Abstract
Strigolactones (SLs) are a class of sesquiterpenoid plant hormones that play a role in the response of plants to various biotic and abiotic stresses. When released into the rhizosphere, they are perceived by both beneficial symbiotic mycorrhizal fungi and parasitic plants. Due to their multiple roles, SLs are potentially interesting agricultural targets. Indeed, the use of SLs as agrochemicals can favor sustainable agriculture via multiple mechanisms, including shaping root architecture, promoting ideal branching, stimulating nutrient assimilation, controlling parasitic weeds, mitigating drought and enhancing mycorrhization. Moreover, over the last few years, a number of studies have shed light onto the effects exerted by SLs on human cells and on their possible applications in medicine. For example, SLs have been demonstrated to play a key role in the control of pathways related to apoptosis and inflammation. The elucidation of the molecular mechanisms behind their action has inspired further investigations into their effects on human cells and their possible uses as anti-cancer and antimicrobial agents.
1. Introduction
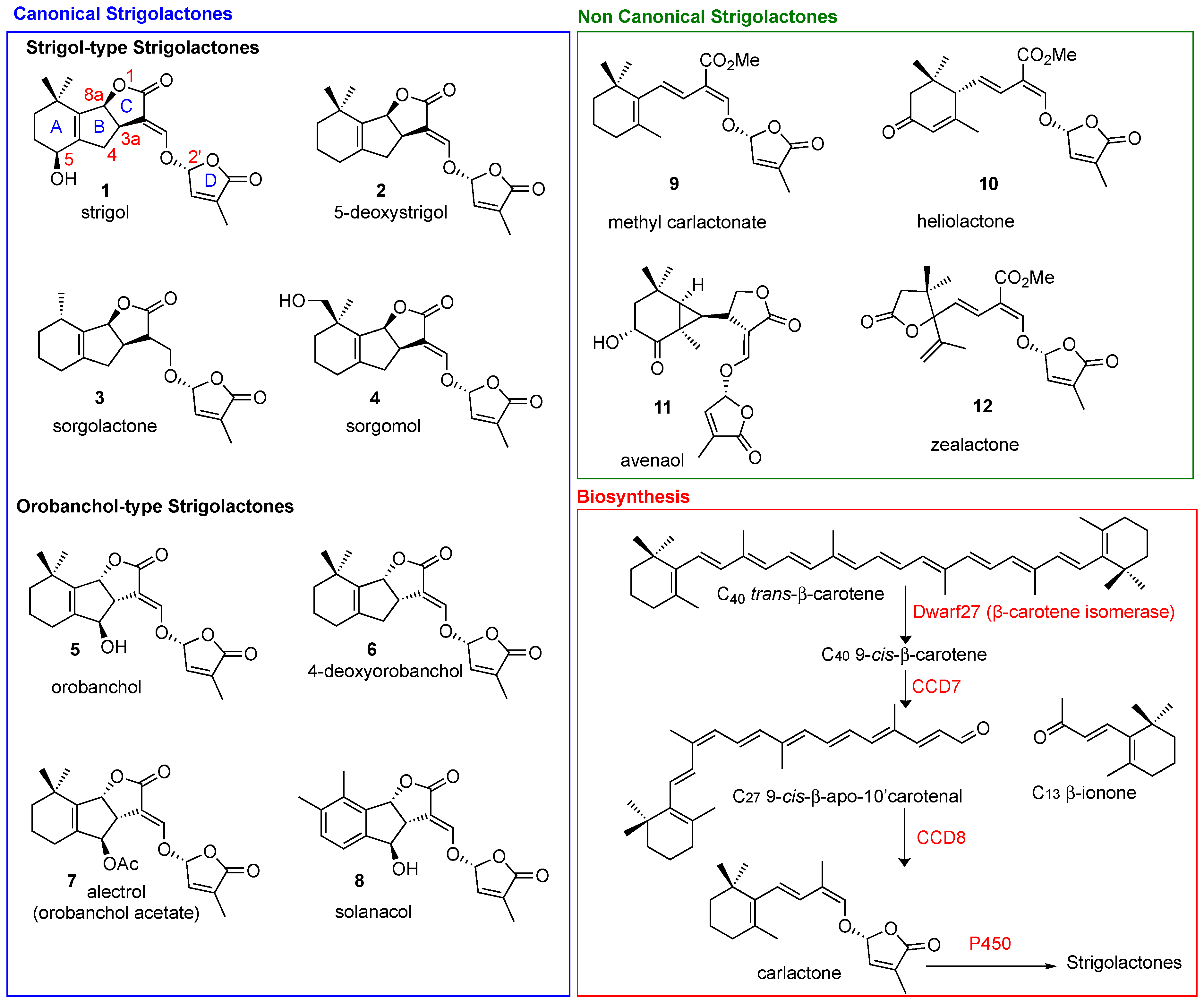
Strigolactones (SLs) are carotenoid-derived sesquiterpene lactones whose structure is characterized by a four-ring system that is generally identified as an ABC tricyclic core linked to a fourth ring, named the D-ring, by means of an enol-ether bridge (Figure 1). The partial elucidation of their biosynthesis in several plant species has identified the involvement of the following genes: DWARF27 (D27; β-carotene isomerase), Carotenoid Cleavage Dioxygenase 7 and 8 (CCD7 and CCD8), and MAX1 homologs (cytochrome P450s) [1]. The first SL, Strigol, was isolated from cotton-root exudate in 1966 [2]; it took over 40 years for its activity as a hyphal branching inducer to be uncovered [3], and for the role of SLs as a new class of phytohormones to be assessed [4,5]. Since then, the boom in interest in the use of these challenging molecules in sustainable agricultural practices indicates that there will be promising forthcoming developments [6,7,8]. Anti-cancer activity has been reported for multiple classes of plant hormones, including cytokinins, methyl jasmonate and brassinosteroids [9], and the first report on the antiproliferative activity of SLs was published in 2012 [10]. An ever-increasing number of references on the exploitation of SLs in the biomedical field have subsequently appeared in the literature. This review highlights the prospects of these future opportunities by outlining the accumulated knowledge on SLs and their potential applications in human health.
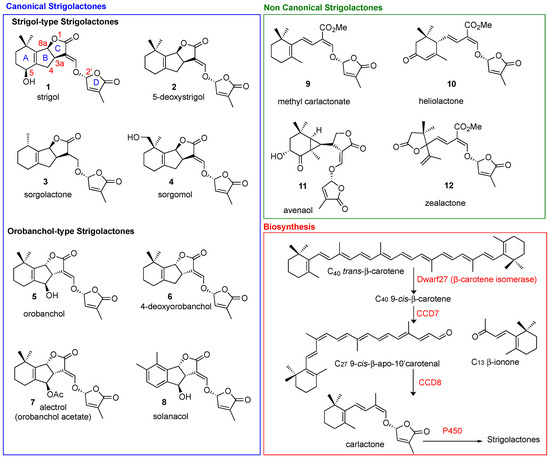
Figure 1.
Structural diversity of natural canonical (blue box) and non-canonical SLs (green box). Biosynthesis in red box.
2. Structure and Synthesis of SLs
2.1. Naturally Occurring SLs
According to recent reviews, more than 25 SLs have been identified across the plant kingdom, with different plant species usually exuding different blends of several SLs [11].
Natural SLs are classified into two main classes: canonical and non-canonical SLs (Figure 1), according to the presence or absence, respectively, of the complete ABC-ring system [12,13]. The D-ring and the enol-ether bridge, which acts as a connection to the ABC core of the molecule, are a conserved feature in both canonical and non-canonical SLs. The structural variations in SLs are reflected in their functional diversity [14]. Stereochemistry plays a crucial role in the fine tuning of the biological properties ascribed to SLs [14,15]. Naturally occurring SLs can be divided into two families, strigol- (3aR,8aS in Strigol, Figure 1) and orobanchol-type SLs, (3aS,8aR in Orobanchol, Figure 1), depending on different orientations of the B/C junction, while the D-ring is always R configured (Figure 1). In biosynthetic pathways, the AB-rings can be modified via demethylation, hydroxylation, epoxidation and acetoxylation [16], giving rise to the structural diversification present in natural SLs.
2.2. SL Analogs and Mimics
Once the potential applications of SLs in agriculture and biomedicine became striking, synthetic SLs turned out to be an important tool with which to elucidate the functions of these signaling molecules and, at the same time, foster research in the field. Chemical synthesis involves either a total synthesis of the entire SL structure, or the synthesis of analogues with simplified structures that retain SL bio-properties [6,17,18].
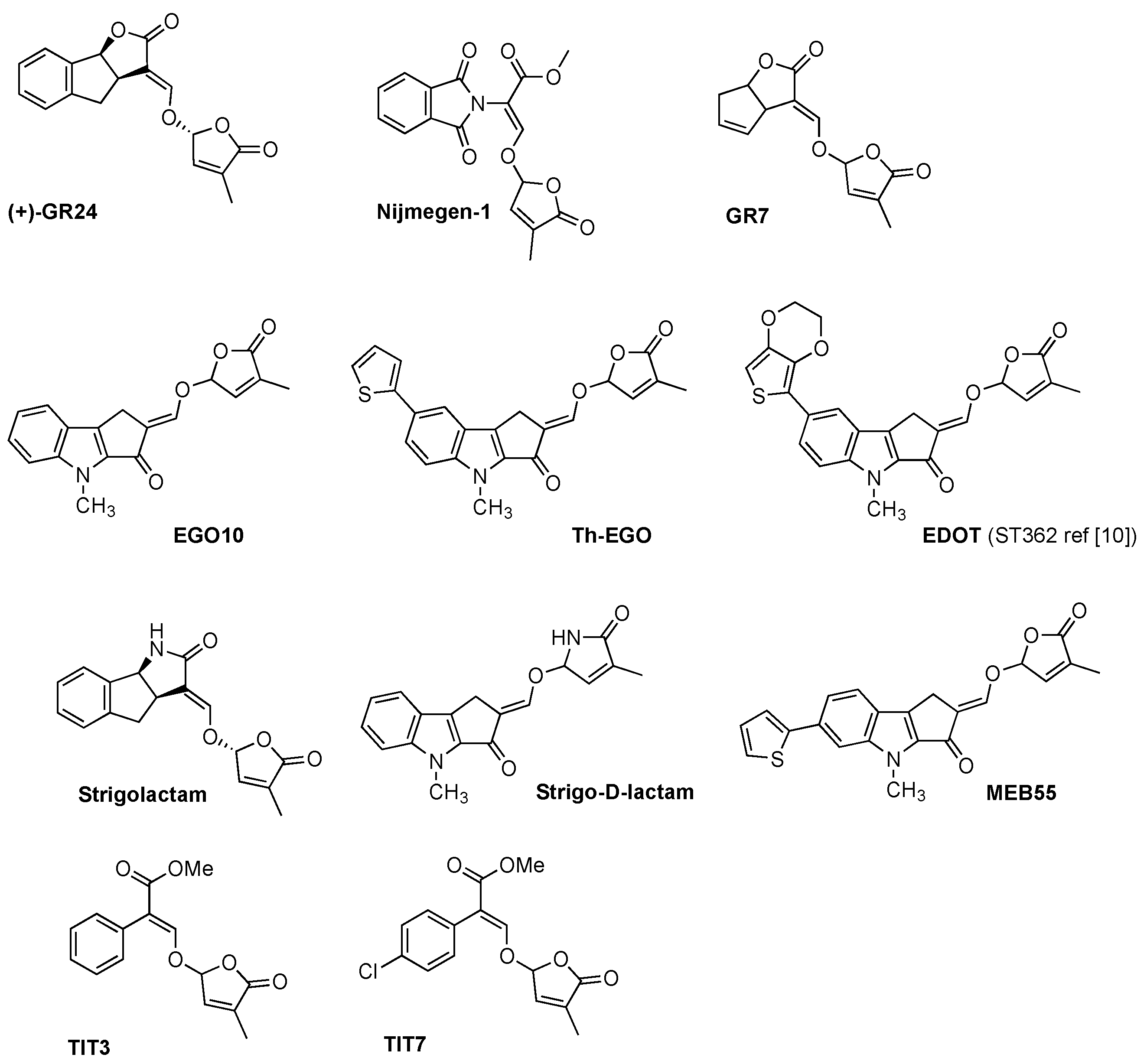
The synthesis of SL analogues is based on the identification of the bioactiphore in SLs. This is the D-ring and the enol-ether bridge connecting C and D-ring (see Strigol 1 in Figure 1), which are required for activity, apparently as a Michael acceptor. Stereochemistry at the D-ring often plays a crucial role, with the most active derivatives showing the same configuration as natural SLs (2′R). A selection of the huge number of synthetic SLs that have been produced so far is provided in Figure 2 and includes GR24 [6], Nijmegen-1 [19], as well as indole derivatives EGO10, TH-EGO and EDOT [20]. Reports have shown that the structural modification of the D-ring into a c-lactam functional group may provide insight into the variations in SL-binding interactions with their receptor [21,22]. Other important analogues are fluorescent SLs, which can be used to track SL perception and trafficking, and include the fluorescence turn-on probe Yoshimulactone Green [23,24,25,26,27]. All these synthetic SLs have greatly contributed to improving our understanding of the biological role of SLs.
