Influence of Freezing and Different Drying Methods on Volatile Profiles of Strawberry and Analysis of Volatile Compounds of Strawberry Commercial Jams
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of HS-SPME
2.2. Application of the HS-SPME/GC-MS Method to Strawberry Fruit Samples
2.2.1. Esters
2.2.2. Furanones
2.2.3. Lactones
2.2.4. Terpenes
2.2.5. Aldehydes and Ketones
2.2.6. Alcohols and Acids
2.3. Application of the HS-SPME/GC-MS Method to Strawberry Jams
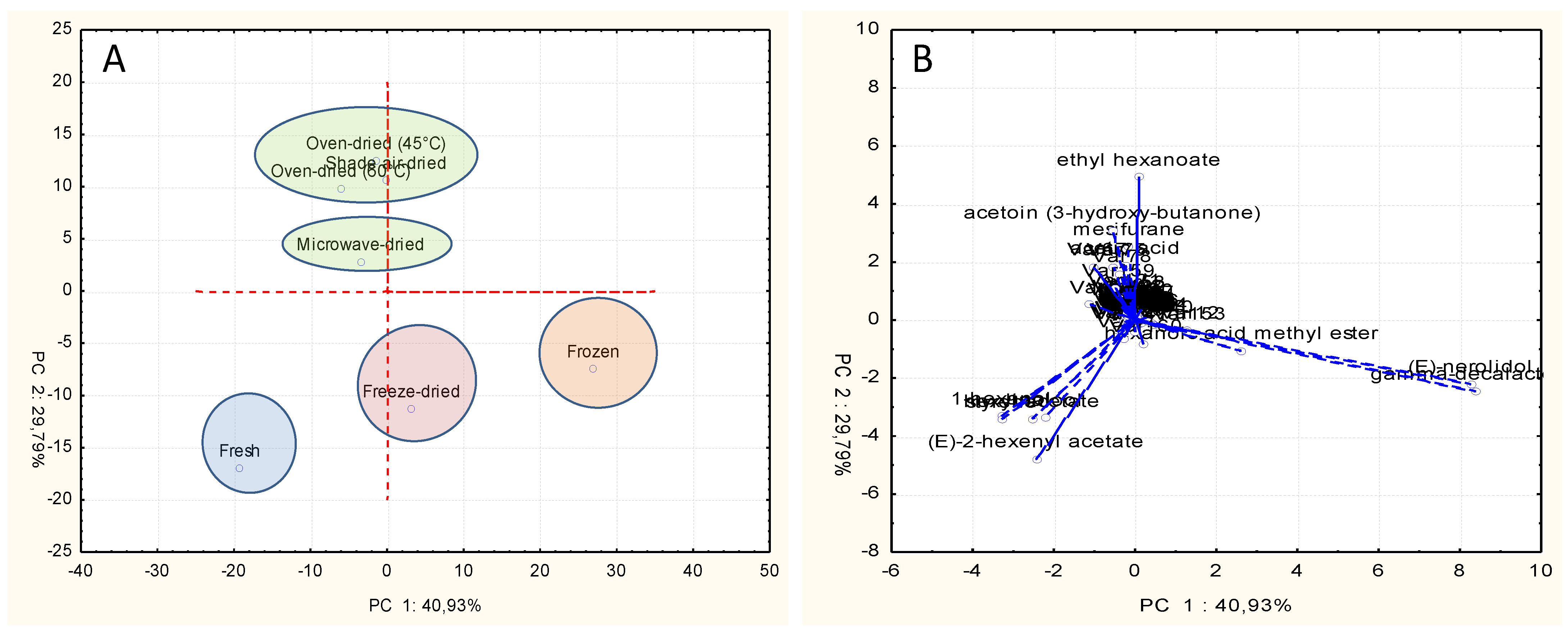
2.4. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Chemicals
3.2. Strawberry Samples
3.3. Headspace Solid-Phase Microextraction (HS-SPME)
3.4. GC-MS Analysis
3.5. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Neamtu, A.-A.; Szoke-Kovacs, R.; Mihok, E.; Georgescu, C.; Turcus, V.; Olah, N.K.; Frum, A.; Tita, O.; Neamtu, C.; Szoke-Kovacs, Z. Bilberry (Vaccinium myrtillus L.) extracts comparative analysis regarding their phytonutrient profiles, antioxidant capacity along with the in vivo rescue effects tested on a Drosophila melanogaster high-sugar diet model. Antioxidants 2020, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Dong, J.; Zhong, C.; Chang, L.; Wang, L. Comparison of aroma compounds of 3 newly-released strawberry cultivars and their parents. Acta Hortic. 2012, 926, 73–78. [Google Scholar] [CrossRef]
- Modise, D. Does freezing and thawing affect the volatile profile of strawberry fruit (Fragaria × ananassa Duch.)? Postharvest Biol. Technol. 2008, 50, 25–30. [Google Scholar] [CrossRef]
- Schwieterman, M.L.; Colquhoun, T.A.; Jaworski, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, H.J. Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS ONE 2014, 9, e88446. [Google Scholar] [CrossRef] [Green Version]
- Forney, C.F.; Kalt, W.; Jordan, M.A. The composition of strawberry aroma is influenced by cultivar, maturity, and storage. HortScience 2000, 35, 1022–1026. [Google Scholar] [CrossRef] [Green Version]
- Sadowska, A.; Świderski, F.; Hallmann, E. Bioactive, physicochemical and sensory properties as well as microstructure of organic strawberry powders obtained by various drying methods. Appl. Sci. 2020, 10, 4706. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of convective and vacuum-microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef] [Green Version]
- Abonyi, B.; Feng, H.; Tang, J.; Edwards, C.; Chew, B.; Mattinson, D.; Fellman, J. Quality retention in strawberry and carrot purees dried with Refractance WindowTM system. J. Food Sci. 2002, 67, 1051–1056. [Google Scholar] [CrossRef]
- Galmarini, M.V.; van Baren, C.; Zamora, M.C.; Chirife, J.; Di Leo Lira, P.; Bandoni, A. Impact of trehalose, sucrose and/or maltodextrin addition on aroma retention in freeze dried strawberry puree. Int. J. Food Sci. Technol. 2011, 46, 1337–1345. [Google Scholar] [CrossRef]
- Kopjar, M.; Hribar, J.; Simčič, M.; Zlatić, E.; Požrl, T.; Piližota, V. Effect of trehalose addition on volatiles responsible for strawberry aroma. Natl. Prod. Commun. 2013, 8, 1934578X1300801229. [Google Scholar] [CrossRef] [Green Version]
- De Boishebert, V.; Giraudel, J.-L.; Montury, M. Characterization of strawberry varieties by SPME–GC–MS and Kohonen self-organizing map. Chemom. Intell. Lab. Syst. 2006, 80, 13–23. [Google Scholar] [CrossRef]
- Reid, L.M.; O’Donnell, C.P.; Downey, G. Potential of SPME-GC and chemometrics to detect adulteration of soft fruit purees. J. Agric. Food Chem. 2004, 52, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Barren, D.; Etiévant, P. The volatile constituents of strawberry jam. Z. Lebensm.-Unters. Forsch. 1990, 191, 279–285. [Google Scholar] [CrossRef]
- Flores, G.; Ruiz del Castillo, M.L. Cancer-related constituents of strawberry jam as compared with fresh fruit. Cancers 2016, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Morales-Quintana, L.; Ramos, P. Chilean strawberry (Fragaria chiloensis): An integrative and comprehensive review. Food Res. Int. 2019, 119, 769–776. [Google Scholar] [CrossRef]
