Zero-to-Two Nanoarchitectonics: Fabrication of Two-Dimensional Materials from Zero-Dimensional Fullerene
Abstract
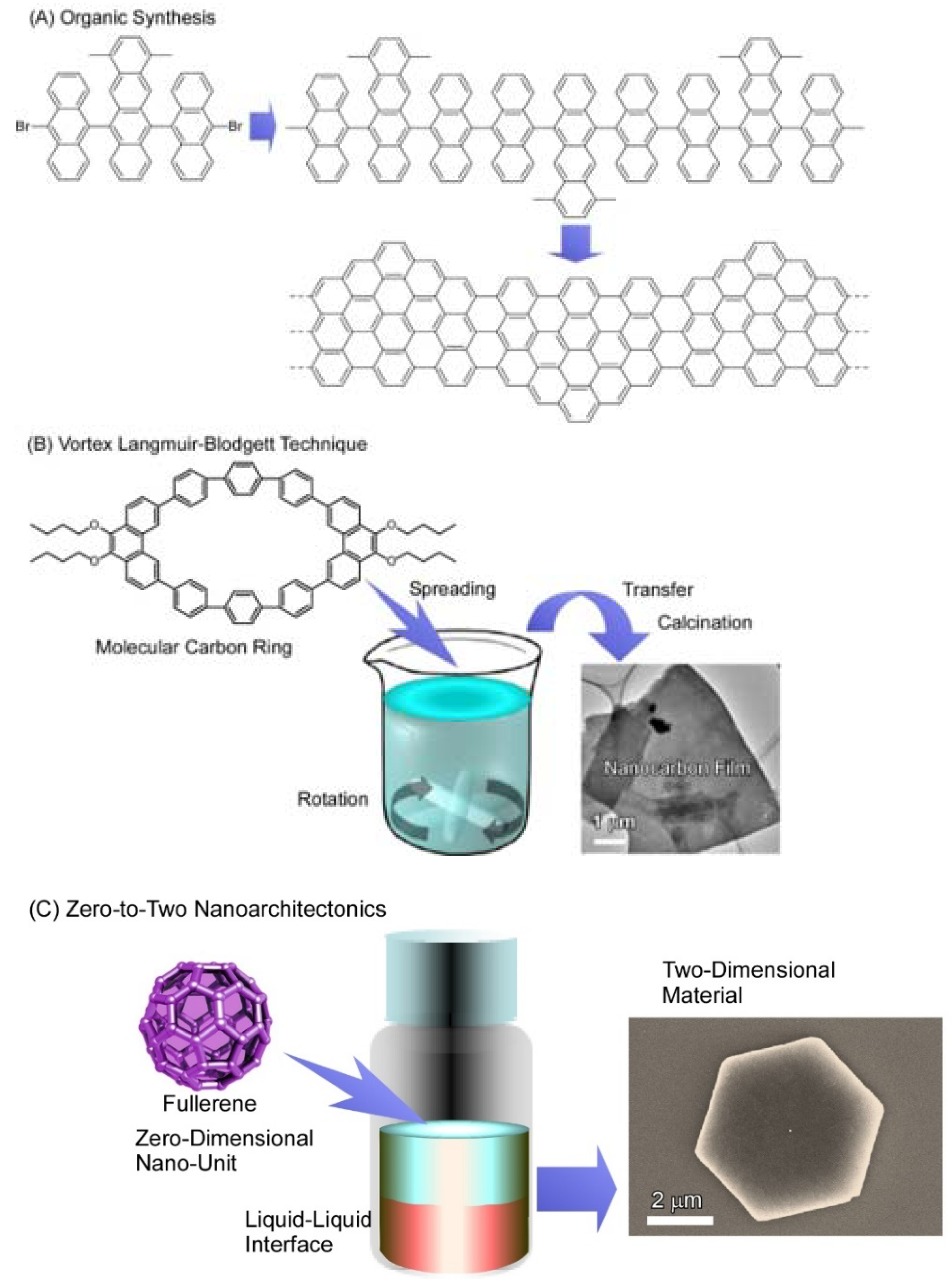
:1. Introduction
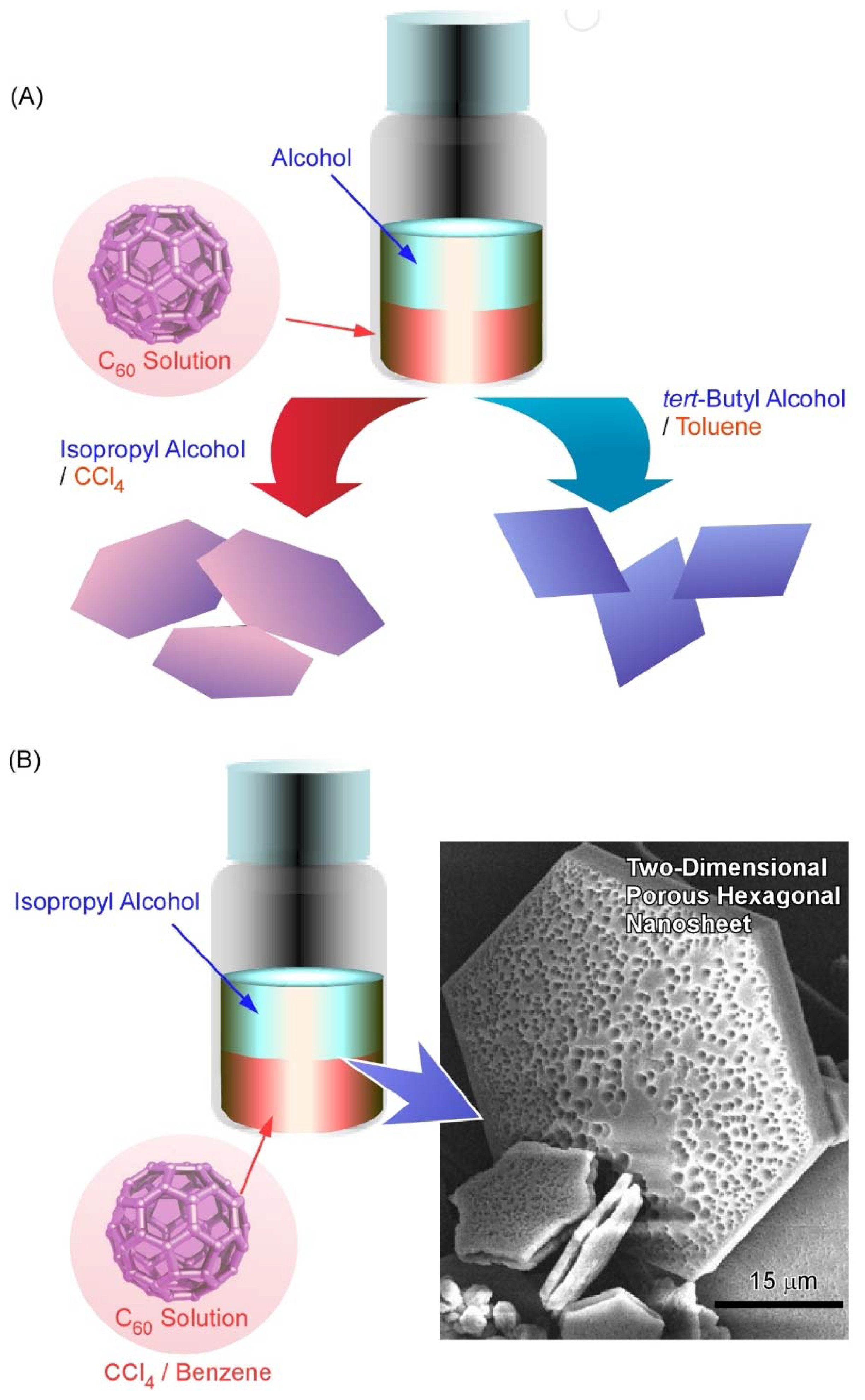
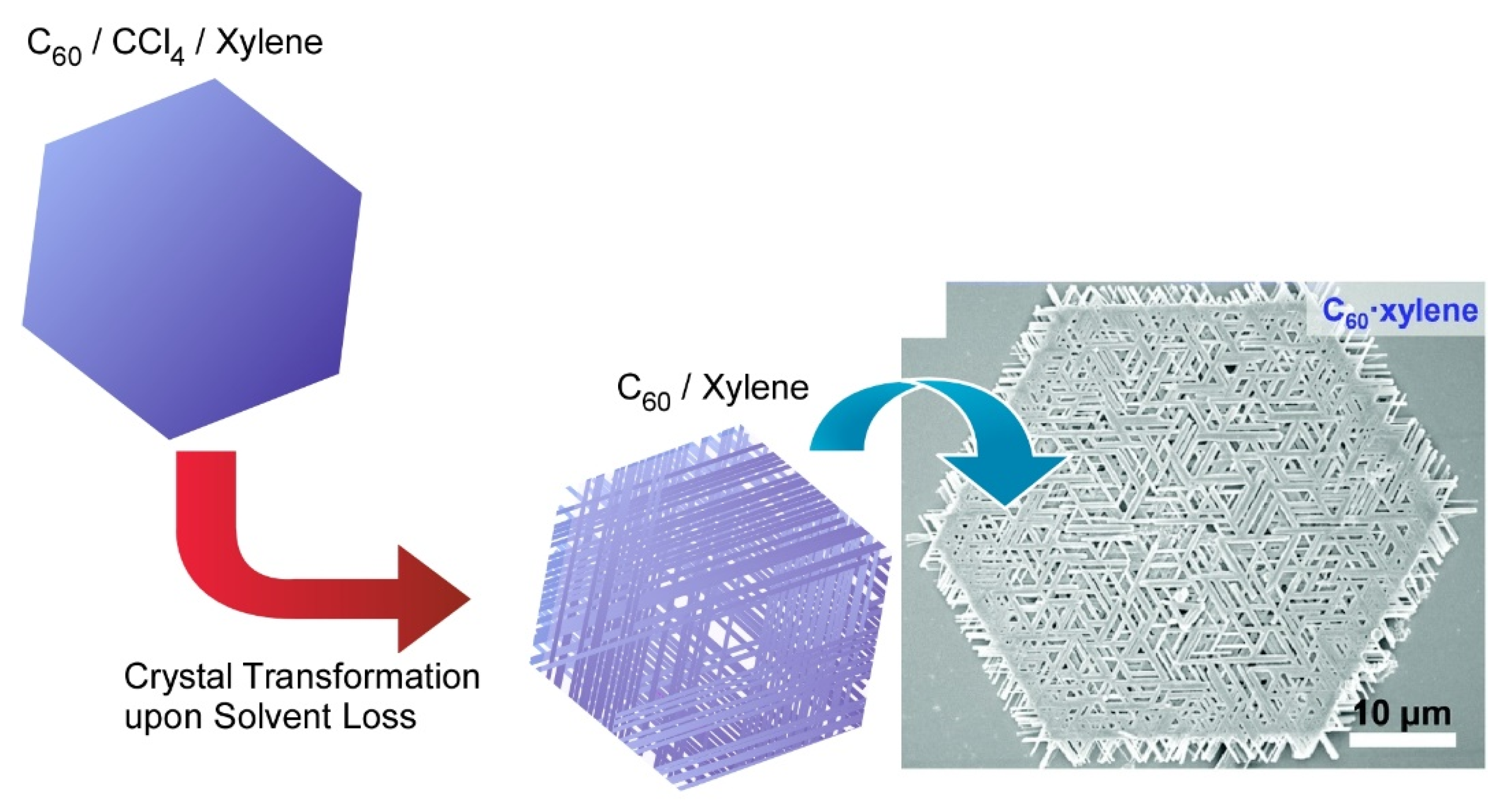
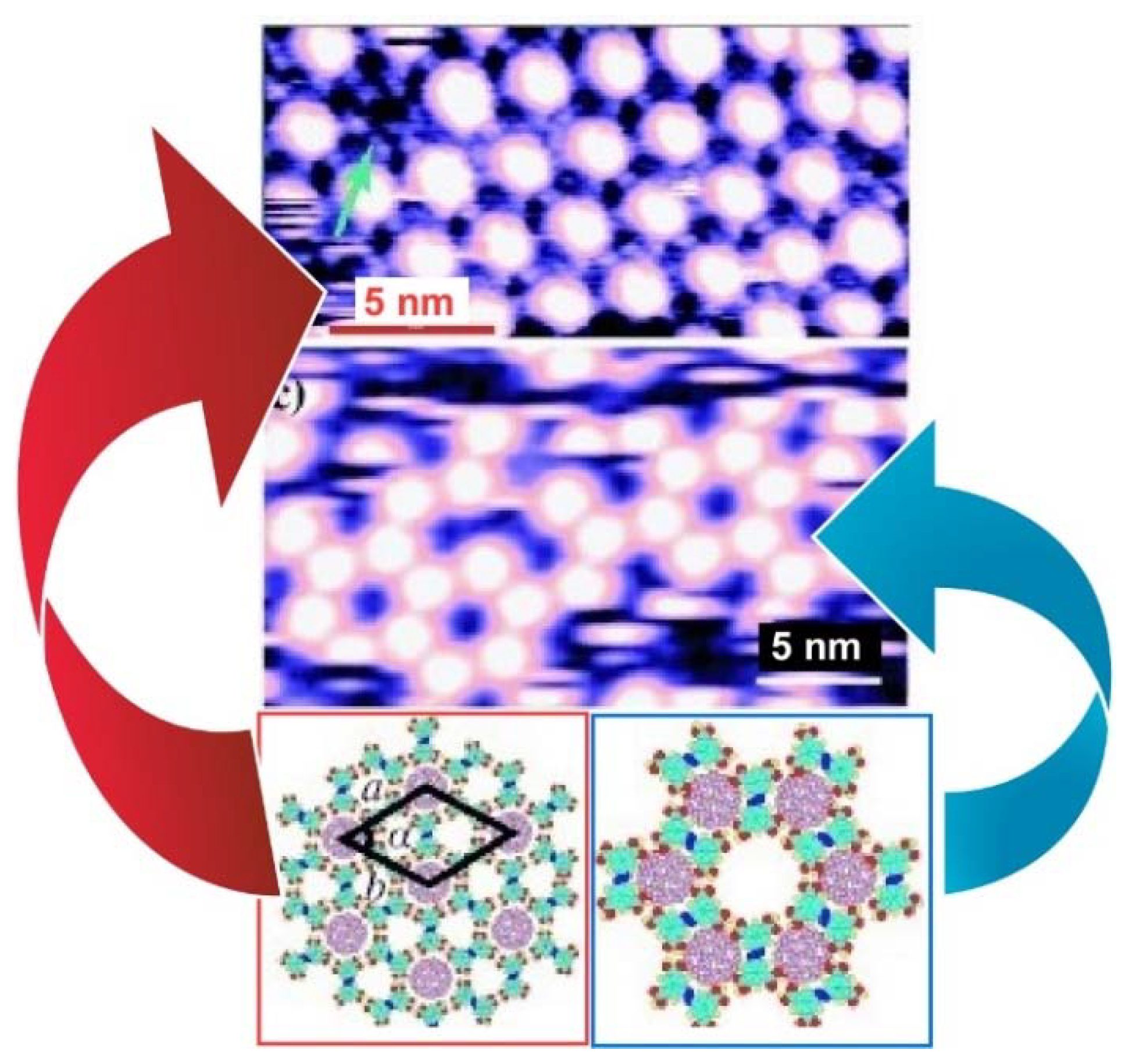
2. Two-Dimensional Fullerene Nanoarchitecture
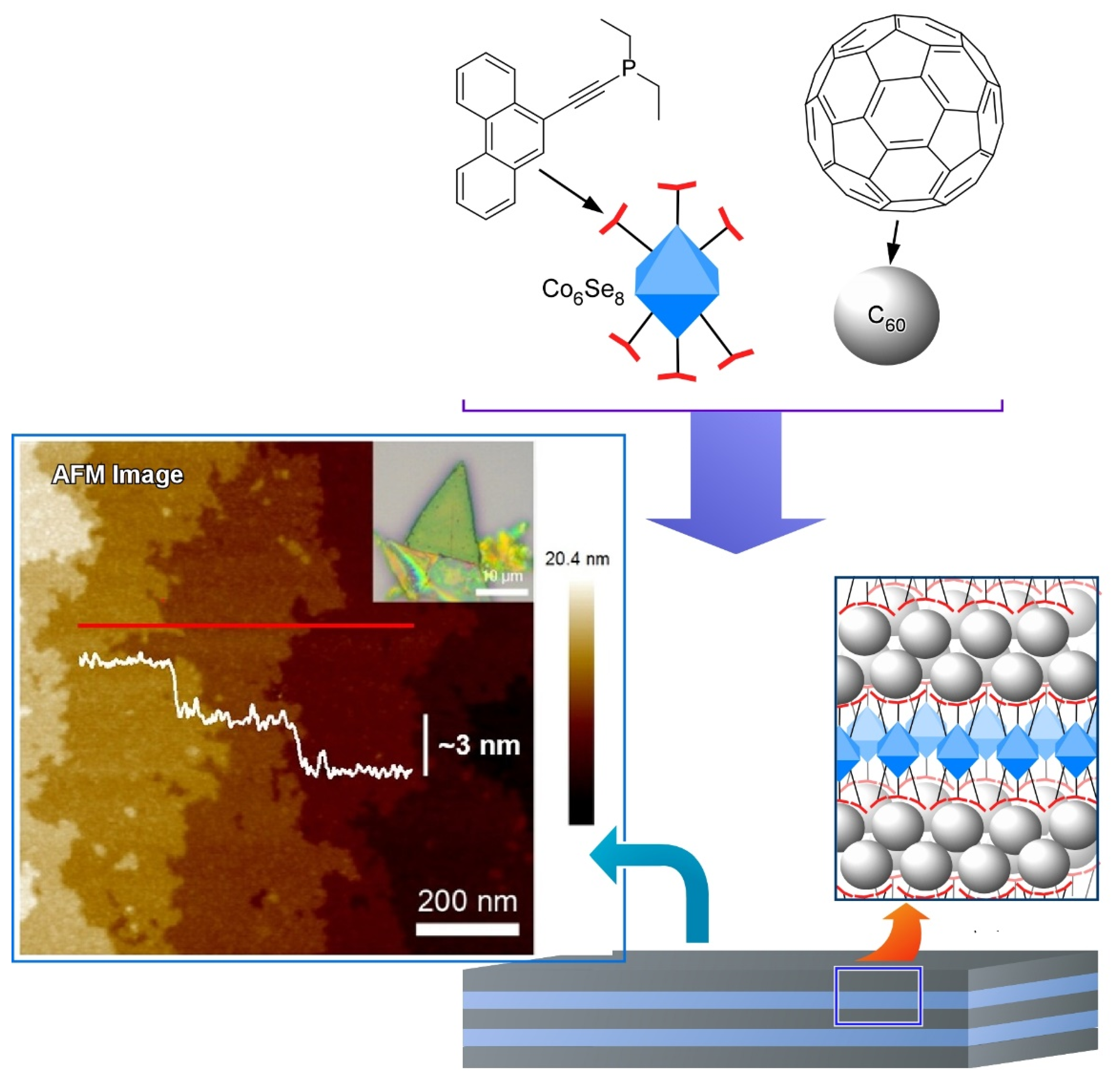
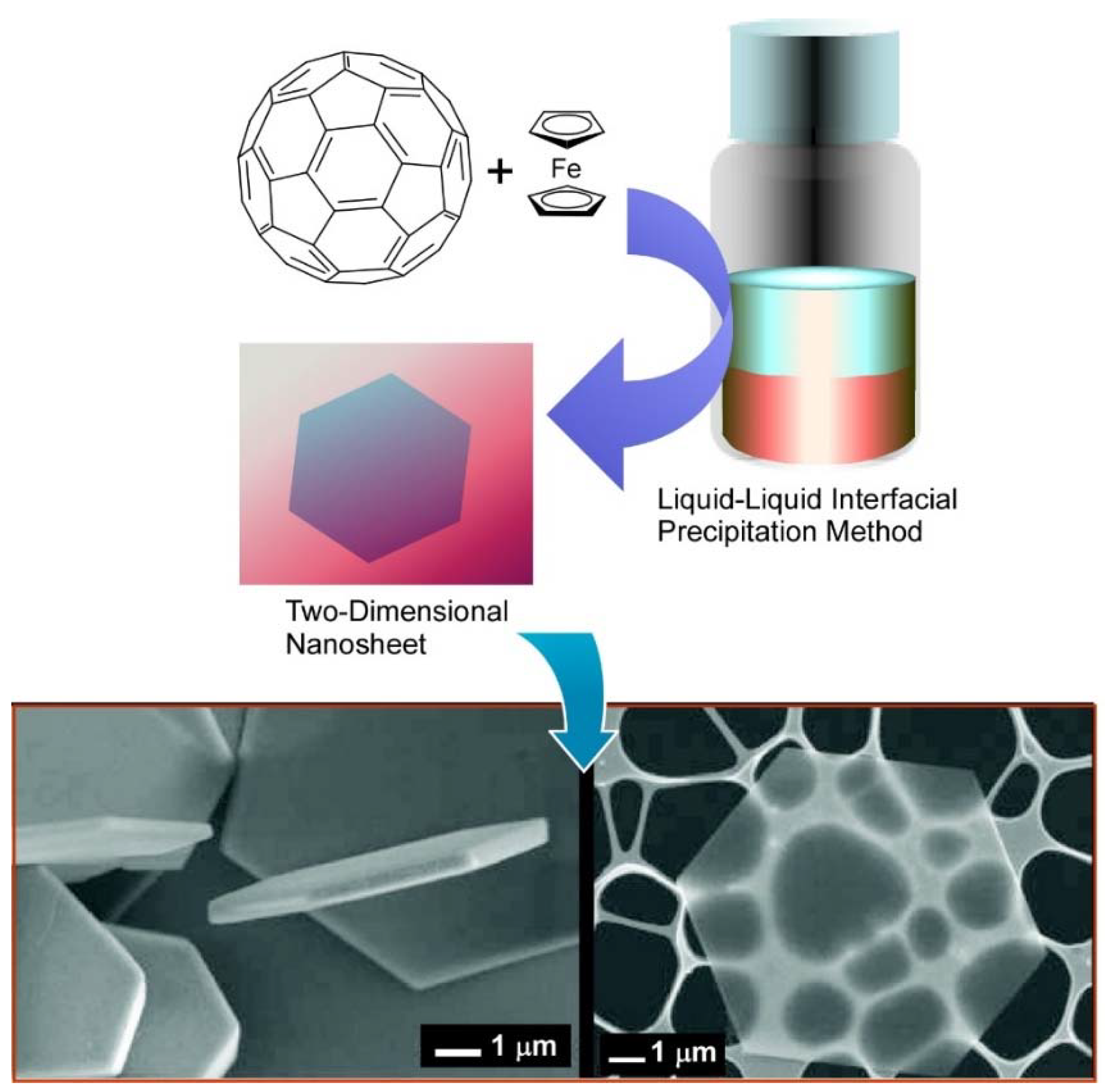
