Single Origin Coffee Aroma: From Optimized Flavor Protocols and Coffee Customization to Instrumental Volatile Characterization and Chemometrics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatile Compounds Profile of the Studied Coffee Samples
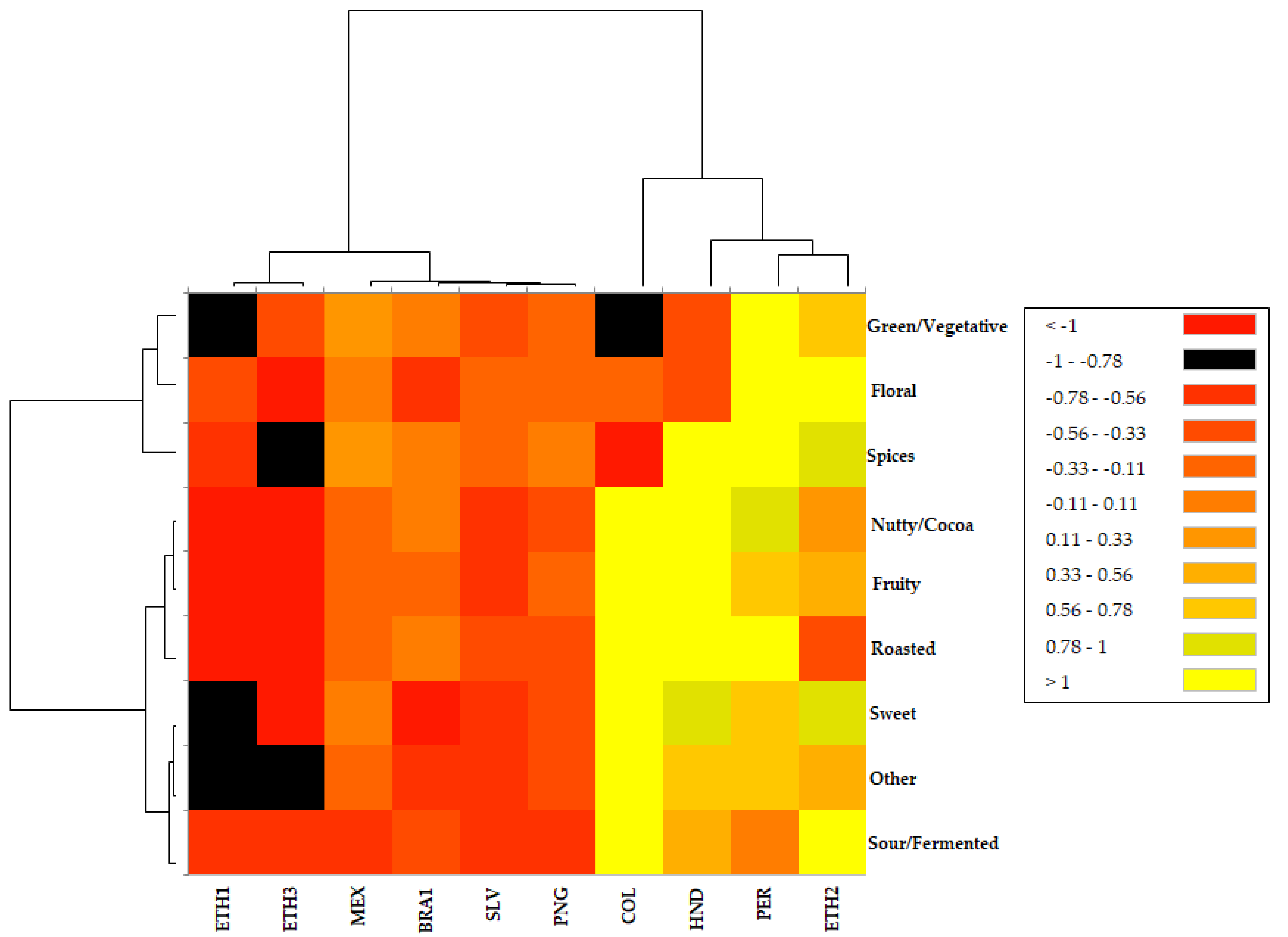
2.2. Volatile Odor Description and Categorization—Heatmap Analysis
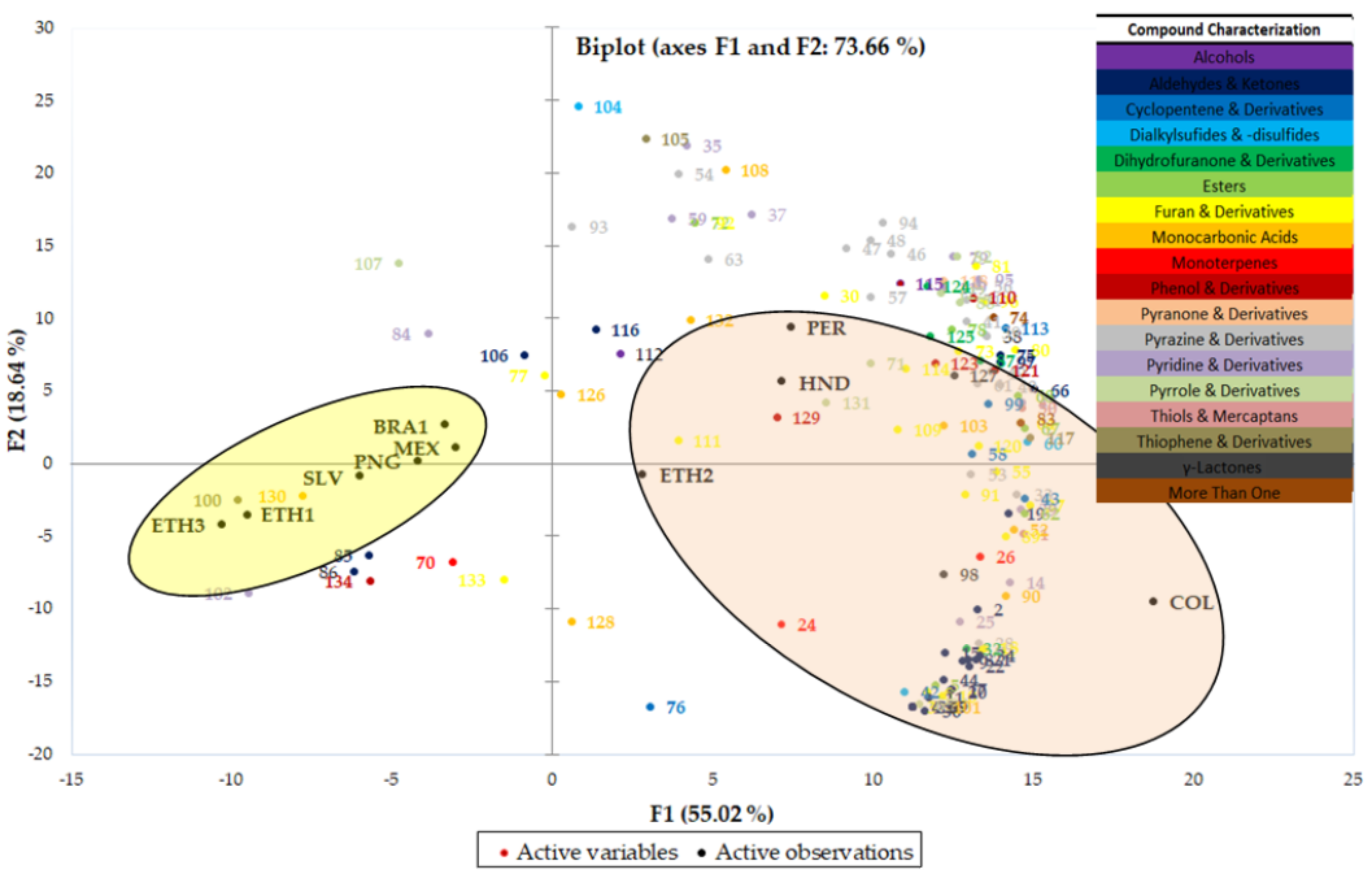
2.3. Sample Differentiation—Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
3. Materials and Methods
3.1. Coffee Samples
3.2. Volatile Compounds Analysis
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- International Coffee Organization. Available online: https://www.ico.org/Market-Report-20-21-e.asp (accessed on 20 June 2021).