- Schieberle, P.; Hofmann, T. Evaluation of the character impact odorants in fresh strawberry juice by quantitative measurements and sensory studies on model mixtures. J. Agric. Food Chem. 1997, 45, 227–232. [Google Scholar] [CrossRef]
- Aharoni, A.; Giri, A.P.; Verstappen, F.W.; Bertea, C.M.; Sevenier, R.; Sun, Z.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 2004, 16, 3110–3131. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.; Ryan, D.; Guasca, A.O.; Marriott, P.; Pang, E. Analysis of strawberry volatiles using comprehensive two-dimensional gas chromatography with headspace solid-phase microextraction. J. Chromatogr. B 2005, 817, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Caprioli, G.; Maggi, F.; Vittori, S.; Sagratini, G. Comparative analysis of the volatile profiles from wild, cultivated, and commercial roots of Gentiana lutea L. by headspace solid phase microextraction (HS–SPME) coupled to gas chromatography mass spectrometry (GC–MS). Food Anal. Methods 2016, 9, 311–321. [Google Scholar] [CrossRef]
- Cannon, R.J.; Agyemang, D.; Curto, N.L.; Yusuf, A.; Chen, M.Z.; Janczuk, A.J. In-depth analysis of Ciflorette strawberries (Fragaria × ananassa ‘Ciflorette’) by multidimensional gas chromatography and gas chromatography-olfactometry. Flavour Fragr. J. 2015, 30, 302–319. [Google Scholar] [CrossRef]
- Ulrich, D.; Kecke, S.; Olbricht, K. What do we know about the chemistry of strawberry aroma? J. Agric. Food Chem. 2018, 66, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Jetti, R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef]
- Ulrich, D.; Komes, D.; Olbricht, K.; Hoberg, E. Diversity of aroma patterns in wild and cultivated Fragaria accessions. Genet. Resour. Crop Evol. 2007, 54, 1185–1196. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Tang, X.; Jin, W.; Han, Z. Differences in volatile ester composition between Fragaria × ananassa and F. vesca and implications for strawberry aroma patterns. Sci. Hortic. 2013, 150, 47–53. [Google Scholar] [CrossRef]
- Rohloff, J. Impact of agricultural and environmental factors on strawberry (Fragaria × ananassa Duch.) aroma—A review. Eur. J. Plant Sci. Biotechnol. 2011, 5, 17–34. [Google Scholar]
- Schreier, P. Quantitative composition of volatile constituents in cultivated strawberries, Fragaria ananassa cv. senga sengana, Senga Litessa and Senga Gourmella. J. Sci. Food Agric. 1980, 31, 487–494. [Google Scholar] [CrossRef]
- Lambert, Y.; Demazeau, G.; Largeteau, A.; Bouvier, J. Changes in aromatic volatile composition of strawberry after high pressure treatment. Food Chem. 1999, 67, 7–16. [Google Scholar] [CrossRef]
- Hirvi, T. Mass fragmentographic and sensory analyses in the evaluation of the aroma of some strawberry varieties. Lebensm. Wiss. Technol. 1983, 16, 157. [Google Scholar]
- Du, X.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of volatiles from two subtropical strawberry cultivars using GC–olfactometry, GC-MS odor activity values, and sensory analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef]
- Ulrich, D.; Hoberg, E.; Rapp, A.; Kecke, S. Analysis of strawberry flavour–discrimination of aroma types by quantification of volatile compounds. Z. Lebensm. Und-Forsch. A 1997, 205, 218–223. [Google Scholar] [CrossRef]
- Golaszewski, R.; Sims, C.; O’keefe, S.; Braddock, R.; Littell, R. Sensory attributes and volatile components of stored strawberry juice. J. Food Sci. 1998, 63, 734–738. [Google Scholar] [CrossRef]
- Hirvi, T. Stability of 2,5-dimethyl-4-hydroxy-3 (2H) furanone and 2,5-dimethyl-4-methoxy-3 (2H) furanone in aqueous buffer solutions. Sci. Technol. Aliment. 1980, 13, 324–325. [Google Scholar]
- Shu, C.K.; Mookherjee, B.D.; Ho, C.T. Volatile components of the thermal degradation of 2,5-dimethyl-4-hydroxy-3 (2H)-furanone. J. Agric. Food Chem. 1985, 33, 446–448. [Google Scholar] [CrossRef]