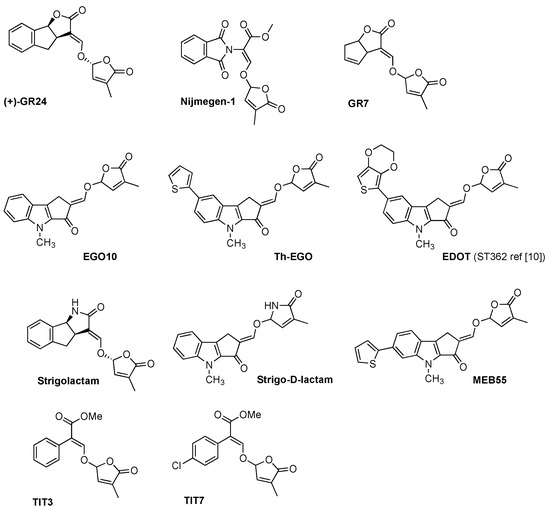
Figure 2.
Panel of synthetic strigolactones. (+)-GR24 [6], Nijmegen-1 [19], GR7 [18], EGO10 [20], TH-EGO [20], EDOT [20], Strigolactam [21], Strigo-D-lactam [22], MEB 55 [10], TIT3 and TIT7 [28].
3. Roles of SLs in Plant Biology
After the first SL was isolated from the root parasite plant Striga lutea (witchweed) for use as a germination stimulant, many other SLs, with similar functions, were identified in Striga spp., broomrapes, Alectrs spp., and other host and non-host plants [2,29,30,31].
All SLs derive from carlactone (CL), which is synthesized in plasmids from all-trans-β-carotene by three different enzymes D27 [32,33], CCD7 and CCD8 [34]. In particular D27, an iron-binding enzyme, catalyzes the isomerization of all-trans-β-carotene to 9-cys-β-carotene, CCD7 converts that to 9-cys-β-apo-10′-carotenal and CCD8 then converts the carotenal into (Z)-(R)-carlactone (CL). The latter is then oxidized by cytochrome P450 monooxygenase MAX1, or other homologous enzymes, to generate the different SLs (Figure 1) [4,5,35]. Interestingly, the genes responsible for SL biosynthesis have been identified in several plants, algae and bryophytes, which suggests that SLs are fundamental molecules that have been maintained by evolution for a very long time [35]. When produced, SLs accumulate in the roots, the main storage organ [36], and then leave them by exudation to reach the rhizosphere, where they can exert their signaling activity [37]. This transport and exudation are regulated by the PhPDR1 transporter, whose mutants have shown a highly reduced level of SLs in root exudate [38].
The biological receptor through which SLs exert their action in plants has been identified as the α/β hydrolase receptor DWARF14 (D14), which is responsible for both the perception and deactivation of hormone signals [39,40]. D14 was first identified in a rice SL-insensitive mutant, but orthologs were soon found in Arabidopsis, petunia and pea [41,42,43]. This enzyme possesses a typical hydrolase catalytic triad, i.e., Ser, His, Asp, and cleaves SLs into the ABC- and the D-ring by performing a nucleophilic attack. The subject has been widely debated [42,44] but, recently, Seto et al., have demonstrated that the active signaling is activated by intact SLs. Upon SL binding, the receptor undergoes a transient conformational adjustment by which it becomes catalytically inactive, but able to interact with D53/SMXLs and D3/MAX2 signaling partners. When the latter are degraded by ubiquitination, the catalytic triad is reconstructed, SLs are hydrolyzed and the signal transduction is interrupted. Thus, the signaling process is triggered by intact SLs and not by hydrolysis or intermediate products [40].
SLs play a number of different roles, which will be explained in more detail hereafter: (i) they control the architecture of above-ground and underground plant organs [4,45,46,47]; (ii) they induce germination in root parasitic plants in genera such as Striga, Orobanche, Alectra and Phelipanche spp. [48], which have limited seed reserves, no photosynthetic activity and represent a real threat for agriculture thanks to SL-mediated activation; (iii) they regulate the symbiosis between plants and arbuscular mycorrhizal fungi (AMF); and (iv) they maintain plant life under hostile ecological conditions [35].
SLs are fundamental to controlling plant growth and architecture. Indeed, they induce root growth and the elongation of root hair, but inhibit secondary shoot branching [49]. They also participate, with auxins, in regulating leaf senescence, stem growth and seed germination [50,51,52]. Other phytohormones, such as abscisic acid (ABA), seem to positively regulate SL biosynthesis [53], while SLs behave antagonistically with respect to cytokinins [54], thus underlining the complex interplay of phytohormones that guarantees proper plant behavior. This integrative pathway also sustains the plant response to stress conditions [55]. SL production is, in fact, also regulated by nutrient starvation, such as salt stress, water stress, temperature and nutrient stress conditions. In the case of water stress, for instance, SLs inhibit shoot growth, but stimulate lateral root growth to increase water uptake from the soil. In the case of nutrient stress, the higher amount of produced SLs leads to shoot-branching suppression and stimulates symbiosis with AMF [4]. This latter can, in fact, guarantee the necessary water, phosphate and nitrogen supply, through hyphal extensions. Interestingly, phosphate starvation, as well as AMF colonization, GR24 treatment and naphthylacetic acid, induces the expression of the PhPDR1 transporter [37].
Similarly, in nitrogen-limited conditions, the expression of SL biosynthesis genes is boosted [56]. Moreover, SLs have been proven to respond to biotic stress [57] caused, in particular, by Rhodococcus fascians, Pectobacterium carotovorum and Pseudomonas syringae [58], whose infection induces the upregulation of genes associated to SL production, such as max1, max3 and max4 [59]. However, no alteration was detected in infections caused by bacteria such as Pythium irregulare and Fusarium oxysporum, thus suggesting that SLs only take part in plant immune response when stimulated by specific bacteria and fungi [35].
4. SLs for Sustainable Agriculture: The First Translation
The application of SLs in sustainable agriculture is a challenging goal, but one that is supported by the widespread use in agriculture of technologies that are based on plant hormones to control crop development [60].
Promising results have been achieved with SLs and SL analogs in agriculture, both when they are used as agrochemicals and via developing crop varieties modified for SL production and signaling [61,62].
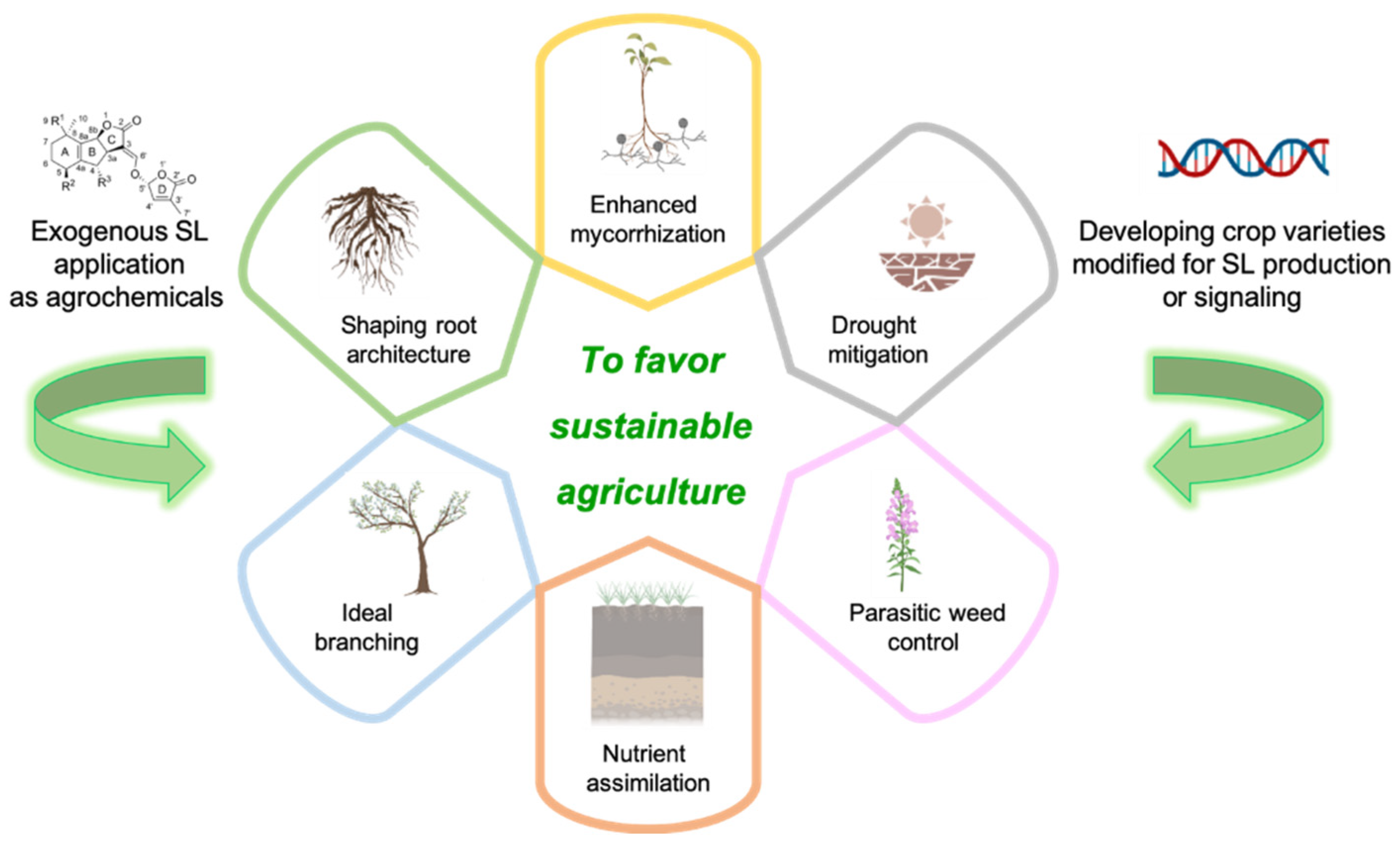
The core components of sustainable agriculture strategies in which SLs display their main application domains are: (i) the control of parasitic weeds; (ii) drought mitigation; (iii) the efficiency of nutrient assimilation and crop development (Figure 3).