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Lolli, V.; Acharjee, A.; Angelino, D.; Tassotti, M.; Rio, D.D.; Mena, P.; Caligiani, A. Chemical characterization of capsule-brewed espresso coffee aroma from the most widespread Italian brands by HS-SPME/GC-MS. Molecules 2020, 25, 1166. [Google Scholar] [CrossRef] [Green Version]
- Olechno, E.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. Impact of Brewing Methods on Total Phenolic Content (TPC) in Various Types of Coffee. Molecules 2020, 25, 5274. [Google Scholar] [CrossRef]
- Risticevic, S.; Carasek, E.; Pawliszyn, J. Headspace solid-phase microextraction-gas chromatographic-time-of-flight mass spectrometric methodology for geographical origin verification of coffee. Anal. Chim. Acta 2008, 617, 72–84. [Google Scholar] [CrossRef]
- Gloess, A.N.; Vietri, A.; Wieland, F.; Smrke, S.; Schönbächler, B.; López, J.A.S.; Petrozzi, S.; Bongers, S.; Koziorowski, T.; Yeretzian, C. Evidence of different flavour formation dynamics by roasting coffee from different origins: On-line analysis with PTR-ToF-MS. Int. J. Mass Spectrom. 2014, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Ongo, E.A.; Montevecchi, G.; Antonelli, A.; Sberveglieri, V.; Sevilla, F. Metabolomics fingerprint of Philippine coffee by SPME-GC-MS for geographical and varietal classification. Food Res. Int. 2020, 134. [Google Scholar] [CrossRef] [PubMed]
- Toledo, P.R.A.B.; Melo, M.M.R.; Pezza, H.R.; Toci, A.T.; Pezza, L.; Silva, C.M. Discriminant analysis for unveiling the origin of roasted coffee samples: A tool for quality control of coffee related products. Food Control. 2017, 73, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Toci, A.T.; Farah, A. Volatile fingerprint of Brazilian defective coffee seeds: Corroboration of potential marker compounds and identification of new low quality indicators. Food Chem. 2014, 153, 298–314. [Google Scholar] [CrossRef]
- Toci, A.T.; Azevedo, D.A.; Farah, A. Effect of roasting speed on the volatile composition of coffees with different cup quality. Food Res. Int. 2020, 137. [Google Scholar] [CrossRef]
- Buffo, R.A.; Cardelli-Freire, C. Coffee flavour: An overview. Flavour Fragr. J. 2004, 19, 99–104. [Google Scholar] [CrossRef]
- Flament, I. Coffee Flavor Chemistry, 1st ed.; John Wiley & Son Ltd.: Chichester, UK, 2002. [Google Scholar]
- Yeretzian, C.; Jordan, A.; Lindinger, W. Analysing the headspace of coffee by proton-transfer-reaction mass-spectrometry. Int. J. Mass Spectrom. 2003, 224, 115–139. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Ohtani, N.; Iwatsuki, K.; Sotoyama, K.; Wada, A.; Tokuno, K.; Iwabuchi, H.; Tanaka, K. Analysis of volatile compounds released during the grinding of roasted coffee beans using solid-phase microextraction. J. Agric. Food Chem. 2003, 51, 1961–1969. [Google Scholar] [CrossRef]
- Blank, I.; Sen, A.; Grosch, W. Potent odorants of the roasted powder and brew of Arabica coffee. Z. Für Lebensm. Unters. Forsch. 1992, 195, 239–245. [Google Scholar] [CrossRef]
- Czerny, M.; Mayer, F.; Grosch, W. Sensory study on the character impact odorants of roasted Arabica coffee. J. Agric. Food 1999, 47, 695–699. [Google Scholar] [CrossRef]
- Maeztu, L.; Sanz, C.; Andueza, S.; Paz De Peña, M.; Bello, J.; Cid, C. Characterization of espresso coffee aroma by static headspace GC-MS and sensory flavor profile. J. Agric. Food Chem. 2001, 49, 5437–5444. [Google Scholar] [CrossRef] [PubMed]
- Specialty Coffee Association. Available online: https://sca.coffee/research/coffee-tasters-flavor-wheel (accessed on 30 June 2021).
- World Coffee Research. Available online: https://worldcoffeeresearch.org/work/sensory-lexicon/ (accessed on 30 June 2021).
- Angeloni, S.; Scortichini, S.; Fiorini, D.; Sagratini, G.; Vittori, S.; Neiens, S.D.; Steinhaus, M.; Zheljazkov, V.D.; Maggi, F.; Caprioli, G. Characterization of odor-active compounds, polyphenols, and fatty acids in coffee silverskin. Molecules 2020, 25, 2993. [Google Scholar] [CrossRef] [PubMed]
- Calva-Estrada, S.J.; Utrilla-Vázquez, M.; Vallejo-Cardona, A.; Roblero-Pérez, D.B.; Lugo-Cervantes, E. Thermal properties and volatile compounds profile of commercial dark-chocolates from different genotypes of cocoa beans (Theobroma cacao L.) from Latin America. Food Res. Int. 2020, 136, 109594. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, P.; Gong, Z.; Feng, L.; Gao, S.; Wang, X.; Teng, J.; Zheng, L.; Liu, Z. Comparing characteristic aroma components of bead-shaped green teas from different regions using headspace solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry combined with chemometrics. Eur. Food Res. Technol. 2020, 246, 1703–1714. [Google Scholar] [CrossRef]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/ (accessed on 11 May 2021).
- Flavornet. Available online: www.flavornet.org (accessed on 11 May 2021).
- The Pherobase: Data of Pheromones and Semiochemicals. Available online: https://www.pherobase.com/ (accessed on 11 May 2021).