3. Two-Dimensional Nanoarchitecture from Fullerene with Other Component
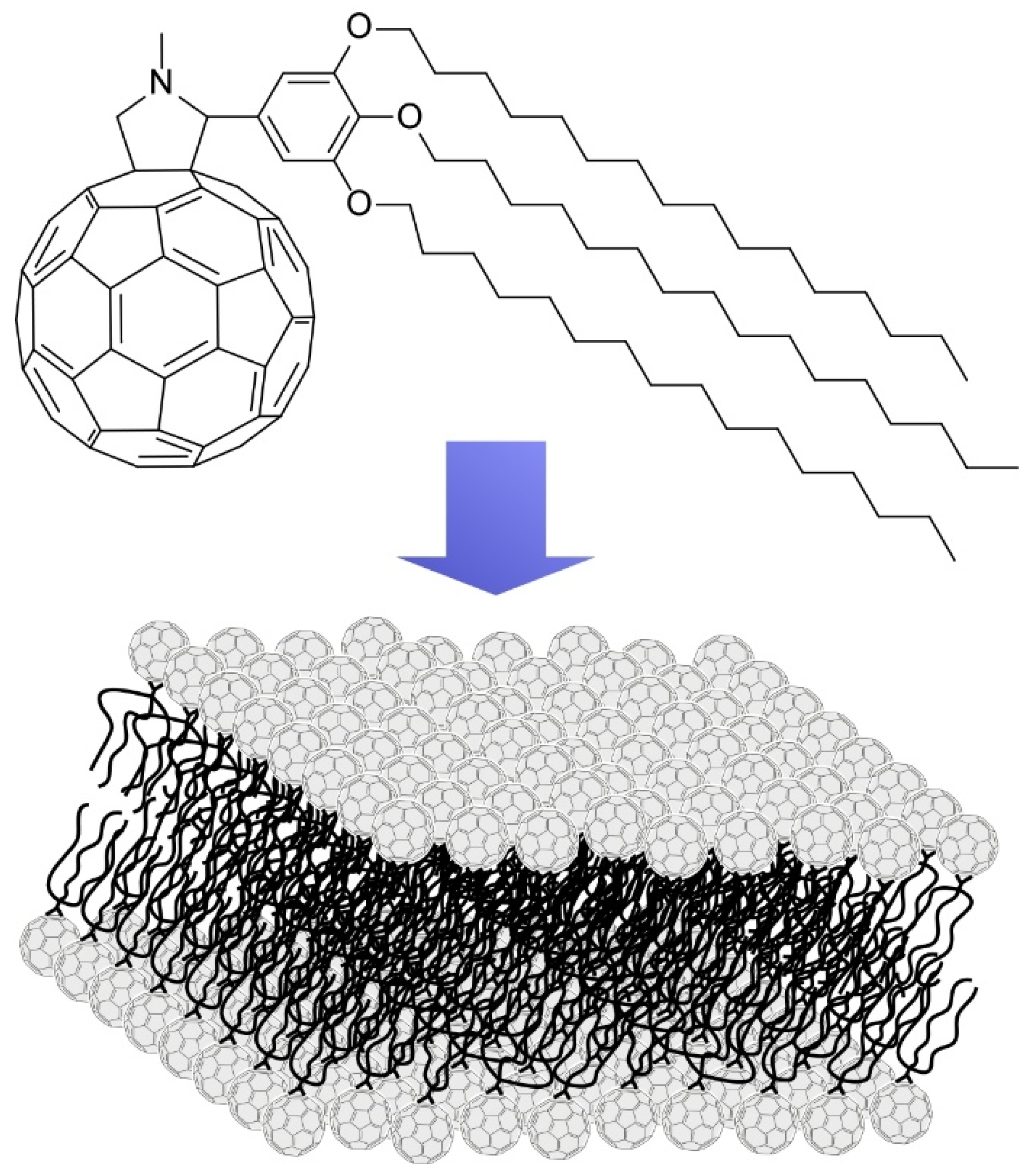
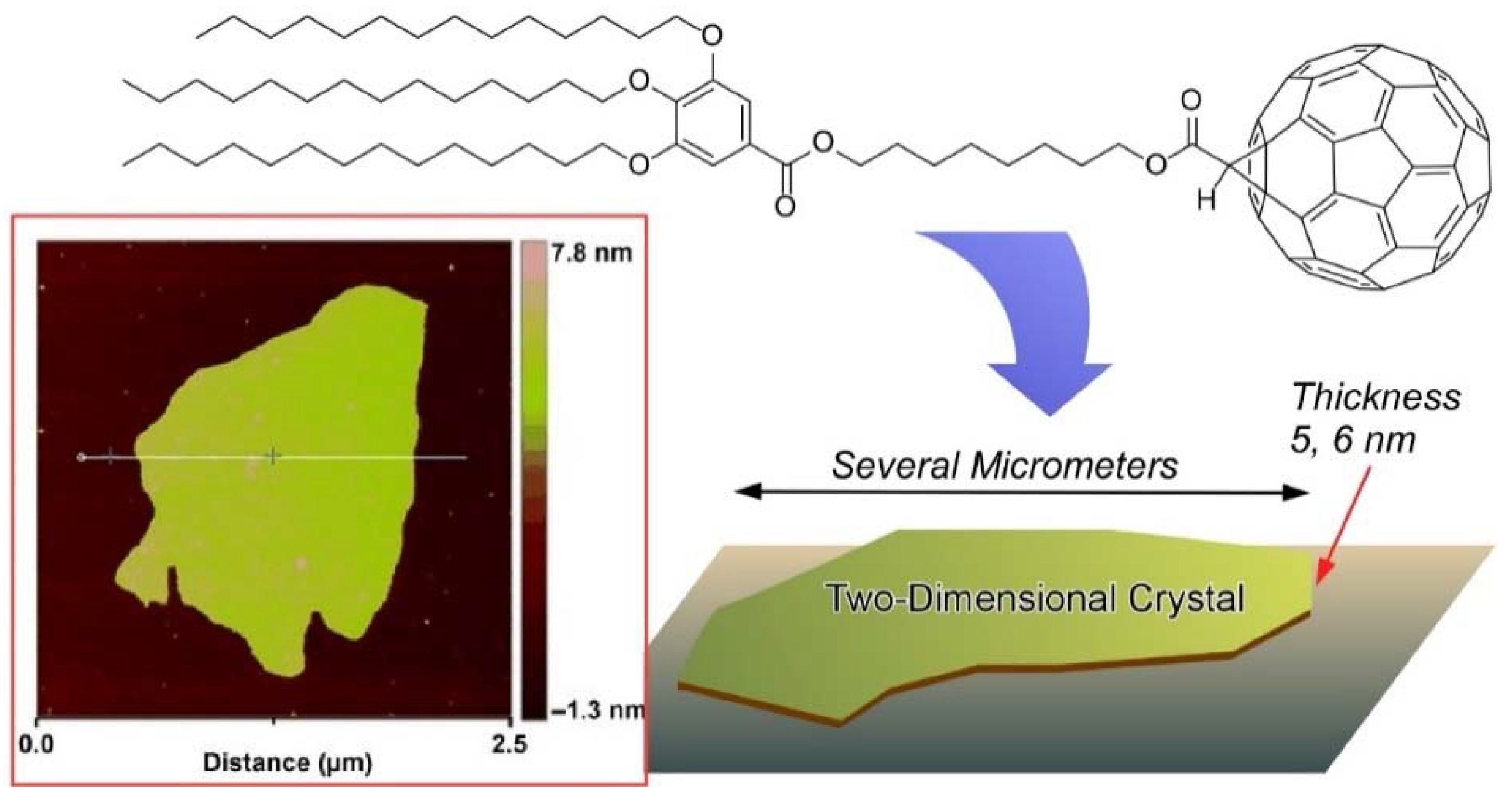
4. Two-Dimensional Nanoarchitecture from Alkylated Fullerene Derivative
5. Two-Dimensional Hierarchical Fullerene Nanoarchitecture from Fullerene
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Shimizu, T.; Lungerich, D.; Stuckner, J.; Murayama, M.; Harano, K.; Nakamura, E. Real-time