- Nuzzi, M.; Scalzo, R.L.; Testoni, A.; Rizzolo, A. Evaluation of fruit aroma quality: Comparison between Gas Chromatography–Olfactometry (GC–O) and Odour Activity Value (OAV) aroma patterns of strawberries. Food Anal. Methods 2008, 1, 270–282. [Google Scholar] [CrossRef]
- Li, L.; Luo, Z.; Huang, X.; Zhang, L.; Zhao, P.; Ma, H.; Li, X.; Ban, Z.; Liu, X. Label-free quantitative proteomics to investigate strawberry fruit proteome changes under controlled atmosphere and low temperature storage. J. Proteom. 2015, 120, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Cheng, S.; Zhang, Y.; Du, B.; Feng, C.; Zhou, Y.; Mei, X.; Jiang, Y.; Duan, X.; Yang, Z. Differential responses of four biosynthetic pathways of aroma compounds in postharvest strawberry (Fragaria × ananassa Duch.) under interaction of light and temperature. Food Chem. 2017, 221, 356–364. [Google Scholar] [CrossRef]
- Pelayo-Zaldívar, C.; Ebeler, S.E.; Kader, A.A. Cultivar and harvest date effects on flavor and other quality attributes of California strawberries. J. Food Qual. 2005, 28, 78–97. [Google Scholar] [CrossRef]
- Mesias, M.; Olombrada, E.; González-Mulero, L.; Morales, F.J.; Delgado-Andrade, C. Investigation on heat-induced chemical indexes in traditional and reformulated biscuits. J. Food Compos. Anal. 2021, 103963. [Google Scholar] [CrossRef]
- Kafkas, E.; Kafkas, S.; Koch-Dean, M.; Schwab, W.; Larkov, O.; Lavid, N.; Bar, E.; Ravid, U.; Lewinsohn, E. Comparison of methodologies for the identification of aroma compounds in strawberry. Turk. J. Agric. For. 2005, 29, 383–390. [Google Scholar]
- Komes, D.; Lovrić, T.; Kovačević Ganić, K.; Gracin, L. Study of trehalose addition on aroma retention in dehydrated strawberry puree. Food Technol. Biotechnol. 2003, 41, 111–119. [Google Scholar]
- Siegmund, B.; Derler, K.; Pfannhauser, W. Changes in the aroma of a strawberry drink during storage. J. Agric. Food Chem. 2001, 49, 3244–3252. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Iuhas, C.I.; Ayvaz, H.; Rugină, D.; Stanilă, A.; Dulf, F.; Bunea, A.; Socaci, S.A.; Socaciu, C.; Pintea, A. Phytochemical characterization of commercial processed blueberry, blackberry, blackcurrant, cranberry, and raspberry and their antioxidant activity. Antioxidants 2019, 8, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellami, I.H.; Wannes, W.A.; Bettaieb, I.; Berrima, S.; Chahed, T.; Marzouk, B.; Limam, F. Qualitative and quantitative changes in the essential oil of Laurus nobilis L. leaves as affected by different drying methods. Food Chem. 2011, 126, 691–697. [Google Scholar] [CrossRef]
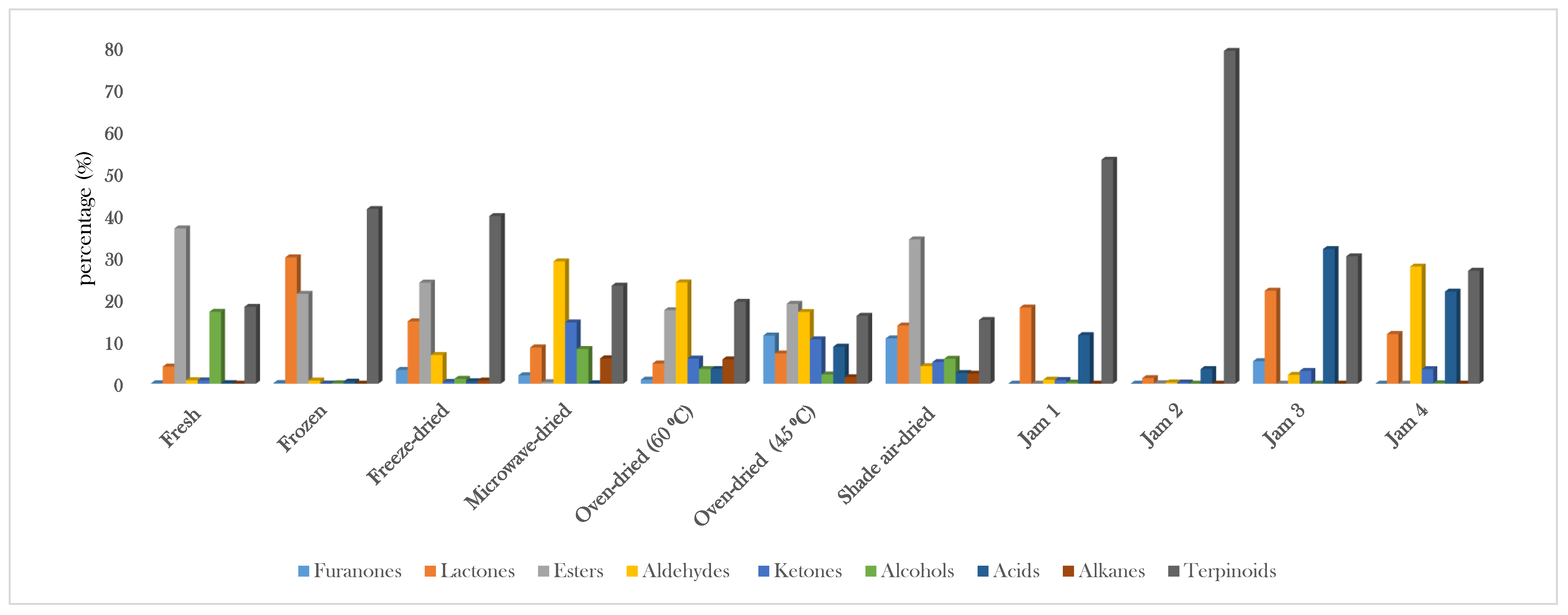


| No. | Compound Name and Class | RI Calc. | Fresh | Frozen | Freeze-Dried | Microwave-Dried | Oven-Dried (60 °C) | Oven-Dried (45 °C) | Shade Air-Dried | Jam 1 | Jam 2 | Jam 3 | Jam 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Furanones | 0.10 | 0.15 | 3.27 | 1.99 | 0.94 | 11.44 | 10.77 | 0.00 | 0.00 | 5.34 | 0.00 | ||
| 1 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | 976 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | Dihydro-5-methyl-5-vinyl-2(3H)-furanone | 1037 | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | 0.00 | 0.38 | 0.00 | 0.00 | 0.00 | 0.00 |
| 3 | Mesifurane | 1058 | 0.08 | 0.13 | 3.23 | 1.58 | 0.81 | 9.30 | 8.47 | 0.00 | 0.00 | 5.34 | 0.00 |
| 4 | Furaneol | 1066 | 0.02 | 0.02 | 0.04 | 0.00 | 0.02 | 1.89 | 1.92 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lactones | 4.07 | 30.05 | 14.82 | 8.62 | 4.80 | 7.18 | 13.83 | 18.12 | 1.32 | 22.11 | 11.81 | ||
| 5 | γ-Butyrolactone | 923 | 3.11 | 0.00 | 1.85 | 4.72 | 3.15 | 1.67 | 4.33 | 0.67 | 0.19 | 0.00 | 3.48 |
| 6 | γ-Caprolactone | 1050 | 0.00 | 0.00 | 0.09 | 0.09 | 0.21 | 0.00 | 0.16 | 0.00 | 0.00 | 0.00 | 0.05 |
| 7 | δ-caprolactone | 1088 | 0.00 | 0.00 | 0.14 | 0.42 | 0.09 | 0.34 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 |