- Frankel, E.N. Lipid Oxidation, 2nd ed.; Woodhead Publishing Limited: Cambridge, UK, 2005. [Google Scholar]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, L.J. OdourThresholds, 2nd ed.; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Rabelo, M.H.S.; Borém, F.M.; de Lima, R.R.; Alves, A.P.C.; Pinheiro, A.C.M.; Ribeiro, D.E.; Santos, C.M.; Pereira, R.G.F.A. Impacts of quaker beans over sensory characteristics and volatile composition of specialty natural coffees. Food Chem. 2021, 342, 128304. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of coffee aroma via the fermentation of green coffee beans with Rhizopus oligosporus: I. Green coffee. Food Chem. 2016, 211, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Paraskevopoulou, A.; Pantazi, F.; Skendi, A. Cake perception, texture and aroma profile as affected by wheat flour and cocoa replacement with carob flour. Foods 2020, 9, 1586. [Google Scholar] [CrossRef] [PubMed]
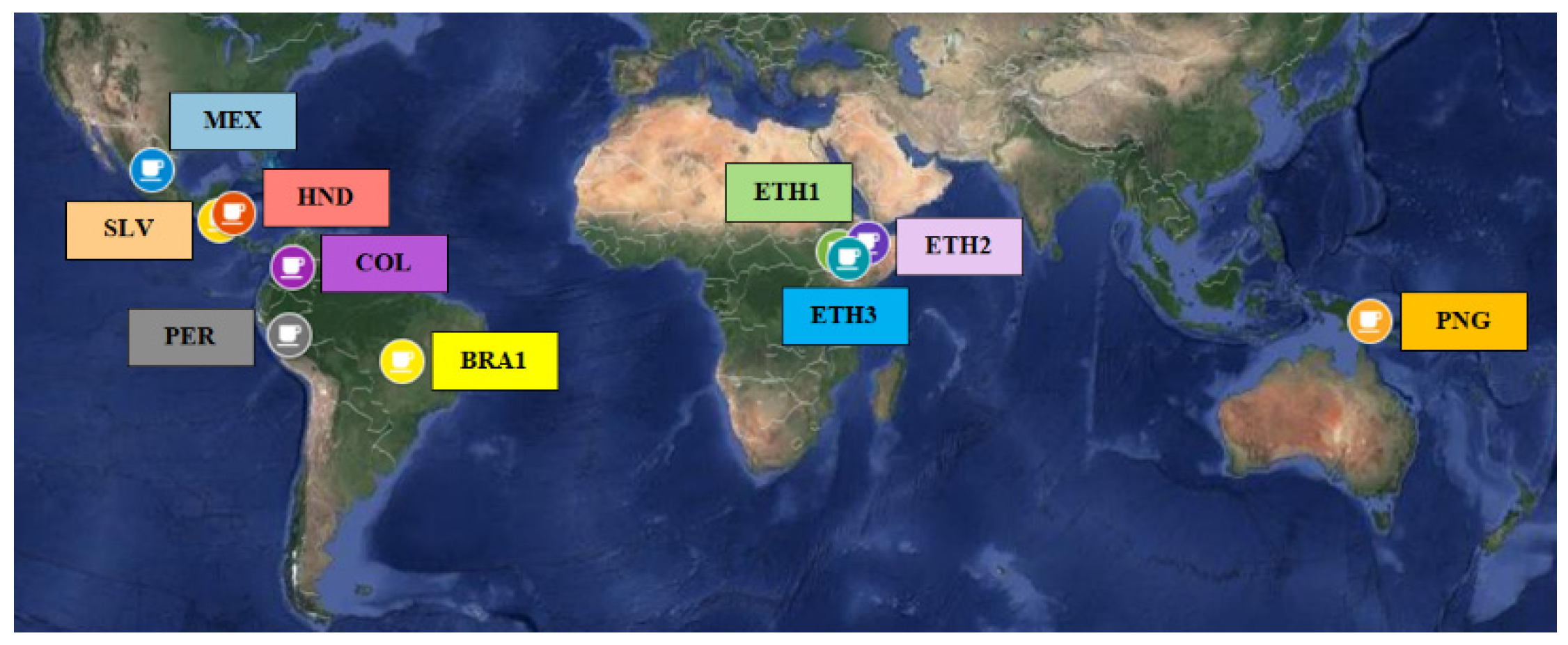

| Sample Label | Origin | Roasting Degree 1 | Brew Characteristics |
|---|---|---|---|
| COL | Colombia | Dark | Aroma: woody, spicy, toasted notes with nutty hints Acidity: gentle brightness Flavor: butter, caramel, chocolate, nuts Body: moderate body Aftertaste: dark chocolate with nutty hints |
| HND | Honduras | Medium | Aroma: vanilla, butter, caramel, nutty notes Acidity: moderate brightness Flavor: butter, caramel, chocolate, spicy, toast-like notes Body: full body Aftertaste: chocolate, nutty, sherry |
| MEX | Mexico | Medium/Light | Aroma: spicy, woody, vanilla notes Acidity: gentle brightness Flavor: sandalwood, honey, pineapple, vanilla Body: medium body Aftertaste: dark chocolate hints, vanilla |
| SLV | El Salvador | Medium/Light | Aroma: spicy, fruity, popcorn, toasted notes Acidity: gentle brightness Flavor: mocha, caramel, apple, nutty, almonds Body: moderate body Aftertaste: dark chocolate hints, nutty |
| PER | Peru | Medium | Aroma: spicy, floral, citrus notes Acidity: gentle brightness Flavor: tobacco, caramel, vanilla, nutty Body: moderate body Aftertaste: velvety, sweet, caramel |
| PNG | Papua New Guinea | Medium/Light | Aroma: spicy, apricot, bouquet notes Acidity: moderate brightness Flavor: woody, spicy, rose, honey, apricot, malty Body: full body Aftertaste: dark chocolate hints, butter, caramel |
| BRA1 | Brazil | Medium | Aroma: toasted notes with nutty hints Acidity: gentle brightness Flavor: caramel, chocolate, nutty Body: full body Aftertaste: dark chocolate |
| ETH1 | Ethiopia, Sidamo | Light | Aroma: citrus, bouquet notes Acidity: sparkle brightness Flavor: lemon, caramel, berries Body: medium body Aftertaste: dark chocolate hints |
| ETH2 | Ethiopia, Harrar | Medium/Dark | Aroma: berries, rose, floral, apricot notes Acidity: moderate brightness Flavor: butter, caramel, apricot, rose bouquet Body: full body Aftertaste: dark chocolate hints, caramel |
| No a | Volatile Compound | RI b | Compound Category c | Odor Description d | Coffee Taster’s Flavor Wheel Classification | Relative Content (%) e | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COL | HND | ETH1 | PER | MEX | SLV | ETH2 | ETH3 | BRA | PNG | ||||||
| Pyrazine and Derivatives | |||||||||||||||
| 28 | Pyrazine | 1223 | PZ | nutty, roasted | nutty/cocoa | 0.49 | 0.14 | -f | 0.09 | - | - | 0.33 | - | - | - |
| 33 | Methylpyrazine | 1281 | PZD | nutty, cocoa, roasted | nutty/cocoa | 5.03 | 4.49 | 0.87 | 3.25 | 2.38 | 1.33 | 4.99 | 0.52 | 0.72 | 1.73 |
| 38 | 2,5-Dimethylpyrazine | 1336 | PZD | cocoa, roasted, nutty | nutty/cocoa | 3.62 | 5.30 | 3.97 | 4.17 | 4.73 | 4.88 | 3.88 | 3.21 | 5.26 | 4.66 |
| 39 | 2,6-Dimethylpyrazine | 1343 | PZD | cocoa, roasted, nutty | nutty/cocoa | 3.63 | 5.51 | 3.01 | 4.12 | 4.35 | 4.07 | 3.64 | 2.53 | 4.96 | 4.35 |
| 40 | Ethylpyrazine | 1348 | PZD | nutty, roasted, cocoa | nutty/cocoa | 1.69 | 2.37 | 0.96 | 1.58 | 1.63 | 1.26 | 1.61 | 0.74 | 1.48 | 1.56 |
| 41 | 2,3-Dimethylpyrazine | 1360 | PZD | nutty, roasted, cocoa | nutty/cocoa | 0.83 | 1.40 | 0.48 | 0.94 | 0.91 | 0.74 | 0.83 | 0.38 | 1.25 | 0.97 |
| 46 | 2-Ethyl-6-methylpyrazine | 1396 | PZD | cocoa, roasted | nutty/cocoa | 1.68 | 3.24 | 2.07 | 2.47 | 2.68 | 2.91 | 1.61 | 2.03 | 4.15 | 2.59 |