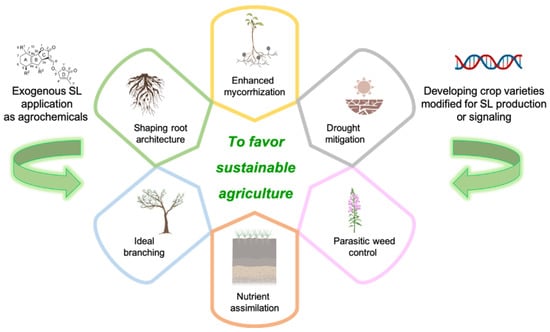
Figure 3.
Agricultural applications of SLs. Exogenous applications of SLs as agrochemicals and the development of crops with modified SL production or signaling have the potential to favor sustainable agriculture via a number of mechanisms: shaping root architecture, promoting ideal branching, stimulating nutrient assimilation, controlling parasitic weeds, mitigating drought and enhancing mycorrhization. Created with BioRender.com.
4.1. SLs in the Control of Parasitic Weeds
One of the most thoroughly investigated applications of SLs is the control of the dangerous parasitic weeds species Striga (witchweeds) and Orobanche (broomrapes), which are estimated to infest upwards of 60 million hectares of farmland worldwide, resulting in severe yield losses every year [63].
Indeed, crops that produce significantly fewer SLs are more resistant to Striga and/or Orobanche infection than other cultivars. This has been observed for different species, including rice [64], tomato (Solanum lycopersicum) [65], the faba bean (Vicia faba) [66,67], and pea (Pisum sativum) [68]. However, the complete loss of SL exudation is not desirable since it can affect some symbiotic mycorrhizal associations, which are particularly needed in soils that are profoundly affected by Striga infestations.
The finding that different SLs have different properties towards mycorrhizae and parasitic weeds has allowed Striga-resistant varieties with normal mycorrhization to be obtained. For example, sorghum species mutated at the Low Germination Stimulant 1 (LGS1) locus are resistant to Striga hermonthica and Striga asiatica, and this resistance can be attributed to a change in profile from strigol-type to orobanchol-type SLs [69]. In field trials, a yield increase in sorghum [70], and maize [71], has been observed in farms across sub-Saharan Africa, where Striga-resistant crops were combined with other control measures, such as fertilization and the procedure of non-host trap crops.
When used as agrochemicals, SL analogs have proven themselves to be a realistic opportunity for controlling Striga and Orobanche via suicidal seed germination. This approach entails the application of SL analogs to soil, followed by the induced germination of the parasites, which cannot survive without the host, thereby depleting the seed bank in the soil. For example, the carbamate SL mimic T-010 reduced S. hermonthica emergence by 94–100% in pots and by 33% in sorghum, and is associated with 187–241% increases in sorghum dry weight [72]. Similarly, the SL analogs Nijmegen-1 and Nijmegen-1 Me were effective in controlling Orobanche ramosa in tobacco (Nicotiana tabacum) crops [73], while a novel class of SLs analogues, derived from dihydroflavonoids, exhibited higher potential in the suicidal germination of the Broomrapes, even compared to the control GR24 [74]. Another approach for the prevention of parasitic seed germination is to antagonize SL responses using SL receptor inhibitors, such as triazole ureas, as agrochemicals [75].
4.2. SLs in Drought Mitigation
Another challenging application of SLs is in the improvement of drought tolerance and decreasing yield losses that are caused by adverse climate conditions that lead to low water availability and high salinity [55,76]
It has been observed that water deprivation increase the expression of SL biosynthesis genes in Arabidopsis leaves [55], tomato shoots [76], and rice [77]. Interestingly, rice root extracts exhibited increased SL content under water deprivation, and the expression of the genes involved in SL biosynthesis was increased in both the roots and shoots of different species, such as in the crown of tall fescue (Festuca arundinacea) [78,79]. By contrast, osmotic stress represses SL biosynthesis in tomato [76], and Lotus japonicus roots [80].
A number of observations have highlighted that the foliar application of GR24, a synthetic SL analog, in SL mutants of Arabidopsis thaliana or grape, can lessen the effects of drought [55,81].
The underlying molecular mechanism is still to be understood, but data are available about the capability of SLs to promote stomata closure to reduce transpiration-associated water loss by interacting with ABA [78,82,83].
Another possibility, in addition to the examples of SL agrochemicals for drought, is the development of drought-tolerant crop varieties via the upregulation of SL signaling. For example, transgenic rice that overexpresses the OsNAC14 transcription factor was observed to upregulate SL biosynthesis genes as well as other genes involved in plant defense, stress response and DNA-damage repair. These transgenic plants had a better survival rate and chlorophyll fluorescence under drought conditions than non-transgenic controls [84].
4.3. SLs in the Promotion of Nutrient Assimilation and Crop Development
SLs can also be optimized to improve nutrient assimilation, thus favoring crop enhancement [85,86].
For example, there is evidence to demonstrate that both natural and synthetic SLs (e.g., GR24) endorse plant growth by positively influencing root vigor in different species [47,49].
One interesting strategy is the shaping of the root microbiome by recruiting specific beneficial microorganisms, such as arbuscular mycorrhizal (AM) fungi that promote hyphal branching [87], spore germination, mitochondrial biogenesis and respiration [88], and the exudation of oligosaccharide and protein signals required for AM recognition by the host [89,90,91]. Interestingly, plants mutated for the petunia hybrida ABC transporter (PDR1), a cellular SL exporter with a key role in regulating the development of AM and axillary branches, displayed reduced symbiotic interactions at the root level, indicating that SLs are critical for the establishment of an appropriate root microbiome [38]. A plant’s genetic background influences the degree of mycorrhization and is a key factor in crop success in low-phosphate soils, as confirmed by experiments on SL transporter overexpression, which led to faster mycorrhization in M. truncatula [92].
This is a critical point as it supports the idea that SLs play a role in the adaptation of root architecture to variable nutrient accessibility in the soil, mainly nitrogen or phosphorus.
Results obtained from field trials have shown that SL analogs increased the capability of maize and sunflower to efficiently uptake nitrogen, when few fertilizers and pesticides are added [93], and of zucchini squash (Cucurbita pepo) [94] and “Hamlin” sweet oranges (Citrus sinensis) [95] to do the same under normal growth conditions.
SLs are also involved in legume nodulation processes, and thereby play an important role in nitrogen acquisition. For instance, the application of GR24 increased nodulation in alfalfa (Medicago sativa) [96], pea [97], and soybean (Glycine max) [98]; conversely, fewer nodules have been observed in SL-biosynthesis mutants than in wild-type plants in L. japonicus, pea and soybean [97,99,100,101].
SLs are increased by nutrient stress, such as low phosphate, nitrogen and sulfur conditions [102,103]. For example, the reduction of phosphate levels induces SLs in different families, including cereals, legumes and nightshades [102].
From a different perspective, crop yield can also be increased upon the reduction of SL biosynthesis or perception. For example, genetic approaches applied to modify a rice allele in order to alter SL signaling lead to an improvement in rice architecture. In particular, the analysis of 147 rice accessions identified the CCD7 gene as causing the partial loss of SL-biosynthesis function. Interestingly, CCD7 is widely co-selected with gibberellin deficiency in rice and contributed to improving grain yields during the green revolution [104]. This observation was further confirmed by the detection of an inverse correlation between different levels of tillering across commercial rice cultivars and SL levels [105], as well as by increased tillering in the Nipponbare background upon the silencing of the CCD7 gene by CRISPR/Cas9 [106].
Even though several “proofs of principle” on the potential application of SL in agriculture are now available, future and continued investments will be crucial for their routine and successful application in agriculture. More data about SL bioavailability and stability in plants and soil are certainly required, and the levels of uptake following application under field conditions should be determined. The fast degradation of natural and synthetic SLs in soil [107], and the limited information on early chemical uptake into seeds are major limits [108]. Finally, more clarity is required in the legislation on the production, commercialization and use of potential future SL-based technologies [7].
The optimization of these aspects could potentially allow SLs to be applied at very low quantities, in a range of 1–10 grams/hectare, with undoubtedly positive implications in terms of costs, environment and human safety [61].
5. Potential of SLs in Human Health
In recent years, a few studies have begun to shed light on the effects of plant hormones on human health. Molecules such as ABA, salicylic acid, indole-3-acetic acid (the best-known auxin) and cytokinins, which have been extensively studied as plant regulators, are also produced by and elicit biological activities in human cells and animal models [9,109].
Interestingly, several phytohormones can also be produced by human gut microbes, in addition to dietary intake, and likely influence many physiological pathways, such as glucose homeostasis, inflammatory responses and other cellular processes [110,111].
Some phytohormones affect human diseases, such as diabetes, inflammatory bowel disease and cancers, which are also modulated by the gut microbiota [112]. For instance, previous findings have revealed the beneficial effects of ABA against inflammation-related diseases such as type 2 diabetes (T2D), colitis, atherosclerosis, glioma and depression [111]. Salicylates, on the other hand, have long been appreciated as pharmacological agents [113].
Considering these effects, the use of phytohormones as multifunctional nutraceuticals against inflammation-associated diseases, in particular metabolic syndrome and its diverse comorbid symptoms, has been proposed [112]. Overall, the optimal formulation and dosage for phytohormone supplements are still to be established, although the ABA extract of fig fruit has recently been proposed for sugar control against T2D [114].
In this context, the value of SLs in the medical field is only emerging recently. The following sections outline the main discoveries in the applications of SLs for human health.
5.1. Modulation of Inflammation
Apart from being involved in the regulation of plant physiology, phytohormones have also been reported to affect human processes including, among others, cell division, glucose metabolism and inflammation [115]. More than ten years ago, it was observed that specific brassinosteroids improve oral glucose tolerance in mice by decreasing the expression of the gluconeogenic enzymes PEPCK and G6Pase and increasing ACT phosphorylation in the liver and muscles [116]. ABA also improves insulin resistance and has a positive effect on neuroinflammation [117], while gibberellic acid (GA) inhibits the release of proinflammatory interleukins, indicating that a GA-enriched diet may alleviate inflammatory disorders [109].
The representative SL GR24 has also been studied for its possible effects on glucose metabolism, and it was found to upregulate and activate SIRT1, a NAD+-dependent deacetylase that plays a key role in glucose homeostasis and energy metabolism, and to enhance insulin signaling, glucose uptake, GLUT4 translocation and mitochondrial biogenesis. It is thus a possible new treatment for insulin resistance in skeletal muscle [118].