| 8 | γ-Octalactone | 1250 | 0.02 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 9 | δ-Octalactone | 1276 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.04 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | γ-Nonalactone | 1353 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 |
| 11 | γ-Decalactone | 1459 | 0.89 | 26.08 | 12.14 | 3.15 | 1.28 | 4.78 | 8.60 | 15.22 | 0.35 | 20.76 | 7.47 |
| 12 | γ-Dodecalactone | 1677 | 0.04 | 3.97 | 0.49 | 0.24 | 0.06 | 0.35 | 0.54 | 2.23 | 0.78 | 1.35 | 0.81 |
| Esters | 36.96 | 21.38 | 24.05 | 0.35 | 17.48 | 19.00 | 34.34 | 0.00 | 0.10 | 0.00 | 0.00 | ||
| 13 | Ethyl acetate | 610 | 2.56 | 0.46 | 0.29 | 0.00 | 0.00 | 0.00 | 2.50 | 0.00 | 0.00 | 0.00 | 0.00 |
| 14 | Methyl butanoate | 721 | 0.24 | 0.46 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 15 | Methyl 2-methylbutanoate | 777 | 0.00 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 16 | Ethyl butanoate | 804 | 0.11 | 0.99 | 0.04 | 0.00 | 0.27 | 0.06 | 1.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| 17 | Butyl acetate | 816 | 0.02 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 18 | Isobutyl butyrate | 843 | 0.01 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 19 | Ethyl 2-methylbutanoate | 850 | 0.00 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.51 | 0.00 | 0.00 | 0.00 | 0.00 |
| 20 | Methyl (S)-(+)-3-hydroxybutyrate | 853 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 1.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 21 | 2-Methylbutyl acetate | 880 | 0.01 | 0.04 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 22 | Methyl hexanoate | 925 | 0.12 | 8.00 | 1.95 | 0.00 | 0.25 | 0.37 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 |
| 23 | Ethyl 3-hydroxybutyrate | 936 | 0.00 | 0.00 | 0.00 | 0.00 | 0.27 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 |
| 24 | Ethyl hexanoate | 1000 | 2.17 | 4.65 | 0.79 | 0.11 | 9.91 | 10.89 | 18.54 | 0.00 | 0.00 | 0.00 | 0.00 |
| 25 | Hexyl acetate | 1014 | 12.68 | 0.82 | 4.66 | 0.00 | 0.63 | 0.76 | 1.52 | 0.00 | 0.00 | 0.00 | 0.00 |
| 26 | (E)-2-Hexenyl acetate | 1016 | 13.70 | 1.15 | 10.76 | 0.00 | 0.73 | 0.75 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 |
| 27 | (Z)-2-Hexenyl acetate | 1020 | 1.52 | 0.00 | 1.82 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| 28 | Isopropyl hexanoate | 1037 | 0.01 | 0.48 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 29 | Ethyl 2-hexenoate | 1043 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 |
| 30 | Formic acid octyl ester | 1069 | 1.51 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 31 | Propyl hexanoate | 1093 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 |
| 32 | Acetic acid heptyl ester | 1111 | 0.11 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 33 | Methyl octanoate | 1122 | 0.02 | 1.35 | 0.59 | 0.00 | 0.05 | 0.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 34 | Ethyl 3-hydroxyhexanoate | 1124 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 |
| 35 | Hexanoic acid-2- methyl propyl ester | 1148 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.29 | 0.00 | 0.00 | 0.00 | 0.00 |
| 36 | Benzoic acid ethyl ester | 1165 | 0.06 | 0.02 | 0.05 | 0.00 | 0.29 | 0.23 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 |
| 37 | Methyl salicylate | 1187 | 0.02 | 0.00 | 0.18 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 38 | Butanoic acid hexyl ester | 1188 | 0.48 | 0.02 | 0.00 | 0.00 | 0.51 | 0.12 | 0.68 | 0.00 | 0.00 | 0.00 | 0.00 |
| 39 | (E)-Butanoic acid-2-hexenyl ester | 1192 | 0.11 | 0.00 | 0.00 | 0.00 | 0.19 | 0.04 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| 40 | Ethyl octanoate | 1193 | 0.01 | 0.59 | 0.28 | 0.00 | 0.54 | 1.16 | 1.76 | 0.00 | 0.00 | 0.00 | 0.00 |