| 47 | 2-Ethyl-5-methylpyrazine | 1404 | PZD | coffee, nutty | nutty/cocoa | 1.18 | 2.10 | 2.45 | 1.74 | 1.92 | 2.28 | 1.27 | 1.76 | 3.47 | 2.41 |
| 48 | 2-Ethyl-3-methylpyrazine | 1408 | PZD | nutty | nutty/cocoa | 1.66 | 3.14 | 2.19 | 2.61 | 2.61 | 3.14 | 1.79 | 2.23 | 4.88 | 2.65 |
| Trimethylpyrazine | nutty, cocoa, potato, roasted | ||||||||||||||
| 49 | Propylpyrazine | 1432 | PZD | green, vegetable, nutty | green/vegetative | 0.18 | 0.22 | 0.12 | 0.18 | 0.14 | 0.17 | 0.14 | 0.13 | 0.30 | 0.14 |
| 51 | Vinylpyrazine | 1448 | PZD | nutty | nutty/cocoa | 0.66 | 0.93 | 0.95 | 0.88 | 0.88 | 1.05 | 0.70 | 1.03 | 1.37 | 0.85 |
| 53 | 3-Ethyl-2,5-dimethylpyrazine | 1462 | PZD | nutty, cocoa, roasted | nutty/cocoa | 1.61 | 1.09 | 1.42 | 1.51 | 1.61 | 2.03 | 0.56 | 1.50 | 1.92 | 1.24 |
| 54 | 2,3-Diethylpyrazine | 1468 | PZD | raw, nutty | green/vegetative | - | 0.08 | 0.08 | 0.08 | - | - | - | - | 0.09 | - |
| 56 | 2-Ethyl-3,5-dimethylpyrazine | 1474 | PZD | nutty, burnt, roasted, coffee | nutty/cocoa | 0.47 | 0.69 | 0.64 | 0.67 | 0.68 | 0.75 | 0.57 | 0.61 | 0.85 | 0.64 |
| 57 | 2-Methyl-6-propyl-pyrazine | 1477 | PZD | burnt, nutty, roasted | nutty/cocoa | 0.17 | 0.25 | 0.00 | 0.23 | 0.21 | - | - | - | 0.43 | 0.23 |
| 61 | 2-Methyl-6-vinylpyrazine | 1499 | PZD | nutty | nutty/cocoa | 0.47 | 0.38 | 0.72 | 0.65 | 0.59 | 0.77 | 0.52 | 0.82 | 0.82 | 0.54 |
| 63 | 2-Methyl-5-vinylpyrazine | 1502 | PZD | coffee | roasted | 0.39 | 0.64 | 0.93 | 0.82 | 0.75 | 1.06 | - | 1.08 | 1.04 | 0.70 |
| 74 | (1-Methylethenyl)-pyrazine | 1574 | PZD | caramel, chocolate, nutty, roasted | sweet | 0.36 | 0.61 | 0.83 | 0.74 | 0.65 | 0.85 | 0.40 | 0.92 | 0.91 | 0.62 |
| 83 | Acetylpyrazine | 1645 | PZD | popcorn, nutty, coffee, roasted | nutty/cocoa | 1.02 | 1.14 | 0.88 | 1.07 | 0.79 | 1.03 | 1.05 | 0.91 | 1.1 | 0.83 |
| 93 | 6-Methyl-2-acetylpyrazine | 1706 | PZD | roasted, coffee, cocoa, popcorn | nutty/cocoa | - | 1.27 | 1.65 | 1.13 | - | - | - | 1.93 | 2.14 | - |
| 94 | 5-Methyl-2-acetylpyrazine | 1715 | PZD | nutty, popcorn | nutty/cocoa | 0.90 | 1.14 | 2.21 | 1.69 | 1.56 | 2.29 | 1.22 | 2.51 | 1.98 | 1.66 |
| 26.04 | 36.13 | 26.43 | 30.62 | 29.07 | 30.61 | 25.11 | 24.84 | 39.12 | 28.37 | ||||||
| Furan and Derivatives | |||||||||||||||
| 3 | Furan | 801 | F | ethereal | other | 0.14 | - | - | - | - | - | - | - | - | - |
| 6 | 2-Methylfuran | 878 | FD | cocoa, nutty, coffee | nutty/cocoa | 1.13 | 0.06 | - | 0.06 | 0.04 | - | - | - | - | 0.04 |
| 10 | 2,5-Dimethylfuran | 959 | FD | meaty, roasted | other | 0.29 | 0.04 | - | - | - | - | 0.06 | - | - | - |
| 18 | Vinylfuran | 1087 | FD | phenolic, coffee | other | 0.25 | 0.07 | - | 0.05 | 0.02 | - | 0.13 | - | - | 0.03 |
| 27 | 2-(2-Propenyl)furan | 1219 | FD | - f | - f | 0.35 | 0.32 | 0.18 | 0.22 | 0.20 | 0.13 | 0.26 | 0.18 | 0.19 | 0.22 |
| 30 | 2-Pentylfuran | 1245 | FD | green, earthy, beany, vegetable | green/vegetative | 0.05 | 0.16 | - | 0.07 | 0.15 | - | 0.05 | - | - | 0.10 |
| 31 | 2-(Methoxymethyl)-furan | 1247 | FD | coffee, roasted | roasted | 0.24 | 0.07 | - | 0.04 | - | - | 0.13 | - | - | - |
| 55 | Furfural | 1470 | FD | sweet, woody, almond | sweet | 7.76 | 7.49 | 5.80 | 6.52 | 8.10 | 4.39 | 9.00 | 3.68 | 1.88 | 7.15 |
| 64 | Acetylfuran | 1510 | FD | nutty, sweet, cocoa, coffee | nutty/cocoa | 3.67 | 4.14 | 3.35 | 3.50 | 4.10 | 3.26 | 3.44 | 2.87 | 2.79 | 4.10 |
| 73 | 5-Methylfurfural | 1573 | FD | caramel, maple, spicy | sweet | 10.36 | 12.34 | 24.99 | 14.53 | 18.80 | 19.06 | 12.85 | 23.68 | 14.07 | 19.52 |
| 74 | 1-(2-Furanyl)-1-propanone | 1574 | FD | fruity | fruity | 0.33 | 0.24 | 0.24 | 0.23 | 0.33 | 0.50 | 0.33 | 0.32 | 0.14 | 0.45 |
| 77 | 2,2’-Bifuran | 1590 | FD | meaty, sulfury | other | - | 0.39 | - | - | 0.45 | - | - | 0.72 | - | - |
| 80 | 2,2’-Methylenebis-furan, | 1618 | FD | roasted | roasted | 0.96 | 1.08 | 1.21 | 1.22 | 1.15 | 1.41 | 0.83 | 1.42 | 1.45 | 1.21 |
| 81 | 2-Acetyl-5-methylfuran | 1625 | FD | nutty, musty | nutty/cocoa | 0.42 | 0.54 | 0.78 | 0.67 | 0.64 | 0.74 | 0.50 | 0.90 | 0.77 | 0.70 |
| 89 | 2-Furanmethanol | 1672 | FD | caramel, coffee | sweet | 8.76 | 6.03 | 1.76 | 4.95 | 3.09 | 1.77 | 9.28 | 1.57 | 1.14 | 2.60 |