Recent studies have reported interesting anti-inflammatory activity for GR24 when tested in vitro and in vivo in RAW263.7 cells and zebrafish larvae, respectively [119]. Two GR24 isomers, in particular, were observed to significantly inhibit the release of the pro-inflammatory mediator NO in lipopolysaccharide (LPS)-stimulated cells, as well as the levels of TNF-α and IL-6, compared to the glucocorticoid dexamethasone. Similarly, the levels of phosphorylated NF-κB p65, IκBα, ERK1/2 and p38 MAPK significantly decreased upon treatment with GR24 isomers in a concentration-dependent manner. Indeed, the suppression of NF-κB and MAPK cascades directly resulted in decreased NO, TNF-α and IL-6 production. Important outcomes in the migration of neutrophils and primitive macrophages in zebrafish injuries were also observed. Apart from widening the many possible roles played by SLs, these results also confirmed the importance of the absolute SL configuration and the unsaturated D-ring, whose absence significantly reduced the aforementioned effects [119].
More recently, the role of SLs in neuroinflammation was studied in more detail when phenotypic screenings were performed on SIM-A9 microglial cell lines treated with a GR24 racemic mixture [120]. Again, a reduction in LPS-induced NO production was observed, and this reduction is comparable to that exerted by 1400W, which is a selective irreversible inhibitor of inducible nitric oxide synthase (iNOS). Both mRNA and iNOS levels, generally elevated in neurodegenerative disorders [121,122], were significantly reduced in a dose-dependent manner. ELISA and Western blot again confirmed the downregulation of the TNF-α gene and the consequent inhibition of TNF-α, known to be involved in the activation of α- and β-secretases, which, in turn, stimulate Aβ deposition and the consequent microglial cytokine storm. The suppression of IL-1β production was been registered. These observations support the potential anti-neuroinflammatory and neuroprotective effects of GR24, and reasonably those of SLs in general, against neurodegenerative disorders and the early events of Alzheimer disease (AD). It was also found that GR24 is able to provide the strong dose-dependent downregulation of COX-2, which is responsible for the production of prostaglandins in inflammatory processes [120]. It has to be noted that a clear correlation exists between COX-2 expression and dementia severity in patients affected by dementia, AD and Parkinson disease [123,124]. Interestingly, the nuclear deposition of LPS-induced NF-κB also decreased 3-fold, while PPARγ protein expression, suppressed by LPS treatment, was restored almost completely. Indeed, it has been reported that PPARγ activation can treat and prevent neurodegenerative diseases, and that PPARγ agonists prevent LPS-induced neuronal death [125]. GR24 has also been proven to increase the accumulation of Nrf2, which is the main transcription factor that controls the expression of several cytoprotective enzymes, in microglia cells. In fact, GR24 treatment induced the increased expression of NADPH quinone dehydrogenase-1 (NQO1) and heme oxygenase-1 (HO-1). GR24 seems to have positive efficacy on BBB endothelial cell permeabilization in reducing the negative effects provided by LPS. In particular, treatment with 20 μM GR24 reduced Evans Blue dye extravasation and increased the expression of tight junction proteins, such as occludins. Overall, Kurt et al., have soundly demonstrated that GR24 promotes the downregulation of proinflammatory genes/proteins and the upregulation of cytoprotective ones in microglia and BBB endothelial cells, thus making it an interesting candidate for the development of new treatments for neurodegenerative and neuroinflammatory diseases [120].
Similar effects were previously reported by the same authors in the treatment of murine RAW macrophages and hepatic Hepa1c1c7 cell lines with GR24 [115]. Having confirmed the potent inhibition exerted by the compound on LPS-induced NO production, molecular docking simulations were performed towards the iNOS enzyme, and confirmed the hypothesis to some extent; better interactions and score values were obtained for GR24 enantiomers compared to the positive control 1400 W [115]. As mentioned above, GR24 has an effect on Nrf2 expression. Nrf2 signaling is regulated by the repressor Kelch-like ECH-associated protein 1 (Keap1), which promotes Nrf2 ubiquitination [126]. The disruption of the Nrf2-Keap1 association allows Nrf2 to translocate within the nucleus and induce the expression of phase II detoxification enzymes. Docking simulations were therefore also performed in the Keap1 crevice bound by the Nrf2 peptide. Again, better poses and interaction energies were obtained compared to the control compounds sulforaphane and curcumin. All these data strongly suggest that there exists a link between the activation of Nrf2 and the increased expression of HO-1 and NQO1 cytoprotective enzymes. Indeed, other phytochemicals, such as resveratrol, carnosol, oroxylin A and epigallocatechin-3-gallate, have been demonstrated to exert their protective role through Nrf2 activation in numerous chronic inflammatory diseases, T2D, neurodegenerative disorders, cancer and cardiovascular diseases [127,128,129].
5.2. SLs as Anti-Cancer Agents
Several plant-derived compounds have shown anti-cancer activity. The most famous of these include curcumin, which is able to suppress NF-κB and cause apoptosis, vinblastine, an alkaloid that targets microtubule, and paclitaxel (Taxol), which also acts on microtubules [130]. More recently, phytohormones enlarged this category with brassinosteroids, which cause G1 arrest and apoptosis [131], methyl jasmonate, which depletes ATP in cancer cells through mitochondrial perturbation [9,132] and cytokinins. SL analogues have also demonstrated anti-cancer activity in vitro and in vivo.
The first anti-tumoral effect of SLs in breast cancer cells was reported by Pollock et al., who found that these natural compounds can specifically inhibit proliferation and induce apoptosis in cancer cells, while sparing non-cancer cells [10]. The effect of GR24 was first evaluated on ER+ tumorigenic, ER- metastatic and normal non-neoplastic fibroblasts. Significant growth reduction was observed at 2.5–5 ppm concentrations in both cancer lines, while no significant effect was observed on fibroblasts. GR24 was also observed to inhibit the growth and reduce the viability of MCF-7 tumorigenic cells that propagated as mammospheres in non-adherent growing conditions. Similar positive results were obtained when five synthetic SL analogues were tested. In particular, ST362 and MEB55 (later renamed TH-EGO and EDOT, respectively), which are characterized by an indolyl-based structure with an enol-ether bridge connecting the C and D ring, were found to be the most potent and to exert a non-reversible reduction in cell viability after only four hours. This effect is likely associated to the inhibition of the phosphorylation of p38 MAPK and JNK1/2, which are stress-activated kinases that play a key role in a stress-signaling cascade and are associated with cell-cycle arrest and apoptosis [133,134]. Indeed, the mechanism by which SLs exerts their anti-tumoral activity has been associated to the blockage of cell-cycle progression and the consequent induction of apoptosis. The authors only observed a dose-dependent increase of cells in the G2/M phase in tumorigenic cell lines, while normal fibroblasts did not show sensitivity to SLs in this context. This may be linked to the higher division rate of cancer cells and to the capacity of SLs to target rapidly dividing cells.
ST362 and MEB55 were also tested, alone and in combination with the breast cancer chemotherapy drug paclitaxel, in xenograft models [135]. The administration of MEB55 led to reductions in tumor volume and tumor-growth rate in mice implanted with MDA-MB-231 xenografts. ST632 also showed promising results, comparable to those of paclitaxel. The co-treatment of cancer cell lines with MEB55 and paclitaxel showed a two-fold decrease in MEB55 IC50, thus suggesting that the two molecules could have an additive effect. However, fewer promising results were obtained on xenografts, as the tumor volume reduction obtained by the co-administration was not significant with respect to treatment with MEB55 alone. The effect of SLs on microtubule bundling has also been studied, and it was found that the phytohormones might affect microtubule network integrity and, consequently, inhibit the migration of the most invasive breast cancer cell lines [136,137]. This might also have an effect on tumor metastatic character, which is strictly related to cell-migration capability. It is interesting to note that paclitaxel also mainly targets microtubules to exert its potent action.
Having demonstrated the inhibition exerted by SLs in breast cancer cells and breast cancer stem cells, the same authors widened the study to other solid and non-solid cancer cell lines, including prostate, colon, lung, melanoma, osteosarcoma and leukemic cells [138]. They found that SLs, in particular the analogues EG5, EG9c, ST357, ST362 and MEB55, were able to inhibit the growth of the cell lines and to induce a cellular-stress response that turned into cell-cycle arrest and apoptosis in all cases, except fibroblasts. In particular, the authors again reported that SLs were able to arrest the cell cycle at the G2 state. This arrest was primarily associated to the down-regulation of cyclin B1, the Cdc25C protein and mRNA levels, and to the activation of stress signaling, such as the induction of multiple heat shock proteins (HSP) and cytokine [139]. The activation of stress signaling exerted by SLs also affects the stress-induced transcription factor FOXO4, p38 MAPK and JNK1/2, which are again involved in the signaling for cell-cycle arrest [133], and apoptosis [134,140]. Moreover, SLs induce the expression of several pro-apoptotic genes and inhibit the expression of survival factors such as ALDH1, which is a key regulator of stem cell viability and self-renewal.
The two most potent molecules were, again, MEB55 and ST362, which were able to induce apoptosis in all tested cell lines and to specifically reduce the viability of prostate tumor conditionally reprogrammed cells (CRC), in which a significant reduction in cyclin B level and a pronounced stress response (pp38 induction) were observed. Similarly, a stronger apoptotic response was observed in CRC tumor cells than in normal cells. These findings support the potential of SL analogues to induce a significant non-reversible apoptotic response in transformed cells and in patient-derived tumor cells, while having significantly lower toxic effects in normal cells [138]. The two compounds were also proven to induce DNA double-strand breaks (DSBs) and consequently activate DNA damage response in osteosarcoma cells [141]. However, at the same time, SLs downregulate the DNA repair protein RAD51 via ubiquitination and, consequently, also the homology-directed repair (HDR) system, which are possibly associated to resistance towards DNA-damaging chemotherapy and radiotherapy. It follows that RAD51 downregulation may be a useful strategy for restoring and enhancing the effectiveness of cancer chemotherapy [141,142]. Importantly, no DSB or cell death was detected in non-transformed fibroblasts, which once again highlights the potential clinical relevance of these molecules.
SLs analogues were also tested in cell lines of hepatocellular carcinoma (HCC), which is the predominant form of liver cancer and the fifth most common type of cancer in men [143]. Two of the tested compounds, namely TIT3 and TIT7 (Figure 2), showed anti-proliferative effects (cell viability reduction) on HepG2 cells, but had a lower effect on hamster kidney cells (BHK cells). The two compounds were also tested on PC3 prostate cancer and T-cell acute lymphoblastic leukemia cell lines, with dose-dependent cell-viability inhibition being shown. This indicates that the two compounds have a capability to inhibit cell proliferation in both solid and hematological tumors. Interestingly, the compounds showed a minimal inhibitory effect on healthy cells compared to cancer cells. The authors also performed a wound-healing assay on HepG2 cells to check cell migration, which was effectively inhibited by both TIT3 and TIT7. A possible mechanism, as already suggested for SLs, could involve the compounds interfering with the microtubular network, but the exact targets and a detailed mechanism of action that explains compound selectivity is currently difficult to define [141].
Taken together, these results clearly support the anti-cancer effects of SLs, which are emerging as a new possible treatment for advanced prostate cancer and other types of tumors.
