Li, X.; Gref, R.; Martineau-Corcos, C. Solid state NMR spectroscopy as a powerful tool to investigate the location of fluorinated lipids in highly porous hybrid organic-inorganic nanoparticles. Magn. Reson. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Aina, V.; Areán, C.O.; Balas, F.; Cauda, V.; Colilla, M.; Delgado, M.R.; Vallet-Regí, M. Studies on MCM-41 mesoporous silica for drug delivery: Effect of particle morphology and amine functionalization. Chem. Eng. J. 2008, 137, 30–37. [Google Scholar] [CrossRef]
- Mellaerts, R.; Roeffaers, M.B.J.; Houthoofd, K.; Van Speybroeck, M.; De Cremer, G.; Jammaer, J.A.G.; Van den Mooter, G.; Augustijns, P.; Hofkens, J.; Martens, J.A. Molecular organization of hydrophobic molecules and co-adsorbed water in SBA-15 ordered mesoporous silica material. Phys. Chem. Chem. Phys. 2011, 13, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Azaïs, T.; Hartmeyer, G.; Quignard, S.; Laurent, G.; Tourné-Péteilh, C.; Devoisselle, J.-M.; Babonneau, F. Solid-state NMR characterization of drug-model molecules encapsulated in MCM-41 silica. Pure Appl. Chem. 2009, 81, 1345–1355. [Google Scholar] [CrossRef]
- Gao, L.; Sun, J.; Zhang, L.; Wang, J.; Ren, B. Influence of alkyl chains as a strategy for controlling drug delivery different structured channels of mesoporous silicate on pattern. Mater. Chem. Phys. 2012, 135, 786–797. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Degradable Drug Carriers: Vanishing Mesoporous Silica Nanoparticles. Chem. Mater. 2019, 31, 4364–4378. [Google Scholar] [CrossRef]
- Folliet, N.; Roiland, C.; Bégu, S.; Aubert, A.; Mineva, T.; Goursot, A.; Selvaraj, K.; Duma, L.; Tielens, F.; Mauri, F.; et al. Investigation of the interface in silica-encapsulated liposomes by combining solid state NMR and first principles calculations. J. Am. Chem. Soc. 2011, 133, 16815–16827. [Google Scholar] [CrossRef]
- Shestakova, P.; Martineau, C.; Mavrodinova, V.; Popova, M. Solid state NMR characterization of zeolite Beta based drug formulations containing Ag and sulfadiazine. RSC Adv. 2015, 5, 81957–81964. [Google Scholar] [CrossRef]
- Crutchfield, M.M.; Callis, C.F.; Irani, R.R.; Roth, G.C. Phosphorus Nuclear Magnetic Resonance Studies of Ortho and Condensed Phosphates. Inorg. Chem. 1962, 4, 813–817. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Rámila, A.; Pérez-Pariente, J.; Babonneau, F.; González-Calbet, J.M. Phosphorous-doped MCM-41 as bioactive material. Solid State Sci. 2005, 7, 233–237. [Google Scholar] [CrossRef]
- Nagashima, H.; Martineau-Corcos, C.; Tricot, G.; Trébosc, J.; Pourpoint, F.; Amoureux, J.-P.; Lafon, O. Recent Development in NMR Studies of Aluminophosphates. In Annual Reports on NMR Spectroscopy; Webb, G., Ed.; Academic Press: London, UK, 2018; Volume 94, Chapter 4. [Google Scholar]
- Porcino, M.; Christodoulou, I.; Le Vuong, M.D.; Gref, R.; Martineau-Corcos, C. New insights on the supramolecular structure of highly porous core-shell drug nanocarriers using solid-state NMR spectroscopy. RSC Adv. 2019, 4, 32472–32475. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Porcino, M.; Qiu, J.; Constantin, D.; Martineau-Corcos, C.; Gref, R. Doxorubicin-loaded metal-organic frameworks nanoparticles with engineered cyclodextrin coatings: Insights on drug location by solid state NMR spectroscopy. Nanomaterials 2021, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Malanga, M.; Manet, I.; Tuza, K.; Aykaç, A.; Ladavière, C.; Fenyvesi, E.; Vargas-Berenguel, A.; Gref, R.; Monti, S. Citric acid–γ-cyclodextrin crosslinked oligomers as carriers for doxorubicin delivery. Photochem. Photobiol. Sci. 2013, 12, 1841–1854. [Google Scholar] [CrossRef]
- Anand, R.; Borghi, F.; Manoli, F.; Manet, I.; Agostoni, V.; Reschiglian, P.; Gref, R.; Monti, S. Host–Guest Interactions in Fe(III)-Trimesate MOF Nanoparticles Loaded with Doxorubicin. J. Phys. Chem. B 2014, 118, 8532–8539. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcino, M.; Li, X.; Gref, R.; Martineau-Corcos, C. Solid-State NMR Spectroscopy: A Key Tool to Unravel the Supramolecular Structure of Drug Delivery Systems. Molecules 2021, 26, 4142. https://doi.org/10.3390/molecules26144142
Porcino M, Li X, Gref R, Martineau-Corcos C. Solid-State NMR Spectroscopy: A Key Tool to Unravel the Supramolecular Structure of Drug Delivery Systems. Molecules. 2021; 26(14):4142. https://doi.org/10.3390/molecules26144142
Chicago/Turabian StylePorcino, Marianna, Xue Li, Ruxandra Gref, and Charlotte Martineau-Corcos. 2021. "Solid-State NMR Spectroscopy: A Key Tool to Unravel the Supramolecular Structure of Drug Delivery Systems" Molecules 26, no. 14: 4142. https://doi.org/10.3390/molecules26144142