| 91 | 2-Furfuryl-5-methylfuran | 1682 | FD | - | - | 0.27 | 0.17 | 0.29 | 0.31 | 0.21 | 0.33 | 0.19 | 0.27 | - | 0.19 |
| 92 | 1-(5-Methyl-2-furanyl)-1-propanone | 1701 | FD | green, nutty | green/vegetative | - | - | - | 0.41 | - | - | - | - | - | - |
| 96 | 3,4-Dimethyl-2,5-furandione | 1757 | FND | - | - | 0.62 | 0.68 | 0.84 | 0.92 | 0.85 | 1.00 | 0.72 | 1.05 | 1.19 | 0.88 |
| 109 | 3-(2-Furanyl)-2-propenal (3-(2-Furyl)acrolein) | 1860 | FD | spicy, woody, cinnamon | spices | 0.23 | 0.18 | 0.52 | 0.33 | 0.38 | 0.45 | 0.30 | 0.57 | 0.28 | 0.35 |
| 111 | 2-Methyl-3(2-furyl) acrolein (cinnamon acrolein) | 1871 | FD | spicy, cinnamon, woody | spices | 0.08 | 0.06 | 0.14 | 0.12 | 0.10 | 0.23 | - | 0.25 | 0.10 | 0.11 |
| 114 | 4-(2-Furanyl)-3-buten-2-one | 1907 | FD | spicy, cinnamon | spices | 0.10 | 0.09 | 0.21 | 0.17 | 0.14 | 0.21 | 0.13 | 0.26 | 0.14 | 0.16 |
| 120 | Furan, 2,2’-[oxybis(methylene)]bis- (Furfuryl ether) | 1985 | FD | coffee, nutty, earthy | nutty/cocoa | 0.12 | 0.08 | 0.16 | 0.16 | 0.12 | 0.19 | 0.14 | 0.19 | 0.13 | 0.13 |
| 133 | 5-Hydroxymethylfurfural | 2517 | FD | buttery, caramel | sweet | 0.04 | 0.03 | 0.19 | 0.06 | 0.08 | 0.11 | 0.06 | 0.15 | 0.01 | 0.08 |
| 36.17 | 34.26 | 40.66 | 34.54 | 38.95 | 33.78 | 38.4 | 38.08 | 24.28 | 38.02 | ||||||
| Esters | |||||||||||||||
| 5 | Methyl acetate | 833 | ES | fruity, fresh, sweet | fruity | 0.20 | 0.02 | - | 0.02 | - | - | - | - | - | - |
| 62 | Furfuryl formate | 1500 | ES | ethereal | other | 1.16 | 0.96 | 0.43 | 0.73 | 0.66 | 0.53 | 1.09 | 0.37 | 0.46 | 0.71 |
| 67 | 1-(Acetyloxy)-2-butanone | 1538 | ES | - | - | 0.76 | 0.87 | 0.48 | 0.66 | 0.63 | 0.57 | 0.73 | 0.50 | 0.66 | 0.77 |
| 68 | 2-Furanmethanol, acetate | 1542 | ES | fruity, sweet | fruity | 4.44 | 5.14 | 3.71 | 4.44 | 4.63 | 4.75 | 4.75 | 3.77 | 5.58 | 5.76 |
| 72 | 2-Furancarbothioic acid, S-methyl ester | 1562 | ES | alliaceous, onion, cabbage | green/vegetative | - | - | - | 0.05 | - | - | - | - | - | - |
| 78 | 2-Furanmethanol propanoate | 1605 | ES | fruity, sweet | fruity | 0.74 | 0.84 | - | 0.98 | 0.93 | 1.29 | 0.77 | 1.26 | 1.34 | 1.05 |
| 7.30 | 7.83 | 4.62 | 6.88 | 6.85 | 7.14 | 7.34 | 5.90 | 8.04 | 8.29 | ||||||
| Pyrrole and Derivatives | |||||||||||||||
| 23 | 1-Methyl-1H-pyrrole | 1149 | PRD | woody, herbal, smoky | other | 0.32 | 0.02 | - | - | - | - | 0.15 | - | - | - |
| 65 | Pyrrole | 1520 | PR | nutty, sweet | nutty/cocoa | 0.15 | - | - | - | - | - | 0.10 | - | - | - |
| 71 | 2-Methylpyrrole | 1560 | PRD | - | - | 0.14 | 0.18 | - | 0.20 | - | - | 0.14 | 0.33 | 0.38 | - |
| 82 | 1-Methyl-1H-pyrrole-2-carboxaldehyde | 1641 | PRD | roasted, nutty | nutty/cocoa | 1.68 | 2.24 | 3.33 | 2.59 | 2.88 | 3.14 | 2.26 | 3.46 | 3.61 | 2.98 |
| 88 | 2-Acetyl-1-methylpyrrole | 1670 | PRD | earthy | other | 0.67 | 0.77 | 1.00 | 0.95 | 0.94 | 1.26 | 0.72 | 1.23 | 1.47 | 1.00 |
| 107 | 1-(Furan-2-ylmethyl)pyrrole (1-Furfurylpyrrole) | 1834 | PRD | vegetable, green | green/vegetative | - | - | 2.27 | 2.07 | 2.09 | 2.16 | 1.61 | 2.45 | 2.08 | 2.05 |
| 119 | 1-(1H-Pyrrol-2-yl)ethenone (2-Acetylpyrrole) | 1975 | PRD | musty, nutty | nutty/cocoa | 1.21 | 1.28 | 2.27 | 1.96 | 1.90 | 2.46 | 1.53 | 2.68 | 2.50 | 1.81 |
| 122 | 1H-Pyrrole-2-carboxaldehyde (2-Formylpyrrole) | 2031 | PRD | musty, coffee | nutty/cocoa | 0.90 | 1.01 | 2.10 | 1.52 | 1.65 | 1.62 | 1.19 | 2.14 | 1.45 | 1.48 |
| 131 | Indole | 2468 | PRD | floral | floral | 0.02 | - | - | 0.04 | 0.03 | - | 0.03 | - | - | 0.04 |
| 5.09 | 5.50 | 10.97 | 9.33 | 9.49 | 10.64 | 7.73 | 12.29 | 11.49 | 9.36 | ||||||
| Pyridine and Derivatives | |||||||||||||||
| 14 | 1,2,3,6-Tetrahydro-1-methyl-pyridine | 1036 | PDD | caramel | sweet | 0.09 | 0.04 | - | 0.04 | - | - | 0.06 | - | - | - |
| 25 | Pyridine | 1196 | PD | fishy, sour | sour/fermented | 4.61 | 1.52 | 0.22 | 1.16 | 0.42 | 0.30 | 4.81 | 0.20 | 0.22 | 0.33 |
| 29 | 2-Methylpyridine | 1236 | PDD | sweaty, sour | sour/fermented | 0.17 | 0.15 | - | 0.09 | 0.05 | - | 0.13 | - | - | 0.04 |
| 35 | 3-Methylpyridine | 1309 | PDD | green, earthy, nutty | green/vegetative | - | 0.13 | - | 0.10 | 0.10 | 0.06 | - | - | 0.09 | 0.05 |