5.3. SLs with Antimicrobial Activity
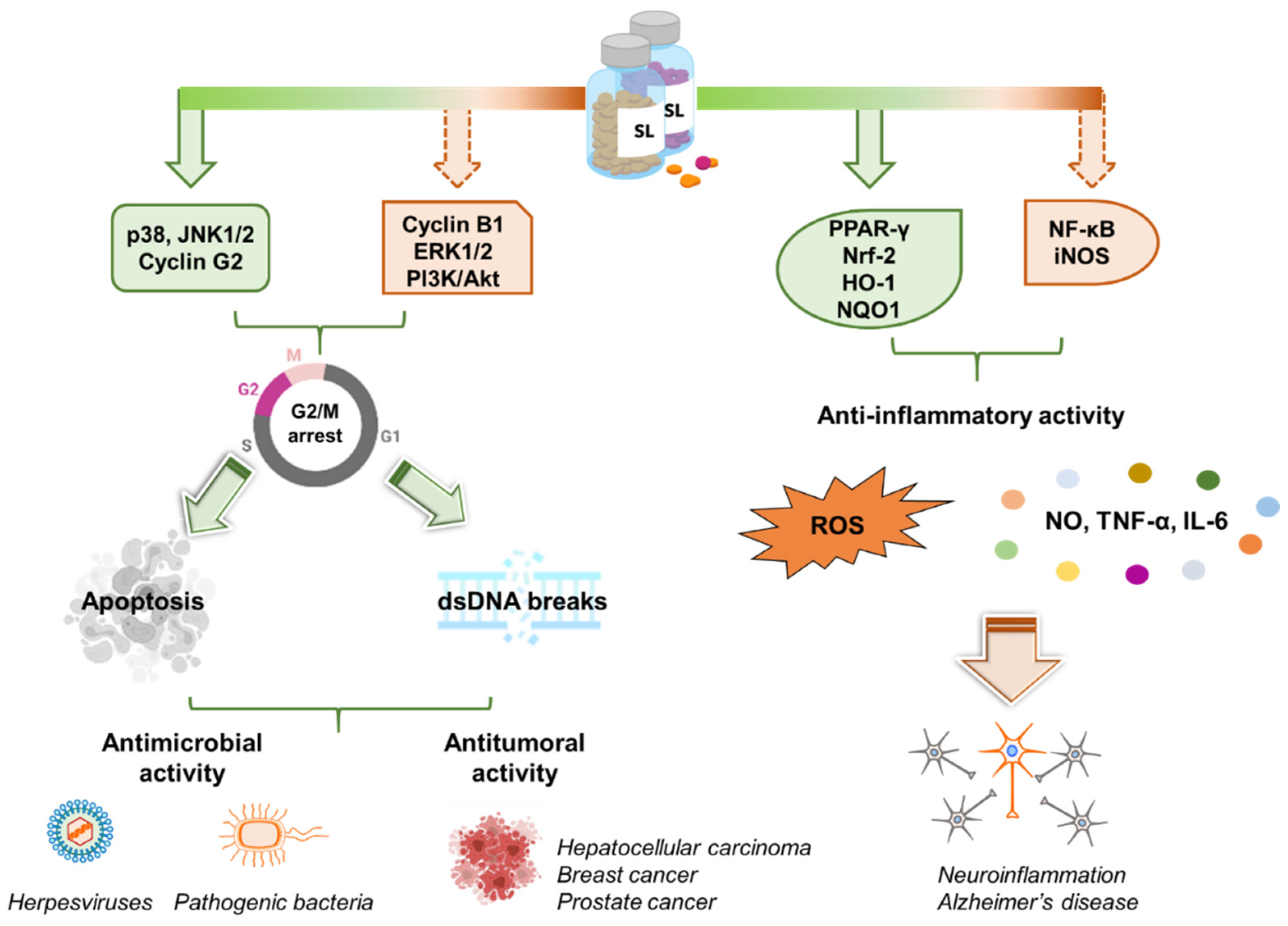
Despite the multifaceted roles of SLs in plant biology and their promising features as drug candidates for different kinds of cancers, their antimicrobial and antiviral activity is still unexplored (Figure 4).
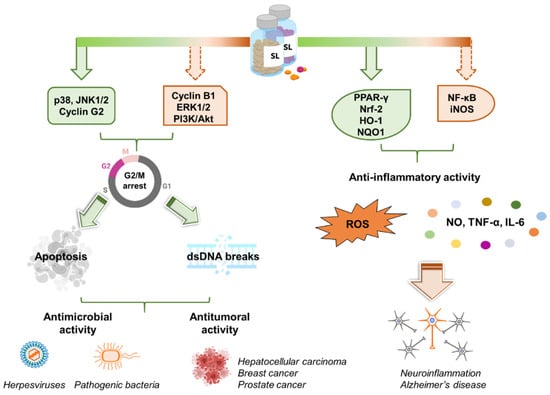
Figure 4.
SL activity in human cells and their potential in medicine. Left panel. Synthetic analogs of SLs control multiple pathways leading to the arrest of the cell cycle in the G2/M phase. Apoptosis and DSBs are then induced. These properties grant SLs antimicrobial as well as anti-tumoral activity. Right panel. SLs exert anti-inflammatory effects by inhibiting the release of inflammatory molecules (e.g., NO, TNF-α, IL-6, ROS). This makes SLs promising scaffolds for the development of novel anti-Alzheimer’s disease candidates. Created with BioRender.com.
The lessons learned from phytopathogenic fungi, in which the SL analog GR24 impairs the growth of root pathogens (e.g., Fusarium oxysporum f. sp. melonis, Fusarium solani f. sp. mango, Sclerotinia sclerotiorum and Macrophomina phaseolina), and the foliar pathogens Alternaria alternata, Colletotrichum acutatum and Botrytis cinerea [144], led to the hypothesis that SL antimicrobial activity could be extended to human pathogens.
The possibility of using SLs as antibiotics has been explored for the novel SL analog TIT3 against different pathogenic bacteria. Promising results were obtained for Staphylococcus aureus, Salmonella typhimurium, Escherichia coli, Klebsiella pneumonia and Bacillus subtilis, indicating that SLs may be a viable alternative for the treatment of different strains of bacteria that are resistant to conventional antibiotics [28].
Recently, our group has demonstrated, for the first time, the efficacy of a group of SL analogues as antivirals against members of the Herpesviridae family, in particular human cytomegalovirus (HCMV) [145]. HCMV is a widespread pathogen that can cause severe disease in immunocompromised individuals [146]. In addition, HCMV infection is the most frequent cause of congenital malformation in developed countries [147]. Although nucleoside analogues have been successfully used against HCMV, their use is hampered by the occurrence of serious side effects, the rapid emergence of resistance and the fact that their efficacy is limited to alleviating symptoms, without eradicating the latent infection [148,149]. There is, therefore, an urgent clinical need for new antiviral drugs that can overcome these limitations. Of the different SL analogs screened, there are two compounds that significantly inhibit HCMV replication in vitro, i.e., TH-EGO and EDOT-EGO. These results are challenging in the field of antiviral research, since, besides inhibiting the late phases of the viral cycle, apoptosis has been shown to be a novel strategy that SLs rely on to exert their inhibitory role against viral replication. These results have been confirmed by in-silico molecular docking simulations, which predict a stable protein-ligand complex between the SL analogs and the modeled structure of the putative target IE1, which is employed by HCMV to escape apoptosis [145].
In this context, further investigations on physiologically relevant targets for HCMV infection, such as endothelial and epithelial cells, and on cells that do not progress to a lytic infection, such as monocytes, will be crucial to corroborating and expanding the data obtained on HFFs. Furthermore, it will be essential to extend the analysis to other HCMV proteins, such as other antiapoptotic HCMV proteins (vMIA, cICA, UL38 and IE2), as well as to other members of the Herpesviridae family and emerging viruses, for which medical demand is an absolute priority at this time.
6. Conclusions
SLs are versatile and challenging molecules. In this review, we have demonstrated how the blooming and interdisciplinary research on SLs continuously unveils exciting, new biological functions and properties for these molecules. The exploitation of these properties is not without challenges: (i) lead compounds with unbiased activity and uncontroversial benefits should be identified; (ii) the synthesis of SLs is complicated, and designing the proper structure to emphasize specific activity is a difficult task; (iii) it is necessary to find the right balance between the stability and reactivity of SLs and effective formulations must be set up. We can foresee that the deep and full understanding of the molecular mechanisms, the elucidation of the transduction signal pathways and the development of their synthetic chemistry will pave the way for a variety of potential applications in agriculture and medicine.
7. Patents
Patent “Strigolattoni per uso nella prevenzione e/o trattamento di infezioni da virus della famiglia Herpesviridae” (No: 102018000010142, PCT/IB2019/059611, E7527/19-EW, University of Turin, Italy).