| 41 | Octyl acetate | 1207 | 0.99 | 0.65 | 0.69 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 42 | 1,4 butanediol diacetate | 1212 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 43 | Isopropyl octanoate | 1229 | 0.01 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 44 | Butanoic acid-3-methylhexyl ester | 1237 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 |
| 45 | Isopentyl hexanoate | 1245 | 0.01 | 0.00 | 0.00 | 0.00 | 0.95 | 0.25 | 0.54 | 0.00 | 0.00 | 0.00 | 0.00 |
| 46 | Pentyl hexanoate | 1282 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| 47 | (Z)-3-Nonenyl acetate | 1288 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 48 | Ethyl nonanoate | 1290 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 |
| 49 | n-Nonyl acetate | 1304 | 0.14 | 0.08 | 0.31 | 0.00 | 0.35 | 0.38 | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 |
| 50 | Methyl decanoate | 1317 | 0.01 | 0.31 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 51 | 1,3 Pentanediol 2,2,4 trimethyl 1 isobutyrate | 1364 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 52 | cis-Methyl cinnamate | 1373 | 0.01 | 0.00 | 0.31 | 0.00 | 0.23 | 0.11 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 |
| 53 | trans-Methyl cinnamate | 1373 | 0.01 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 54 | Hexyl hexanoate | 1379 | 0.04 | 0.05 | 0.28 | 0.08 | 0.74 | 0.33 | 0.55 | 0.00 | 0.00 | 0.00 | 0.00 |
| 55 | (2E)-2-Hexenyl hexanoate | 1381 | 0.05 | 0.06 | 0.03 | 0.16 | 0.38 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 56 | Octyl butyrate | 1382 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.36 | 0.00 | 0.00 | 0.00 | 0.00 |
| 57 | Ethyl decanoate | 1388 | 0.00 | 0.14 | 0.05 | 0.00 | 0.22 | 0.33 | 0.32 | 0.00 | 0.00 | 0.00 | 0.00 |
| 58 | Ethyl cinnamate | 1457 | 0.01 | 0.13 | 0.00 | 0.00 | 0.97 | 1.10 | 2.63 | 0.00 | 0.00 | 0.00 | 0.00 |
| 59 | Propyl decanoate | 1522 | 0.01 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 60 | Octyl hexanoate | 1581 | 0.01 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 |
| 61 | Methyl tetradecanoate | 1722 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 62 | Ethyl-9-tetradecanoate | 1771 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 |
| 63 | Ethyl tetradecanoate | 1792 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.75 | 0.00 | 0.00 | 0.00 | 0.00 |
| 64 | Ethyl hexadecanoate | 1985 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 |
| Aldehydes | 0.82 | 0.74 | 6.81 | 29.09 | 24.10 | 17.05 | 4.16 | 0.94 | 0.30 | 2.10 | 27.86 | ||
| 65 | 3-Methyl butanal | 656 | 0.00 | 0.00 | 0.07 | 0.22 | 0.28 | 0.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 66 | Hexanal | 801 | 0.14 | 0.11 | 0.15 | 1.25 | 0.32 | 0.50 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 |
| 67 | Furfural | 820 | 0.00 | 0.00 | 0.00 | 3.41 | 11.75 | 0.44 | 0.00 | 0.59 | 0.21 | 0.68 | 17.22 |
| 68 | (E)-2-Hexenal | 851 | 0.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 69 | Heptanal | 902 | 0.00 | 0.00 | 0.12 | 0.11 | 0.22 | 0.17 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| 70 | (Z)-2-Heptenal | 955 | 0.00 | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 71 | Benzaldehyde | 955 | 0.20 | 0.30 | 3.94 | 10.80 | 0.00 | 0.47 | 0.86 | 0.33 | 0.08 | 1.15 | 9.68 |
| 72 | Octanal | 1002 | 0.00 | 0.00 | 0.00 | 1.26 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 73 | Benzeneacetaldehyde | 1039 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 |
| 74 | (E)-2-Octenal | 1055 | 0.00 | 0.06 | 0.00 | 0.00 | 0.77 | 0.18 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 75 | Nonanal | 1101 | 0.18 | 0.17 | 1.83 | 4.27 | 4.37 | 6.48 | 1.76 | 0.00 | 0.00 | 0.25 | 0.37 |
| 76 | (2E,4E)-2,4-Octadienal | 1104 | 0.00 | 0.00 | 0.00 | 0.46 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 77 | (E)-2-Nonenal | 1155 | 0.05 | 0.01 | 0.05 | 0.45 | 0.66 | 1.27 | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 |
| 78 | Decanal | 1200 | 0.01 | 0.09 | 0.60 | 1.74 | 3.81 | 5.54 | 0.92 | 0.00 | 0.00 | 0.00 | 0.00 |