| 37 | 4-Methylpyridine | 1317 | PDD | green | green/vegetative | - | 0.08 | - | 0.07 | 0.07 | - | 0.09 | - | - | - |
| 45 | 3-Ethylpyridine | 1390 | PDD | tobacco, oakmoss | roasted | 0.26 | 0.26 | 0.14 | 0.26 | 0.22 | 0.26 | 0.27 | 0.17 | 0.37 | 0.21 |
| 59 | 3-Vinylpyridine | 1488 | PDD | - | - | - | - | - | 0.23 | 0.13 | - | - | - | - | - |
| 79 | 2-Acetylpyridine | 1613 | PDD | popcorn, tobacco | roasted | 0.34 | 0.44 | 0.62 | 0.55 | 0.53 | 0.67 | 0.41 | 0.73 | 0.75 | 0.56 |
| 84 | 2-Acetyl-6-methylpyridine | 1649 | PDD | roasted, coffee, cocoa | roasted | - | - | 0.24 | 0.20 | 0.17 | 0.25 | - | 0.30 | - | - |
| 95 | N-Acetyl-4(H)-pyridine | 1720 | PDD | - | - | 0.82 | 1.11 | 1.36 | 1.24 | 1.20 | 1.51 | 1.07 | 1.38 | 1.51 | 1.38 |
| 102 | 4-Acetylpyridine | 1805 | PDD | burnt, coffee | roasted | - | - | 0.33 | - | - | 0.37 | - | 0.39 | - | - |
| 6.29 | 3.73 | 2.91 | 3.94 | 2.89 | 3.42 | 6.84 | 3.17 | 2.94 | 2.57 | ||||||
| Carbonic Acids | |||||||||||||||
| 52 | Acetic acid | 1458 | MA | acidic, pungent, cheesy | sour/fermented | 1.20 | 0.88 | 0.19 | 0.58 | 0.26 | 0.31 | 0.99 | 0.25 | 1.00 | 0.40 |
| 69 | Propanoic acid | 1544 | MA | acidic, pungent, cheesy | sour/fermented | 0.24 | - | - | - | - | - | - | - | - | - |
| 83 | 4-Hydroxy-butanoic acid | 1648 | HCA | - | - | 0.33 | 0.25 | 0.37 | 0.42 | 0.44 | 0.52 | 0.59 | 0.32 | 0.78 | 0.38 |
| 90 | 3-Methyl-butanoic acid | 1675 | MA | cheesy, sour, sweaty | sour/fermented | 0.98 | 0.55 | - | 0.24 | - | - | 0.47 | - | 0.21 | - |
| 101 | 2-Butenoic acid (Crotonic acid) | 1765 | MA | milky | sour/fermented | 0.21 | - | - | - | - | - | - | - | - | - |
| 103 | 3-Methyl-2-butenoic acid | 1823 | MA | phenolic, dairy, green | other | 0.71 | 0.79 | 0.66 | 0.86 | 0.80 | 0.93 | 1.10 | 0.52 | - | 0.82 |
| 108 | Hexanoic acid | 1858 | FA | sour, fatty, sweaty | sour/fermented | - | 0.10 | - | 0.08 | - | - | - | - | 0.04 | 0.07 |
| 126 | Octanoic Acid | 2073 | FA | sour, fatty, sweaty | sour/fermented | - | 0.07 | 0.14 | - | - | - | - | - | - | - |
| 128 | Nonanoic acid | 2182 | FA | sour, fatty, sweaty | sour/fermented | 0.07 | 0.07 | 0.12 | - | 0.14 | 0.26 | 0.05 | 0.14 | 0.08 | 0.04 |
| 130 | Decanoic acid | 2288 | FA | unpleasant rancid sour fatty citrus | sour/fermented | - | 0.03 | 0.08 | - | 0.07 | 0.12 | 0.04 | 0.10 | 0.03 | - |
| 132 | Dodecanoic acid | 2510 | FA | fatty, waxy | sour/fermented | - | 0.02 | - | - | - | - | - | - | - | - |
| 3.74 | 2.76 | 1.56 | 2.18 | 1.71 | 2.14 | 3.24 | 1.33 | 2.14 | 1.71 | ||||||
| Aldehydes and Ketones | |||||||||||||||
| 2 | Acetaldehyde | 720 | AA | fruity, fresh, aldehydic | fruity | 0.13 | 0.02 | - | 0.05 | - | - | - | - | - | - |
| 4 | 2-Methylpropanal | 817 | AA | aldehydic, fresh, floral, pungent | other | 0.52 | - | - | - | - | - | - | - | - | - |
| 7 | 2-Butanone | 906 | AO | acetone, ethereal | other | 0.22 | - | - | - | - | - | - | - | - | - |
| 8 | 2-Methylbutanal | 920 | AA | musty, cocoa, coffee, nutty | other | 0.59 | 0.15 | - | 0.07 | 0.01 | - | 0.07 | - | - | 0.04 |
| 9 | 3-Methylbutanal | 924 | AA | aldehydic, fruity, green | fruity | 0.56 | 0.13 | - | 0.07 | 0.02 | - | 0.04 | - | - | 0.03 |
| 11 | 2,3-Butanedione | 983 | αDK | sweet, caramel, buttery | sweet | 0.39 | 0.02 | - | 0.02 | - | - | 0.02 | - | - | 0.02 |
| 12 | 3-Pentanone | 986 | AO | ethereal, acetone | other | 0.10 | - | - | - | - | - | - | - | - | - |
| 15 | (Z)-2-Butenal | 1041 | AE | pungent, suffocating | other | 0.12 | - | - | 0.04 | - | - | - | - | - | - |
| 16 | 3-Hexanone | 1067 | AO | fruity, sweet | fruity | 0.05 | - | - | - | - | - | - | - | - | - |
| 17 | 2,3-Pentanedione | 1072 | αDK | sweet, caramel, buttery | sweet | 0.96 | 0.13 | 0.07 | 0.10 | 0.04 | 0.05 | 0.39 | 0.10 | - | 0.09 |
| 19 | Hexanal | 1093 | AA | green, fresh, fatty | green/vegetative | 0.04 | 0.04 | - | 0.02 | 0.01 | - | 0.03 | - | - | 0.01 |
| 20 | (E)-2-Methyl-2-butenal, | 1103 | AE | green, fresh, fruity | green/vegetative | 0.14 | 0.03 | - | - | - | - | 0.06 | - | - | - |
| 21 | 2,3-Hexanedione | 1137 | αHK | buttery, sweet, caramel | sweet | 0.19 | 0.05 | - | 0.03 | - | - | 0.09 | - | - | 0.02 |
| 22 | 3,4-Hexanedione | 1146 | αDK | buttery, sweet, caramel | sweet | 0.12 | 0.04 | - | 0.01 | - | - | 0.05 | - | - | 0.01 |