Author Contributions
F.S., V.D., C.P.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are actively involved in research on the topic of the Review, funded by: Proof of Concept (PoC)-TOINPROVE/2020, the “Cassa di Risparmio” Foundation of Turin (V.D.), the Italian Ministry of Education, University and Research-MIUR (PRIN 2015RMNSTA) (V.D.), the University of Turin (RILO2020) (V.D.) (C.P.) (F.S.), and the National Agency for the evaluation of Universities and Research Institutes (ANVUR, Basic Research 2017 to P.C.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lopez-Obando, M.; Ligerot, Y.; Bonhomme, S.; Boyer, F.D.; Rameau, C. Strigolactone biosynthesis and signaling in plant development. Development 2015, 142, 3615–3619. [Google Scholar] [CrossRef]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (striga lutea lour.): Isolation and properties of a potent stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef]
- Akiyama, K.; Ogasawara, S.; Ito, S.; Hayashi, H. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 2010, 51, 1104–1117. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Blanco-Ania, D. Strigolactones: New plant hormones in the spotlight. J. Exp. Bot. 2018, 69, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Vurro, M.; Prandi, C.; Baroccio, F. Strigolactones: How far is their commercial use for agricultural purposes? Pest Manag. Sci. 2016, 72, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Aliche, E.B.; Screpanti, C.; De Mesmaeker, A.; Munnik, T.; Bouwmeester, H.J. Science and application of strigolactones. New Phytol. 2020, 227, 1001–1011. [Google Scholar] [CrossRef]
- Lin, L.; Tan, R.X. Cross-kingdom actions of phytohormones: A functional scaffold exploration. Chem. Rev. 2011, 111, 2734–2760. [Google Scholar] [CrossRef]
- Pollock, C.B.; Koltai, H.; Kapulnik, Y.; Prandi, C.; Yarden, R.I. Strigolactones: A novel class of phytohormones that inhibit the growth and survival of breast cancer cells and breast cancer stem-like enriched mammosphere cells. Breast Cancer Res. Treat. 2012, 134, 1041–1055. [Google Scholar] [CrossRef]
- Bürger, M.; Chory, J. The Many Models of Strigolactone Signaling. Trends Plant Sci. 2020, 25, 395–405. [Google Scholar] [CrossRef]
- Wang, Y.; Bouwmeester, H.J. Structural diversity in the strigolactones. J. Exp. Bot. 2018, 69, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Kisugi, T.; Nomura, T.; Nakatani, Y.; Akiyama, K.; McErlean, C.S.P. Which are the major players, canonical or non-canonical strigolactones? J. Exp. Bot. 2018, 69, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, A.; Waters, M.T.; Sun, Y.K.; Skelton, B.W.; Dixon, K.W.; Ghisalberti, E.L.; Flematti, G.R.; Smith, S.M. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in arabidopsis. Plant Physiol. 2014, 165, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Flematti, G.R.; Scaffidi, A.; Waters, M.T.; Smith, S.M. Stereospecificity in strigolactone biosynthesis and perception. Planta 2016, 243, 1361–1373. [Google Scholar] [CrossRef]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef]
- Prandi, C.; McErlean, C.S.P. The chemistry of strigolactones. In Strigolactones—Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 163–198. [Google Scholar]
- Zwanenburg, B.; Ćavar Zeljković, S.; Pospíšil, T. Synthesis of strigolactones, a strategic account. Pest Manag. Sci. 2016, 72, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Nefkens, G.H.L.; Thuring, J.W.J.F.; Beenakkers, M.F.M.; Zwanenburg, B. Synthesis of a Phthaloylglycine-Derived Strigol Analogue and Its Germination Stimulatory Activity toward Seeds of the Parasitic Weeds Striga hermonthica and Orobanche crenata. J. Agric. Food Chem. 1997, 45, 2273–2277. [Google Scholar] [CrossRef]
- Prandi, C.; Occhiato, E.G.; Tabasso, S.; Bonfante, P.; Novero, M.; Scarpi, D.; Bova, M.E.; Miletto, I. New potent fluorescent analogues of strigolactones: Synthesis and biological activity in parasitic weed germination and fungal branching. Eur. J. Org. Chem. 2011, 2011, 3781–3793. [Google Scholar] [CrossRef]
- De Mesmaeker, A.; Screpanti, C.; Fonné-Pfister, R.; Lachia, M.; Lumbroso, A.; Bouwmeester, H. Design, synthesis and biological evaluation of strigolactone and strigolactam derivatives for potential crop enhancement applications in modern agriculture. Chimia 2019, 73, 549–560. [Google Scholar] [CrossRef]
- Lombardi, C.; Artuso, E.; Grandi, E.; Lolli, M.; Spyrakis, F.; Priola, E.; Prandi, C. Recent advances in the synthesis of analogues of phytohormones strigolactones with ring-closing metathesis as a key step. Org. Biomol. Chem. 2017, 15, 8218–8231. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Yoshimura, M.; Sato, Y.; Kuwata, K.; Toh, S.; Holbrook-Smith, D.; Zhang, H.; McCourt, P.; Itami, K.; Kinoshita, T.; et al. Probing strigolactone receptors in Striga hermonthica with fluorescence. Science 2015, 349, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Prandi, C.; Ghigo, G.; Occhiato, E.G.; Scarpi, D.; Begliomini, S.; Lace, B.; Alberto, G.; Artuso, E.; Blangetti, M. Tailoring fluorescent strigolactones for in vivo investigations: A computational and experimental study. Org. Biomol. Chem. 2014, 12, 2960–2968. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Heugebaert, T.; Matthys, C.; Van Deun, R.; Boyer, F.D.; Goormachtig, S.; Stevens, C.; Geelen, D. A fluorescent alternative to the synthetic strigolactone GR24. Mol. Plant 2013, 6, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Lace, B.; Wininger, S.; Dam, A.; Kumari, P.; Belausov, E.; Tsemach, H.; Kapulnik, Y.; Prandi, C.; Koltai, H. Influx and Efflux of Strigolactones Are Actively Regulated and Involve the Cell-Trafficking System. Mol. Plant 2015, 8, 1809–1812. [Google Scholar] [CrossRef][Green Version]
- Prandi, C.; Rosso, H.; Lace, B.; Occhiato, E.G.; Oppedisano, A.; Tabasso, S.; Alberto, G.; Blangetti, M. Strigolactone analogs as molecular probes in chasing the (SLs) receptor/s: Design and synthesis of fluorescent labeled molecules. Mol. Plant 2013, 6, 113–127. [Google Scholar] [CrossRef]
- Al-Malki, A.L.; Huwait, E.A.; Moselhy, S.S. Synthesis, characterization and in vitro antibacterial activity of a novel strigolactones analogues TIT3. J. Pure Appl. Microbiol. 2020, 14, 2425–2430. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Zwanenburg, B. Tentative Molecular Mechanism for Germination Stimulation of Striga and Orobanche Seeds by Strigol and Its Synthetic Analogues. J. Agric. Food Chem. 1992, 40, 1066–1070. [Google Scholar] [CrossRef]
- Yokota, T.; Sakai, H.; Okuno, K.; Yoneyama, K.; Takeuchi, Y. Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry 1998, 49, 1967–1973. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Branching in rice. Curr. Opin. Plant Biol. 2011, 14, 94–99. [Google Scholar] [CrossRef]
- Hao, L.; Renxiao, W.; Qian, Q.; Meixian, Y.; Xiangbing, M.; Zhiming, F.; Cunyu, Y.; Biao, J.; Zhen, S.; Jiayang, L.; et al. DWARF27,an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar] [CrossRef]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, S.; Shukla, R.K. The role of strigolactones and their potential cross-talk under hostile ecological conditions in plants. Front. Physiol. 2017, 7, 691. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kusumoto, D.; Sekimoto, H.; Sugimoto, Y.; Takeuchi, Y.; Yoneyama, K. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 2007, 227, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ruyter-Spira, C.; Al-Babili, S.; van der Krol, S.; Bouwmeester, H. The biology of strigolactones. Trends Plant Sci. 2013, 18, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Reinhardt, D.; Bours, R.; Bouwmeester, H.J.; Martinoia, E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 483, 341–344. [Google Scholar] [CrossRef]
- Sanchez, E.; Artuso, E.; Lombardi, C.; Visentin, I.; Lace, B.; Saeed, W.; Lolli, M.L.; Kobauri, P.; Ali, Z.; Spyrakis, F.; et al. Structure–activity relationships of strigolactones via a novel, quantitative in planta bioassay. J. Exp. Bot. 2018, 69, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Yasui, R.; Kameoka, H.; Tamiru, M.; Cao, M.; Terauchi, R.; Sakurada, A.; Hirano, R.; Kisugi, T.; Hanada, A.; et al. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 2019, 10, 191. [Google Scholar] [CrossRef]
- Hamiaux, C.; Drummond, R.S.M.; Janssen, B.J.; Ledger, S.E.; Cooney, J.M.; Newcomb, R.D.; Snowden, K.C. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 2012, 22, 2032–2036. [Google Scholar] [CrossRef]
- De Saint Germain, A.; Clavé, G.; Badet-Denisot, M.A.; Pillot, J.P.; Cornu, D.; Le Caer, J.P.; Burger, M.; Pelissier, F.; Retailleau, P.; Turnbull, C.; et al. An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat. Chem. Biol. 2016, 12, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Nelson, D.C.; Scaffidi, A.; Flematti, G.R.; Sun, Y.K.; Dixon, K.W.; Smith, S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 2012, 139, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Ming, Z.; Yan, L.; Li, S.; Wang, F.; Ma, S.; Yu, C.; Yang, M.; Chen, L.; Chen, L.; et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 2016, 536, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Resnick, N.; Mayzlish-Gati, E.; Kaplan, Y.; Wininger, S.; Hershenhorn, J.; Koltai, H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J. Exp. Bot. 2011, 62, 2915–2924. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host arabidopsis. Plant Physiol. 2011, 155, 974–987. [Google Scholar] [CrossRef]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R.; et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011, 155, 721–734. [Google Scholar] [CrossRef]
- Yoneyama, K.; Kisugi, T.; Xie, X.; Yoneyama, K. Chemistry of Strigolactones: Why and How do Plants Produce so Many Strigolactones? In Molecular Microbial Ecology of the Rhizosphere; John Wiley and Sons: Hoboken, NJ, USA, 2013; Volume 1, ISBN 9781118296172. [Google Scholar]
- Koltai, H. Strigolactones are regulators of root development. New Phytol. 2011, 190, 545–549. [Google Scholar] [CrossRef]
- Yamada, Y.; Furusawa, S.; Nagasaka, S.; Shimomura, K.; Yamaguchi, S.; Umehara, M. Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 2014, 240, 399–408. [Google Scholar] [CrossRef]
- Agusti, J.; Herold, S.; Schwarz, M.; Sanchez, P.; Ljung, K.; Dun, E.A.; Brewer, P.B.; Beveridge, C.A.; Sieberer, T.; Sehr, E.M.; et al. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. USA 2011, 108. [Google Scholar] [CrossRef]