| 79 | 3,4-Dimethyl benzaldehyde | 1205 | 0.00 | 0.00 | 0.00 | 3.96 | 1.02 | 0.98 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 80 | 5-Hydroxymethylfurfural | 1220 | 0.00 | 0.00 | 0.00 | 0.06 | 0.15 | 0.04 | 0.00 | 0.02 | 0.01 | 0.02 | 0.50 |
| 81 | (E)-2-Decenal | 1255 | 0.00 | 0.00 | 0.00 | 0.96 | 0.16 | 0.06 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 |
| 82 | Undecanal | 1299 | 0.00 | 0.00 | 0.05 | 0.00 | 0.06 | 0.35 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 83 | 2-Undecenal | 1355 | 0.00 | 0.00 | 0.00 | 0.14 | 0.06 | 0.01 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ketones | 0.78 | 0.02 | 0.43 | 14.57 | 5.97 | 10.54 | 5.14 | 0.87 | 0.28 | 3.03 | 3.45 | ||
| 84 | 3-Pentanone | 701 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 85 | Acetoin | 715 | 0.00 | 0.00 | 0.00 | 0.00 | 5.43 | 9.25 | 4.85 | 0.00 | 0.00 | 0.00 | 0.00 |
| 86 | 2-Methyl-1-penten-3-one | 767 | 0.00 | 0.00 | 0.00 | 1.29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 87 | 2-Heptanone | 891 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.80 | 0.00 |
| 88 | 2-Acetoxy-3-butanone | 896 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.09 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 |
| 89 | 4-Methyl-2-Heptanone | 938 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 90 | 1-Octen-3-one | 977 | 0.00 | 0.00 | 0.04 | 6.42 | 0.20 | 0.32 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 91 | 2-Methyl-3-octanone | 984 | 0.00 | 0.00 | 0.06 | 0.91 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 92 | 6-Methyl-5-hepten-2-one | 986 | 0.16 | 0.02 | 0.26 | 4.97 | 0.27 | 0.88 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 |
| 93 | 2H-Pyran-2,6(3H)-dione | 998 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.87 | 0.28 | 0.00 | 0.00 |
| 94 | Acetophenone | 1060 | 0.59 | 0.00 | 0.00 | 0.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.64 |
| 95 | 2-Furyl hydroxy methyl ketone | 1077 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.81 |
| 96 | 2-Nonanone | 1090 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.23 | 0.00 |
| 97 | 6-Methyl-3,5-heptadiene-2-one | 1110 | 0.00 | 0.00 | 0.00 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 98 | Ketoisophorone | 1138 | 0.00 | 0.00 | 0.07 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Alcohols | 17.10 | 0.09 | 1.15 | 8.24 | 3.46 | 2.18 | 5.91 | 0.25 | 0.00 | 0.00 | 0.09 | ||
| 99 | 2-Pentanol | 730 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 100 | 3-Methyl-1-Butanol | 732 | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.61 | 0.00 | 0.00 | 0.00 | 0.00 |
| 101 | 1-Pentanol | 764 | 0.07 | 0.00 | 0.07 | 0.08 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 102 | 2,3-Butandiol | 789 | 0.00 | 0.00 | 0.00 | 0.00 | 1.37 | 1.40 | 0.93 | 0.00 | 0.00 | 0.00 | 0.00 |
| 103 | (Z)-3-Hexen-1-ol | 854 | 0.16 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 104 | (E)-2-Hexen-1-ol | 866 | 0.75 | 0.04 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 105 | 1-Hexanol | 869 | 14.37 | 0.03 | 0.35 | 0.00 | 0.00 | 0.00 | 1.14 | 0.25 | 0.00 | 0.00 | 0.00 |
| 106 | 1-Heptanol | 970 | 0.23 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 107 | 1-Octen-3-ol | 979 | 0.00 | 0.00 | 0.04 | 6.42 | 0.88 | 0.30 | 0.26 | 0.00 | 0.00 | 0.00 | 0.00 |
| 108 | Benzyl alcohol | 1030 | 0.00 | 0.00 | 0.13 | 0.00 | 0.28 | 0.21 | 0.23 | 0.00 | 0.00 | 0.00 | 0.00 |
| 109 | (Z)-2-Octen-1-ol | 1066 | 0.03 | 0.00 | 0.04 | 1.10 | 0.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 110 | 1-Octanol | 1070 | 1.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 111 | Phenyl ethyl alcohol | 1105 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.42 | 0.00 | 0.00 | 0.00 | 0.09 |