| 34 | 3-Hydroxy-2-butanone (acetoin) | 1293 | αHK | buttery, creamy, dairy | sour/fermented | 0.46 | 0.11 | - | 0.08 | - | - | 0.17 | - | - | - |
| 36 | 1-Hydroxy-2-propanone | 1312 | αHK | caramel, sweet | sweet | 0.74 | - | - | - | - | - | 0.27 | - | - | - |
| 44 | 1-Hydroxy-2-butanone | 1386 | αHK | coffee, sweet | sweet | 0.20 | 0.04 | - | - | - | - | 0.14 | - | - | - |
| 66 | Benzaldehyde | 1528 | AAD | fruity, sweet, bitter almond | fruity | 0.39 | 0.39 | 0.48 | 0.46 | 0.47 | 0.52 | 0.40 | 0.55 | 0.57 | 0.45 |
| 75 | 3,6-Heptanedione | 1577 | γDK | - | - | 0.22 | 0.22 | 0.34 | 0.28 | 0.27 | 0.26 | 0.22 | 0.33 | 0.38 | 0.35 |
| 85 | Benzeneacetaldehyde | 1658 | AD | honey, sweet, floral, green | sweet | - | - | 0.26 | - | - | - | - | - | - | - |
| 86 | 2,4-Nonadienal, (E,E)- | 1661 | AKD | fatty, green | green/vegetative | - | - | - | - | - | - | - | 1.16 | - | - |
| 97 | 2-Cyclohexene-1,4-dione | 1760 | CD | - | - | 0.23 | 0.22 | 0.39 | 0.31 | 0.32 | 0.38 | 0.28 | 0.41 | 0.39 | 0.32 |
| 106 | trans-β-Damascenone | 1830 | NK | floral, sweet, fruity | floral | - | - | 0.20 | 0.14 | - | 0.14 | 0.12 | 0.13 | - | - |
| 116 | α-Εthylidene-benzeneacetaldehyde | 1934 | AD | musty, sweet, cocoa | other | - | - | 0.07 | 0.08 | - | - | 0.06 | 0.09 | - | - |
| 6.37 | 1.59 | 1.81 | 1.76 | 1.14 | 1.35 | 2.41 | 2.77 | 1.34 | 1.34 | ||||||
| Phenol and Derivatives | |||||||||||||||
| 110 | 2-Methoxy-phenol (guaiacol) | 1867 | PHD | smoky, burnt, spicy | roasted | 0.78 | 0.83 | 1.45 | 1.26 | 1.08 | 1.15 | 0.84 | 1.58 | 1.32 | 1.18 |
| 121 | Phenol | 2010 | PH | phenolic, plastic, rubbery | other | 0.62 | 0.55 | 1.04 | 0.89 | 0.84 | 0.94 | 0.72 | 1.07 | 0.82 | 0.83 |
| 123 | 4-Ethyl-2-methoxy-phenol (4-ethylguaiacol) | 2036 | PHD | spicy, phenolic | spices | 0.14 | 0.12 | 0.22 | 0.23 | 0.16 | 0.29 | 0.18 | 0.25 | 0.20 | 0.22 |
| 129 | 2-Methoxy-4-vinylphenol (4-Vinylguaiacol) | 2202 | PHD | spicy, clove, phenolic | spices | 0.48 | 0.38 | 1.41 | 0.84 | 0.71 | 1.25 | 0.70 | 1.22 | 0.52 | 0.92 |
| 134 | Vanillin | 2591 | PHD | sweet, vanilla | sweet | 0.01 | 0.01 | 0.04 | 0.02 | 0.03 | 0.04 | 0.02 | 0.07 | 0.03 | 0.03 |
| 2.03 | 1.89 | 4.16 | 3.24 | 2.82 | 3.67 | 2.46 | 4.19 | 2.89 | 3.18 | ||||||
| Cyclopentene and Derivatives | |||||||||||||||
| 42 | 2-Cyclopenten-1-one | 1368 | CP | - | - | 0.18 | - | - | - | - | - | 0.16 | - | - | - |
| 43 | 2-Methyl-2-cyclopenten-1-one | 1383 | CPD | - | - | 0.13 | 0.11 | - | 0.08 | 0.06 | - | 0.10 | - | - | 0.05 |
| 58 | 3,4,5-Trimethyl-2-cyclopenten-1-one | 1483 | CPD | - | - | 0.16 | 0.17 | - | 0.09 | 0.08 | 0.08 | - | - | 0.12 | 0.10 |
| 76 | 2-Cyclopentene-1,4-dione | 1583 | CPD | - | - | 0.33 | 0.00 | 0.67 | - | 0.62 | - | - | 0.55 | - | - |
| 99 | 2-Hydroxy-2-cyclopenten-1-one | 1771 | CPD | maple, caramel | sweet | 0.35 | 0.35 | - | 0.40 | - | - | - | - | - | - |
| 113 | 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 1897 | CPD | sweet, caramel, maple | sweet | 0.35 | 0.37 | 0.53 | 0.51 | 0.48 | 0.52 | 0.40 | 0.57 | 0.56 | 0.48 |
| 1.50 | 1.00 | 1.20 | 1.08 | 1.24 | 0.60 | 0.66 | 1.12 | 0.68 | 0.63 | ||||||
| Pyranone Derivatives | |||||||||||||||
| 118 | Maltol | 1972 | PND | sweet, caramel, cotton candy | sweet | 1.69 | 1.82 | 2.91 | 2.79 | 2.54 | 3.49 | 2.11 | 3.76 | 3.42 | 2.74 |
| 1.69 | 1.82 | 2.91 | 2.79 | 2.54 | 3.49 | 2.11 | 3.76 | 3.42 | 2.74 | ||||||
| Dihydrofuranone Derivatives | |||||||||||||||
| 32 | Dihydro-2-methyl-3(2H)-Furanone | 1277 | DFD | nutty, bready, sweet | nutty/cocoa | 0.72 | 0.18 | - | 0.12 | - | 0.05 | 0.54 | - | 0.08 | 0.06 |
| 87 | 2,5-Dihydro-3,5-dimethyl-2-furanone | 1665 | DFD | - | - | 0.36 | 0.41 | - | 0.38 | 0.50 | - | 0.31 | - | 0.41 | 0.47 |
| 124 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone (furaneol) | 2041 | DFD | caramel, sweet, cotton candy | sweet | 0.30 | 0.34 | 0.65 | 0.55 | 0.49 | 0.56 | 0.35 | 0.53 | 0.38 | 0.47 |
| 125 | 5-Acetyldihydro-2(3H)-furanone | 2068 | DFD | winey | sour/fermented | 0.21 | 0.18 | - | 0.30 | 0.33 | 0.38 | 0.29 | 0.00 | 0.28 | 0.33 |
| 1.59 | 1.11 | 0.65 | 1.35 | 1.32 | 0.99 | 1.49 | 0.53 | 1.15 | 1.33 | ||||||