- de Saint Germain, A.; Ligerot, Y.; Dun, E.A.; Pillot, J.-P.; Ross, J.J.; Beveridge, C.A.; Rameau, C. Strigolactones Stimulate Internode Elongation Independently of Gibberellins. Plant Physiol. 2013, 163, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- López-Ráez, J.A.; Kohlen, W.; Charnikhova, T.; Mulder, P.; Undas, A.K.; Sergeant, M.J.; Verstappen, F.; Bugg, T.D.H.; Thompson, A.J.; Ruyter-Spira, C.; et al. Does abscisic acid affect strigolactone biosynthesis? New Phytol. 2010, 187, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.A.; de Saint Germain, A.; Rameau, C.; Beveridge, C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012, 158, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Van Ha, C.; Leyva-Gonzalez, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Van Dong, N.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ito, K.; Abeta, N.; Takahashi, R.; Sasaki, Y.; Yajima, S. Effects of strigolactone signaling on Arabidopsis growth under nitrogen deficient stress condition. Plant Signal. Behav. 2016, 11, e1126031. [Google Scholar] [CrossRef]
- Marzec, M. Strigolactones as Part of the Plant Defence System. Trends Plant Sci. 2016, 21, 900–903. [Google Scholar] [CrossRef]
- Piisilä, M.; Keceli, M.A.; Brader, G.; Jakobson, L.; Jöesaar, I.; Sipari, N.; Kollist, H.; Palva, E.T.; Kariola, T. The F-box protein MAX2 contributes to resistance to bacterial phytopathogens in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 53. [Google Scholar] [CrossRef]
- Stes, E.; Depuydt, S.; De Keyser, A.; Matthys, C.; Audenaert, K.; Yoneyama, K.; Werbrouck, S.; Goormachtig, S.; Vereecke, D. Strigolactones as an auxiliary hormonal defence mechanism against leafy gall syndrome in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 5123–5134. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Screpanti, C.; Fonné-Pfister, R.; Lumbroso, A.; Rendine, S.; Lachia, M.; De Mesmaeker, A. Strigolactone derivatives for potential crop enhancement applications. Bioorg. Med. Chem. Lett. 2016, 26, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.S.P. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef]
- Parker, C. Observations on the current status of orobanche and striga problems worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef]
- Jamil, M.; Rodenburg, J.; Charnikhova, T.; Bouwmeester, H.J. Pre-attachment Striga hermonthica resistance of New Rice for Africa (NERICA) cultivars based on low strigolactone production. New Phytol. 2011, 192, 964–975. [Google Scholar] [CrossRef]
- Dor, E.; Yoneyama, K.; Wininger, S.; Kapulnik, Y.; Yoneyama, K.; Koltai, H.; Xie, X.; Hershenhorn, J. Strigolactone deficiency confers resistance in tomato line SL-ORT1 to the parasitic weeds Phelipanche and Orobanche spp. Phytopathology 2011, 101, 213–222. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Kisugi, T.; Xie, X.; Rubiales, D.; Yoneyama, K. Low strigolactone root exudation: A novel mechanism of broomrape (Orobanche and Phelipanche spp.) resistance available for faba bean breeding. J. Agric. Food Chem. 2014, 62, 7063–7071. [Google Scholar] [CrossRef]
- Trabelsi, I.; Yoneyama, K.; Abbes, Z.; Amri, M.; Xie, X.; Kisugi, T.; Kim, H.I.; Kharrat, M.; Yoneyama, K. Characterization of strigolactones produced by Orobanche foetida and Orobanche crenata resistant faba bean (Vicia faba L.) genotypes and effects of phosphorous, nitrogen, and potassium deficiencies on strigolactone production. S. Afr. J. Bot. 2017, 108, 15–22. [Google Scholar] [CrossRef]
- Pavan, S.; Schiavulli, A.; Marcotrigiano, A.R.; Bardaro, N.; Bracuto, V.; Ricciardi, F.; Charnikhova, T.; Lotti, C.; Bouwmeester, H.; Ricciardi, L. Characterization of low-strigolactone germplasm in pea (Pisum sativum L.) resistant to crenate broomrape (Orobanche crenata Forsk.). Mol. Plant-Microbe Interact. 2016, 29, 743–749. [Google Scholar] [CrossRef]
- Gobena, D.; Shimels, M.; Rich, P.J.; Ruyter-Spira, C.; Bouwmeester, H.; Kanuganti, S.; Mengiste, T.; Ejeta, G. Mutation in sorghum LOW GERMINATION STIMULANT 1 alters strigolactones and causes Striga resistance. Proc. Natl. Acad. Sci. USA 2017, 114, 4471–4476. [Google Scholar] [CrossRef]
- Tesso, T.; Gutema, Z.; Deressa, A.; Ejeta, G. An integrated Striga management option offers effective control of Striga in Ethiopia. In Integrating New Technologies for Striga Control: Towards Ending the Witch-Hunt; World Scientific Publishing Co.: Singapore, 2007; pp. 199–212. ISBN 9789812771506. [Google Scholar]
- Douthwaite, B.; Schulz, S.; Olanrewaju, A.S.; Ellis-Jones, J. Impact pathway evaluation of an integrated Striga hermonthica control project in Northern Nigeria. Agric. Syst. 2007, 92, 201–222. [Google Scholar] [CrossRef]
- Samejima, H.; Babiker, A.G.; Takikawa, H.; Sasaki, M.; Sugimoto, Y. Practicality of the suicidal germination approach for controlling Striga hermonthica. Pest Manag. Sci. 2016, 72, 2035–2042. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Mwakaboko, A.S.; Kannan, C. Suicidal germination for parasitic weed control. Pest Manag. Sci. 2016, 72, 2016–2025. [Google Scholar] [CrossRef]
- Jin, Z.; Xu, X.; Kang, Y.; Pang, Z.; Xu, N.; Chen, F. Strigolactone analogues derived from dihydroflavonoids as potent seed germinators for the broomrapes. J. Agric. Food Chem. 2020, 68, 11077–11087. [Google Scholar] [CrossRef]
- Nakamura, H.; Hirabayashi, K.; Miyakawa, T.; Kikuzato, K.; Hu, W.; Xu, Y.; Jiang, K.; Takahashi, I.; Niiyama, R.; Dohmae, N.; et al. Triazole Ureas Covalently Bind to Strigolactone Receptor and Antagonize Strigolactone Responses. Mol. Plant 2019, 12, 44–58. [Google Scholar] [CrossRef]
- Visentin, I.; Vitali, M.; Ferrero, M.; Zhang, Y.; Ruyter-Spira, C.; Novák, O.; Strnad, M.; Lovisolo, C.; Schubert, A.; Cardinale, F. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol. 2016, 212, 954–963. [Google Scholar] [CrossRef]
- Du, H.; Huang, F.; Wu, N.; Li, X.; Hu, H.; Xiong, L. Integrative Regulation of Drought Escape through ABA-Dependent and -Independent Pathways in Rice. Mol. Plant 2018, 11, 584–597. [Google Scholar] [CrossRef]
- Haider, I.; Andreo-Jimenez, B.; Bruno, M.; Bimbo, A.; Floková, K.; Abuauf, H.; Ntui, V.O.; Guo, X.; Charnikhova, T.; Al-Babili, S.; et al. The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 2018, 69, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Wang, J.; Huang, B. Drought inhibition of tillering in Festuca arundinacea associated with axillary bud development and strigolactone signaling. Environ. Exp. Bot. 2017, 142, 15–23. [Google Scholar] [CrossRef]
- Liu, J.; He, H.; Vitali, M.; Visentin, I.; Charnikhova, T.; Haider, I.; Schubert, A.; Ruyter-Spira, C.; Bouwmeester, H.J.; Lovisolo, C.; et al. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: Exploring the interaction between strigolactones and ABA under abiotic stress. Planta 2015, 241, 1435–1451. [Google Scholar] [CrossRef]
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2019, 135, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zhang, Y.; Li, C.; Liu, Z.; Yang, N.; Pan, L.; Wu, J.; Wang, J.; Yang, J.; Lv, Y.; et al. Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol. 2018, 217, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, S.; Wang, G. Strigolactones are common regulators in induction of stomatal closure in planta. Plant Signal. Behav. 2018, 13, e1444322. [Google Scholar] [CrossRef]
- Shim, J.S.; Oh, N.; Chung, P.J.; Kim, Y.S.; Choi, Y.D.; Kim, J.K. Overexpression of OsNAC14 improves drought tolerance in rice. Front. Plant Sci. 2018, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Chesterfield, R.J.; Vickers, C.E.; Beveridge, C.A. Translation of Strigolactones from Plant Hormone to Agriculture: Achievements, Future Perspectives, and Challenges. Trends Plant Sci. 2020, 25, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.J.; Fonne-Pfister, R.; Screpanti, C.; De Mesmaeker, A. Strigolactones: Plant Hormones with Promising Features. Angew. Chem. Int. Ed. 2019, 58, 12778–12786. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, T.R.; Leite, M.F.A.; Lucheta, A.R.; Shimels, M.; Bouwmeester, H.J.; van Veen, J.A.; Kuramae, E.E. Rhizobacterial community structure differences among sorghum cultivars in different growth stages and soils. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Carvalhais, L.C.; Rincon-Florez, V.A.; Brewer, P.B.; Beveridge, C.A.; Dennis, P.G.; Schenk, P.M. The ability of plants to produce strigolactones affects rhizosphere community composition of fungi but not bacteria. Rhizosphere 2019, 9, 18–26. [Google Scholar] [CrossRef]
- Banasiak, J.; Borghi, L.; Stec, N.; Martinoia, E.; Jasiński, M. The Full-Size ABCG Transporter of Medicago truncatula Is Involved in Strigolactone Secretion, Affecting Arbuscular Mycorrhiza. Front. Plant Sci. 2020, 11, 1. [Google Scholar] [CrossRef]
- Liu, G.; Pfeifer, J.; de Brito Francisco, R.; Emonet, A.; Stirnemann, M.; Gübeli, C.; Hutter, O.; Sasse, J.; Mattheyer, C.; Stelzer, E.; et al. Changes in the allocation of endogenous strigolactone improve plant biomass production on phosphate-poor soils. New Phytol. 2018, 217, 784–798. [Google Scholar] [CrossRef]
- Pokluda, R.; Shehata, S.M.; Kopta, T. Vegetativer chemischer Status und Produktivität von Gartenkürbispflanzen (Cucurbita pepo L.) in Reaktion auf die Blattanwendung von Pentakeep und Strigolactonen unter NPK-Raten. Gesunde Pflanz. 2018, 70, 21–29. [Google Scholar] [CrossRef]
- Zheng, Y.; Kumar, N.; Gonzalez, P.; Etxeberria, E. Strigolactones restore vegetative and reproductive developments in Huanglongbing (HLB) affected, greenhouse-grown citrus trees by modulating carbohydrate distribution. Sci. Hortic. 2018, 237, 89–95. [Google Scholar] [CrossRef]
- Soto, M.J.; Fernández-Aparicio, M.; Castellanos-Morales, V.; García-Garrido, J.M.; Ocampo, J.A.; Delgado, M.J.; Vierheilig, H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem. 2010, 42, 383–385. [Google Scholar] [CrossRef]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073–1081. [Google Scholar] [CrossRef]
- Rehman, N.u.; Ali, M.; Ahmad, M.Z.; Liang, G.; Zhao, J. Strigolactones promote rhizobia interaction and increase nodulation in soybean (Glycine max). Microb. Pathog. 2018, 114, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Yoneyama, K.; Hugill, C.J.; Quittenden, L.J.; Reid, J.B. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant 2013, 6, 76–87. [Google Scholar] [CrossRef]
- Liu, J.; Novero, M.; Charnikhova, T.; Ferrandino, A.; Schubert, A.; Ruyter-Spira, C.; Bonfante, P.; Lovisolo, C.; Bouwmeester, H.J.; Cardinale, F. Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 2013, 64, 1967–1981. [Google Scholar] [CrossRef]
- Haq, B.U.I.; Ahmad, M.Z.; Ur Rehman, N.; Wang, J.; Li, P.; Li, D.; Zhao, J. Functional characterization of soybean strigolactone biosynthesis and signaling genes in Arabidopsis MAX mutants and GmMAX3 in soybean nodulation. BMC Plant Biol. 2017, 17, 1–20. [Google Scholar] [CrossRef]
- Yoneyama, K.K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K.K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef]