| 112 | 1-Nonanol | 1167 | 0.22 | 0.00 | 0.17 | 0.31 | 0.24 | 0.13 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| 113 | (Z)-2,6-octadien-1-ol 3,7dimethyl | 1222 | 0.00 | 0.00 | 0.23 | 0.33 | 0.21 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 114 | 3-phenylpropanol | 1222 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 | 0.00 | 0.00 | 0.00 | 0.00 |
| Acids | 0.12 | 0.48 | 0.58 | 0.13 | 3.48 | 8.79 | 2.51 | 11.54 | 3.48 | 32.03 | 21.89 | ||
| 115 | Acetic acid | 604 | 0.06 | 0.21 | 0.00 | 0.00 | 2.15 | 7.83 | 1.69 | 9.98 | 3.48 | 0.00 | 3.63 |
| 116 | 2-Methyl butanoic acid | 861 | 0.04 | 0.00 | 0.03 | 0.00 | 0.44 | 0.19 | 0.09 | 0.00 | 0.00 | 1.23 | 0.00 |
| 117 | Hexanoic acid | 997 | 0.02 | 0.27 | 0.42 | 0.02 | 0.89 | 0.20 | 0.64 | 1.56 | 0.00 | 19.96 | 17.59 |
| 118 | Octanoic acid | 1170 | 0.00 | 0.00 | 0.13 | 0.11 | 0.00 | 0.57 | 0.09 | 0.00 | 0.00 | 10.84 | 0.67 |
| Alkanes | 0.00 | 0.00 | 0.74 | 6.01 | 5.78 | 1.53 | 2.36 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 119 | 2,3-Dimethyl hexane | 761 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 120 | Octane | 820 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 121 | 4-Methyl octane | 861 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 122 | Nonane | 900 | 0.00 | 0.00 | 0.05 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 123 | Decane | 998 | 0.00 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 124 | Octyl cyclopropane | 1069 | 0.00 | 0.00 | 0.43 | 0.00 | 1.20 | 0.38 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 125 | Dodecane | 1195 | 0.00 | 0.00 | 0.00 | 2.72 | 2.87 | 0.69 | 0.98 | 0.00 | 0.00 | 0.00 | 0.00 |
| 126 | Tetradecane | 1391 | 0.00 | 0.00 | 0.07 | 2.37 | 1.59 | 0.39 | 1.28 | 0.00 | 0.00 | 0.00 | 0.00 |
| 127 | Hexadecane | 1593 | 0.00 | 0.00 | 0.14 | 0.55 | 0.10 | 0.06 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| 128 | Heptadecane | 1695 | 0.00 | 0.00 | 0.05 | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Terpenoids | 18.28 | 41.57 | 39.89 | 23.32 | 19.48 | 16.14 | 15.14 | 53.31 | 79.24 | 30.31 | 26.86 | ||
| 129 | Sabinen | 971 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 130 | β-Myrcene | 989 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.47 | 0.00 | 0.00 |
| 131 | d-Limonene | 1024 | 0.03 | 0.00 | 0.51 | 0.23 | 0.29 | 0.16 | 0.07 | 0.19 | 58.05 | 0.00 | 0.15 |
| 132 | β-Ocimene | 1046 | 0.02 | 0.06 | 0.24 | 0.17 | 0.17 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 133 | Terpinolene | 1083 | 0.02 | 0.15 | 0.31 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 134 | cis-Linalol oxide | 1084 | 0.00 | 0.00 | 0.00 | 0.00 | 0.45 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.83 |
| 135 | Linalol | 1096 | 11.96 | 2.30 | 16.43 | 7.15 | 6.99 | 2.46 | 4.40 | 0.70 | 0.00 | 5.18 | 0.49 |
| 136 | Terpinen-4-ol | 1172 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 137 | α-Terpineol | 1183 | 0.24 | 0.05 | 1.72 | 2.10 | 1.45 | 0.57 | 0.35 | 3.46 | 7.98 | 8.75 | 2.09 |
| 138 | Myrtenol | 1188 | 0.67 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 139 | Geraniol | 1248 | 0.01 | 0.00 | 0.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 140 | Myrtenyl acetate | 1317 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 141 | α-Cubebene | 1366 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.38 | 0.00 | 0.00 |
| 142 | Caryophyllene | 1408 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.24 | 0.00 | 0.00 |
| 143 | trans-Geranyl acetone | 1445 | 0.00 | 0.04 | 0.50 | 1.30 | 0.26 | 0.82 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 |
| 144 | cis-β-Farnesene | 1450 | 0.05 | 1.50 | 0.77 | 0.23 | 0.17 | 0.32 | 0.40 | 0.00 | 0.00 | 0.00 | 0.23 |
| 145 | α-Curcumene | 1476 | 0.00 | 0.04 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 146 | (Z,E)-α-Farnesene | 1489 | 0.00 | 0.33 | 0.22 | 0.00 | 0.07 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 147 | α-Muurolene | 1493 | 0.02 | 0.00 | 0.34 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 148 | α-Farnesene | 1500 | 0.01 | 1.10 | 0.50 | 0.35 | 0.29 | 0.47 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 |