video imaging of mechanical motions of a single molecular shuttle with sub-millisecond sub-angstrom precision. Bull. Chem. Soc. Jpn. 2020, 93, 1079–1085. [Google Scholar] [CrossRef]
- Kazuma, E. Real-space studies of plasmon-induced dissociation reactions with an STM. Bull. Chem. Soc. Jpn. 2020, 93, 1552–1557. [Google Scholar] [CrossRef]
- Kamei, K.; Shimizu, T.; Harano, K.; Nakamura, E. Aryl radical addition to curvatures of carbon nanohorns for single-molecule-level molecular imaging. Bull. Chem. Soc. Jpn. 2020, 93, 1603–1608. [Google Scholar] [CrossRef]
- Kaur, G.; Lewis, J.S.; van Oijen, A.M. Shining a spotlight on DNA: Single-molecule methods to visualise DNA. Molecules 2019, 24, 491. [Google Scholar] [CrossRef] [Green Version]
- Hishikawa, A.; Matsuda, A.; Fushitani, M. Ultrafast reaction imaging and control by ultrashort intense laser pulses. Bull. Chem. Soc. Jpn. 2020, 93, 1293–1304. [Google Scholar] [CrossRef]
- Muraoka, T. Biofunctional molecules inspired by protein mimicry and manipulation. Bull. Chem. Soc. Jpn. 2020, 93, 138–153. [Google Scholar] [CrossRef]
- Sun, Z.; Ikemoto, K.; Fukunaga, T.M.; Koretsune, T.; Arita, R.; Sato, S.; Isobe, H. Finite