- Shindo, M.; Shimomura, K.; Yamaguchi, S.; Umehara, M. Upregulation of DWARF27 is associated with increased strigolactone levels under sulfur deficiency in rice. Plant Direct 2018, 2, e00050. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, L.; Yu, H.; Zeng, L.; Hu, J.; Ni, S.; Rao, Y.; Li, S.; Chu, J.; Meng, X.; et al. A Strigolactone Biosynthesis Gene Contributed to the Green Revolution in Rice. Mol. Plant 2020, 13, 923–932. [Google Scholar] [CrossRef]
- Jamil, M.; Charnikhova, T.; Houshyani, B.; van Ast, A.; Bouwmeester, H.J. Genetic variation in strigolactone production and tillering in rice and its effect on Striga hermonthica infection. Planta 2012, 235, 473–484. [Google Scholar] [CrossRef]
- Butt, H.; Jamil, M.; Wang, J.Y.; Al-Babili, S.; Mahfouz, M. Engineering plant architecture via CRISPR/Cas9-mediated alteration of strigolactone biosynthesis. BMC Plant Biol. 2018, 18, 174. [Google Scholar] [CrossRef]
- Lumbroso, A.; Villedieu-Percheron, E.; Zurwerra, D.; Screpanti, C.; Lachia, M.; Dakas, P.Y.; Castelli, L.; Paul, V.; Wolf, H.C.; Sayer, D.; et al. Simplified strigolactams as potent analogues of strigolactones for the seed germination induction of Orobanche cumana Wallr. Pest Manag. Sci. 2016, 72, 2054–2068. [Google Scholar] [CrossRef] [PubMed]
- Lachia, M.; Fonne-Pfister, R.; Screpanti, C.; Rendine, S.; Renold, P.; Witmer, D.; Lumbroso, A.; Godineau, E.; Hueber, D.; De Mesmaeker, A. New and scalable access to karrikin (KAR1) and evaluation of its potential application on corn germination. Helv. Chim. Acta 2018, 101, e201800081. [Google Scholar] [CrossRef]
- Chanclud, E.; Lacombe, B. Plant Hormones: Key Players in Gut Microbiota and Human Diseases? Trends Plant Sci. 2017, 22, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, A.; Topcuoğlu, Ş.F.; İnan, S. Auxin, Gibberellin, Cytokinin and Abscisic Acid Production in Some Bacteria. World J. Microbiol. Biotechnol. 2006, 22, 1061–1064. [Google Scholar] [CrossRef]
- Lievens, L.; Pollier, J.; Goossens, A.; Beyaert, R.; Staal, J. Abscisic Acid as Pathogen Effector and Immune Regulator. Front. Plant Sci. 2017, 8, 587. [Google Scholar] [CrossRef]
- Kim, S.W.; Goossens, A.; Libert, C.; Van Immerseel, F.; Staal, J.; Beyaert, R. Phytohormones: Multifunctional nutraceuticals against metabolic syndrome and comorbid diseases. Biochem. Pharmacol. 2020, 175, 113866. [Google Scholar] [CrossRef]
- Klessig, D.F.; Tian, M.; Choi, H.W. Multiple Targets of Salicylic Acid and Its Derivatives in Plants and Animals. Front. Immunol. 2016, 7, 206. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Villar, A.; Mulà, A.; Zangara, A.; Risco, E.; Smidt, C.R.; Hontecillas, R.; Leber, A.; Bassaganya-Riera, J. Abscisic Acid Standardized Fig (Ficus carica) Extracts Ameliorate Postprandial Glycemic and Insulinemic Responses in Healthy Adults. Nutrients 2019, 11, 1757. [Google Scholar] [CrossRef]
- Tumer, T.B.; Yılmaz, B.; Ozleyen, A.; Kurt, B.; Tok, T.T.; Taskin, K.M.; Kulabas, S.S. GR24, a synthetic analog of Strigolactones, alleviates inflammation and promotes Nrf2 cytoprotective response: In vitro and in silico evidences. Comput. Biol. Chem. 2018, 76, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Kizelsztein, P.; Komarnytsky, S.; Raskin, I. Hypoglycemic effects of brassinosteroid in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E652. [Google Scholar] [CrossRef]
- Sánchez-Sarasúa, S.; Moustafa, S.; García-Avilés, Á.; López-Climent, M.F.; Gómez-Cadenas, A.; Olucha-Bordonau, F.E.; Sánchez-Pérez, A.M. The effect of abscisic acid chronic treatment on neuroinflammatory markers and memory in a rat model of high-fat diet induced neuroinflammation. Nutr. Metab. 2016, 13, 1–11. [Google Scholar] [CrossRef]
- Modi, S.; Yaluri, N.; Kokkola, T.; Laakso, M. Plant-derived compounds strigolactone GR24 and pinosylvin activate SIRT1 and enhance glucose uptake in rat skeletal muscle cells. Sci. Rep. 2017, 7, 17606. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.X.; Han, Y.S.; Wang, J.C.; Yang, H.; Kong, H.; Liu, K.J.; Chen, S.Y.; Chen, Y.R.; Chang, Y.Q.; Chen, W.M.; et al. Strigolactones: A plant phytohormone as novel anti-inflammatory agents. MedChemComm 2018, 9, 181–188. [Google Scholar] [CrossRef]
- Kurt, B.; Ozleyen, A.; Antika, G.; Yilmaz, Y.B.; Tumer, T.B. Multitarget Profiling of a Strigolactone Analogue for Early Events of Alzheimer’s Disease: In Vitro Therapeutic Activities against Neuroinflammation. ACS Chem. Neurosci. 2020, 11, 501–507. [Google Scholar] [CrossRef]
- Bagasra, O.; Michaels, F.H.; Zheng, Y.M.; Bobroski, L.E.; Spitsin, S.V.; Fu, Z.F.; Tawadros, R.; Koprowski, H. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA 1995, 92, 12041–12045. [Google Scholar] [CrossRef]
- Brosnan, C.F.; Battistini, L.; Raine, C.S.; Dickson, D.W.; Casadevall, A.; Lee, S.C. Reactive nitrogen intermediates in human neuropathology: An overview. Dev. Neurosci. 1994, 16, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L. Role of COX-2 in inflammatory and degenerative brain diseases. Subcell. Biochem. 2007, 42, 127–141. [Google Scholar] [CrossRef]
- Wang, P.; Guan, P.P.; Wang, T.; Yu, X.; Guo, J.J.; Wang, Z.Y. Aggravation of Alzheimer’s disease due to the COX-2-mediated reciprocal regulation of IL-1β and Aβ between glial and neuron cells. Aging Cell 2014, 13, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wu, J.S.; Tsai, H.D.; Huang, C.Y.; Chen, J.J.; Sun, G.Y.; Lin, T.N. Peroxisome proliferator-activated receptor gamma (PPAR-γ) and neurodegenerative disorders. Mol. Neurobiol. 2012, 46, 114–124. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed]
- Tumer, T.B.; Rojas-Silva, P.; Poulev, A.; Raskin, I.; Waterman, C. Direct and indirect antioxidant activity of polyphenol- and isothiocyanate-enriched fractions from moringa oleifera. J. Agric. Food Chem. 2015, 63, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wang, Q.; Zhang, W.; Li, Z.; Wang, Y.; Hu, R. Oroxylin A exerts anti-inflammatory activity on lipopolysaccharide-induced mouse macrophage via Nrf2/ARE activation. Biochem. Cell Biol. 2014, 92, 337–348. [Google Scholar] [CrossRef]
- Hasan, M.N.; Razvi, S.S.I.; Kuerban, A.; Balamash, K.S.; Al-Bishri, W.M.; Abulnaja, K.O.; Choudhry, H.; Khan, J.A.; Moselhy, S.S.; Ma, Z.; et al. Strigolactones—A novel class of phytohormones as anti-cancer agents. J. Pest. Sci. 2018, 43, 168. [Google Scholar] [CrossRef]
- Steigerová, J.; Oklestkova, J.; Levková, M.; Rárová, L.; Kolář, Z.; Strnad, M. Brassinosteroids cause cell cycle arrest and apoptosis of human breast cancer cells. Chem. Biol. Interact. 2010, 188, 487–496. [Google Scholar] [CrossRef]
- Cohen, S.; Flescher, E. Methyl jasmonate: A plant stress hormone as an anti-cancer drug. Phytochemistry 2009, 70, 1600–1609. [Google Scholar] [CrossRef]
- Corrèze, C.; Blondeau, J.P.; Pomérance, M. p38 mitogen-activated protein kinase contributes to cell cycle regulation by cAMP in FRTL-5 thyroid cells. Eur. J. Endocrinol. 2005, 153, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Iyoda, K.; Sasaki, Y.; Horimoto, M.; Toyama, T.; Yakushijin, T.; Sakakibara, M.; Takehara, T.; Fujimoto, J.; Hori, M.; Wands, J.R.; et al. Involvement of the p38 mitogen-activated protein kinase cascade in hepatocellular carcinoma. Cancer 2003, 97, 3017–3026. [Google Scholar] [CrossRef]
- Mayzlish-Gati, E.; Laufer, D.; Grivas, C.F.; Shaknof, J.; Sananes, A.; Bier, A.; Ben-Harosh, S.; Belausov, E.; Johnson, M.D.; Artuso, E.; et al. Strigolactone analogs act as new anti-cancer agents in inhibition of breast cancer in xenograft model. Cancer Biol. Ther. 2015, 16, 1682–1688. [Google Scholar] [CrossRef]
- Kaverina, I.; Straube, A. Regulation of cell migration by dynamic microtubules. Semin. Cell Dev. Biol. 2011, 22, 968–974. [Google Scholar] [CrossRef]
- Schiff, P.B.; Horwitz, S.B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. USA 1980, 77, 1561–1565. [Google Scholar] [CrossRef]
- Pollock, C.B.; McDonough, S.; Wang, V.S.; Lee, H.; Ringer, L.; Li, X.; Prandi, C.; Lee, R.J.; Feldman, A.S.; Koltai, H.; et al. Strigolactone analogues induce apoptosis through activation of p38 and the stress response pathway in cancer cell lines and in conditionally reprogrammed primary prostate cancer cells. Oncotarget 2014, 5, 1683–1698. [Google Scholar] [CrossRef]
- Murphy, M.E. The HSP70 family and cancer. Carcinogenesis 2013, 34, 1181–1188. [Google Scholar] [CrossRef]
- Chang, H.L.; Wu, Y.C.; Su, J.H.; Yeh, Y.T.; Yuan, S.S.F. Protoapigenone, a novel flavonoid, induces apoptosis in human prostate cancer cells through activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase 1/2. J. Pharmacol. Exp. Ther. 2008, 325, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Croglio, M.P.; Haake, J.M.; Ryan, C.P.; Wang, V.S.; Lapier, J.; Schlarbaum, J.P.; Dayani, Y.; Artuso, E.; Prandi, C.; Koltai, H.; et al. Analogs of the novel phytohormone, strigolactone, trigger apoptosis and synergize with PARP inhibitors by inducing DNA damage and inhibiting DNA repair. Oncotarget 2016, 7, 13984–14001. [Google Scholar] [CrossRef][Green Version]
- Ward, A.; Khanna, K.K.; Wiegmans, A.P. Targeting homologous recombination, new pre-clinical and clinical therapeutic combinations inhibiting RAD51. Cancer Treat. Rev. 2015, 41, 35–45. [Google Scholar] [CrossRef]
- Hasan, M.N.; Choudhry, H.; Razvi, S.S.; Moselhy, S.S.; Kumosani, T.A.; Zamzami, M.A.; Omran, Z.; Halwani, M.A.; Al-Babili, S.; Abualnaja, K.O.; et al. Synthetic strigolactone analogues reveal anti-cancer activities on hepatocellular carcinoma cells. Bioorg. Med. Chem. Lett. 2018, 28, 1077–1083. [Google Scholar] [CrossRef]
- Dor, E.; Joel, D.M.; Kapulnik, Y.; Koltai, H.; Hershenhorn, J. The synthetic strigolactone GR24 influences the growth pattern of phytopathogenic fungi. Planta 2011, 234, 419–427. [Google Scholar] [CrossRef]
- Biolatti, M.; Blangetti, M.; D’arrigo, G.; Spyrakis, F.; Cappello, P.; Albano, C.; Ravanini, P.; Landolfo, S.; De Andrea, M.; Prandi, C.; et al. Strigolactone analogs are promising antiviral agents for the treatment of human cytomegalovirus infection. Microorganisms 2020, 8, 703. [Google Scholar] [CrossRef]
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021. [Google Scholar] [CrossRef]
- Gugliesi, F.; Coscia, A.; Griffante, G.; Galitska, G.; Pasquero, S.; Albano, C.; Biolatti, M. Where do we Stand after Decades of Studying Human Cytomegalovirus? Microorganisms 2020, 8, 685. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.R.; Wills, M.R.; Sinclair, J.H. HCMV Antivirals and Strategies to Target the Latent Reservoir. Viruses 2021, 13, 817. [Google Scholar] [CrossRef]
- Chen, S.-J.; Wang, S.-C.; Chen, Y.-C. Antiviral Agents as Therapeutic Strategies Against Cytomegalovirus Infections. Viruses 2019, 12, 21. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).