| Dialkylsufides and Disulfides | |||||||||||||||
| 60 | 2-(Methylthio)methylfuran (Furfuryl methyl sulfide) | 1496 | DS | alliaceous, vegetable | green/vegetative | 0.40 | 0.43 | 0.16 | 0.31 | 0.27 | 0.28 | 0.27 | 0.20 | 0.37 | 0.30 |
| 104 | 2-[(Methyldithio)methyl]furan (Furfuryl methyl disulfide) | 1825 | DS | sulfurous, coffee, roasted | roasted | - | 0.23 | 0.19 | 0.24 | 0.22 | 0.26 | 0.22 | 0.25 | 0.29 | 0.23 |
| 0.40 | 0.66 | 0.35 | 0.55 | 0.49 | 0.54 | 0.49 | 0.45 | 0.66 | 0.53 | ||||||
| Thiols | |||||||||||||||
| 1 | Methanethiol | 699 | TH | alliaceous, egg, cabbage, garlic | other | 0.07 | 0.04 | - | 0.04 | 0.01 | 0.01 | 0.03 | - | - | 0.04 |
| 50 | 2-Furfurylthiol | 1440 | TH | roasted, fresh coffee | roasted | 0.31 | 0.32 | 0.16 | 0.32 | 0.15 | 0.25 | 0.16 | 0.15 | 0.21 | 0.18 |
| 0.38 | 0.36 | 0.16 | 0.36 | 0.16 | 0.26 | 0.19 | 0.15 | 0.21 | 0.22 | ||||||
| Thiophene and Derivatives | |||||||||||||||
| 13 | Thiophene | 1031 | TPH | sulfurus, alliaceous, garlic | other | 0.02 | - | - | - | - | - | - | - | - | - |
| 100 | 3-Acetylthiophene | 1773 | TPHD | - | - | - | - | 0.27 | - | - | 0.35 | 0.00 | 0.28 | 0.32 | 0.32 |
| 105 | 3-Acetyl-2,5-dimethylthiophene | 1827 | TPHD | burnt, roasted | roasted | - | 0.10 | - | 0.22 | 0.18 | 0.00 | 0.00 | 0.00 | 0.20 | 0.20 |
| 117 | 2-Thiophenemethanol | 1947 | TPHD | savory, coffee, roasted | roasted | 0.23 | 0.19 | 0.25 | 0.26 | 0.23 | 0.29 | 0.23 | 0.24 | 0.19 | 0.24 |
| 0.25 | 0.29 | 0.52 | 0.48 | 0.41 | 0.64 | 0.23 | 0.52 | 0.71 | 0.76 | ||||||
| Alcohols | |||||||||||||||
| 112 | Benzyl alcohol | 1878 | ALC | floral | floral | - | - | - | 0.10 | 0.08 | - | 0.24 | - | 0.10 | 0.09 |
| 115 | Phenylethyl Alcohol | 1916 | ALC | floral, rose | floral | 0.22 | 0.25 | 0.37 | 0.42 | 0.41 | 0.45 | 0.36 | - | 0.33 | 0.40 |
| 0.22 | 0.25 | 0.37 | 0.52 | 0.49 | 0.45 | 0.60 | - | 0.43 | 0.49 | ||||||
| Monoterpenes | |||||||||||||||
| 24 | β-Myrcene | 1175 | MT | spicy, peppery, terpenic | spices | 0.07 | 0.07 | 0.19 | 0.05 | 0.05 | 0.05 | 0.08 | 0.20 | - | 0.07 |
| 26 | d-Limonene | 1210 | MT | citrus, orange, fresh | fruity | 0.23 | 0.20 | 0.28 | 0.16 | 0.10 | 0.08 | 0.23 | 0.29 | 0.08 | 0.14 |
| 70 | Linalool | 1555 | MT | floral, sweet, citrus | floral | - | - | 0.16 | - | - | - | 0.14 | 0.16 | - | - |
| 0.30 | 0.27 | 0.63 | 0.21 | 0.15 | 0.13 | 0.45 | 0.65 | 0.08 | 0.21 | ||||||
| γ-Lactones | |||||||||||||||
| 83 | γ-Butyrolactone | 1646 | γLC | creamy, fatty, caramel | sweet | 0.31 | 0.25 | 0.12 | 0.15 | 0.21 | 0.19 | 0.26 | 0.29 | 0.42 | 0,17 |
| 98 | 2(3H)-Furanone (γ-Crotonolactone) | 1762 | DHF | buttery | sour/fermented | 0.28 | 0.27 | - | - | - | - | - | - | - | - |
| 127 | 2-Hydroxy-γ-butyrolactone | 2180 | γLC | - | - | 0.05 | 0.05 | - | 0.06 | 0.05 | - | - | - | - | 0.04 |
| 0.64 | 0.57 | 0.12 | 0.21 | 0.26 | 0.19 | 0.26 | 0.29 | 0.42 | 0.21 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakidou, P.; Plati, F.; Matsakidou, A.; Varka, E.-M.; Blekas, G.; Paraskevopoulou, A. Single Origin Coffee Aroma: From Optimized Flavor Protocols and Coffee Customization to Instrumental Volatile Characterization and Chemometrics. Molecules 2021, 26, 4609. https://doi.org/10.3390/molecules26154609
Zakidou P, Plati F, Matsakidou A, Varka E-M, Blekas G, Paraskevopoulou A. Single Origin Coffee Aroma: From Optimized Flavor Protocols and Coffee Customization to Instrumental Volatile Characterization and Chemometrics. Molecules. 2021; 26(15):4609. https://doi.org/10.3390/molecules26154609
Chicago/Turabian StyleZakidou, Panagiota, Fotini Plati, Anthia Matsakidou, Evdoxia-Maria Varka, Georgios Blekas, and Adamantini Paraskevopoulou. 2021. "Single Origin Coffee Aroma: From Optimized Flavor Protocols and Coffee Customization to Instrumental Volatile Characterization and Chemometrics" Molecules 26, no. 15: 4609. https://doi.org/10.3390/molecules26154609
APA StyleZakidou, P., Plati, F., Matsakidou, A., Varka, E.-M., Blekas, G., & Paraskevopoulou, A. (2021). Single Origin Coffee Aroma: From Optimized Flavor Protocols and Coffee Customization to Instrumental Volatile Characterization and Chemometrics. Molecules, 26(15), 4609. https://doi.org/10.3390/molecules26154609