| 149 | (Z)-γ-Bisabolene | 1508 | 0.00 | 0.07 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 150 | δ-Cadenine | 1519 | 0.00 | 0.00 | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 151 | β-Sesquiphellandrene | 1519 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 152 | (E)-γ-Bisabolene | 1529 | 0.00 | 0.05 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 153 | (E)-α-Bisabolene | 1542 | 0.00 | 0.13 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 |
| 154 | Myrtenyl-2-methyl butyrate | 1556 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 155 | (E)-Nerolidol | 1563 | 5.13 | 31.17 | 17.14 | 11.04 | 8.97 | 10.78 | 9.65 | 40.99 | 9.94 | 13.75 | 17.92 |
| 156 | Bisabolol oxide II | 1655 | 0.00 | 0.03 | 0.06 | 0.62 | 0.15 | 0.06 | 0.04 | 5.32 | 1.51 | 2.00 | 3.27 |
| 157 | α-Bisabolol | 1683 | 0.01 | 3.97 | 0.00 | 0.11 | 0.08 | 0.09 | 0.00 | 2.65 | 0.67 | 0.63 | 1.63 |
| 158 | Nerolidyl acetate | 1711 | 0.00 | 0.25 | 0.00 | 0.02 | 0.00 | 0.10 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| 159 | Farnesol | 1720 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Others | 14.56 | 0.33 | 5.14 | 4.53 | 4.17 | 1.39 | 1.72 | 0.00 | 2.09 | 0.22 | 0.00 | ||
| 160 | Styrene | 887 | 14.29 | 0.02 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.09 | 0.22 | 0.00 |
| 161 | 2-Pentyl furan | 990 | 0.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 162 | Pyranone | 1135 | 0.00 | 0.00 | 0.00 | 0.09 | 0.28 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 163 | m-Di-tert-butyl benzene | 1247 | 0.00 | 0.00 | 0.00 | 4.43 | 3.75 | 1.00 | 1.56 | 0.00 | 0.00 | 0.00 | 0.00 |
| 164 | 1-Methoxy-4-(1-propenyl) benzene | 1277 | 0.00 | 0.00 | 5.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 165 | Sulfur | 2049 | 0.03 | 0.31 | 0.00 | 0.01 | 0.14 | 0.39 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total identified | 92.79 | 94.81 | 96.88 | 96.85 | 89.66 | 95.24 | 95.88 | 85.03 | 86.81 | 95.14 | 91.96 |
| Jam | Ingredients (100 g Jam Product) |
|---|---|
| Jam 1 (Coop Vivi verde jam) | 60 g strawberry, sugar, lemon juice, pectin, citric acid |
| Jam 2 (Coop jam) | 50 g strawberry, sugar, lemon juice, pectin, citric acid, elderberry juice |
| Jam 3 (Zuegg jam) | 50 g strawberry, sugar, lemon juice, pectin |
| Jam 4 (Alce Nero jam) | 100 g strawberry, lemon juice, grapes juice |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abouelenein, D.; Mustafa, A.M.; Angeloni, S.; Borsetta, G.; Vittori, S.; Maggi, F.; Sagratini, G.; Caprioli, G. Influence of Freezing and Different Drying Methods on Volatile Profiles of Strawberry and Analysis of Volatile Compounds of Strawberry Commercial Jams. Molecules 2021, 26, 4153. https://doi.org/10.3390/molecules26144153
Abouelenein D, Mustafa AM, Angeloni S, Borsetta G, Vittori S, Maggi F, Sagratini G, Caprioli G. Influence of Freezing and Different Drying Methods on Volatile Profiles of Strawberry and Analysis of Volatile Compounds of Strawberry Commercial Jams. Molecules. 2021; 26(14):4153. https://doi.org/10.3390/molecules26144153
Chicago/Turabian StyleAbouelenein, Doaa, Ahmed M. Mustafa, Simone Angeloni, Germana Borsetta, Sauro Vittori, Filippo Maggi, Gianni Sagratini, and Giovanni Caprioli. 2021. "Influence of Freezing and Different Drying Methods on Volatile Profiles of Strawberry and Analysis of Volatile Compounds of Strawberry Commercial Jams" Molecules 26, no. 14: 4153. https://doi.org/10.3390/molecules26144153
APA StyleAbouelenein, D., Mustafa, A. M., Angeloni, S., Borsetta, G., Vittori, S., Maggi, F., Sagratini, G., & Caprioli, G. (2021). Influence of Freezing and Different Drying Methods on Volatile Profiles of Strawberry and Analysis of Volatile Compounds of Strawberry Commercial Jams. Molecules, 26(14), 4153. https://doi.org/10.3390/molecules26144153