phenine nanotubes with periodic vacancy defects. Science 2019, 363, 151–155. [Google Scholar] [CrossRef]
- Niwa, T.; Hosoya, T. Molecular renovation strategy for expeditious synthesis of molecular probes. Bull. Chem. Soc. Jpn. 2020, 93, 230–248. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, W.; Hattori, T.; Yamamoto, H. Game change from reagent- to substrate-controlled peptide synthesis. Bull. Chem. Soc. Jpn. 2020, 93, 759–767. [Google Scholar] [CrossRef]
- Yamada, H.; Kuzuhara, D.; Suzuki, M.; Hayashi, H.; Aratani, N. Synthesis and morphological control of organic semiconducting materials using the precursor approach. Bull. Chem. Soc. Jpn. 2020, 93, 1234–1267. [Google Scholar] [CrossRef]
- Akagi, K. Interdisciplinary chemistry based on integration of liquid crystals and conjugated polymers: Development and progress. Bull. Chem. Soc. Jpn. 2019, 92, 1509–1655. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Kobayashi, S.; Murakami, D.; Aratsu, F.; Kashiwazaki, A.; Hoshiba, T.; Fukushima, K. Design of polymeric biomaterials: The “intermediate water concept”. Bull. Chem. Soc. Jpn. 2019, 92, 2043–2057. [Google Scholar] [CrossRef]
- Yamago, S. Photoactivation of organotellurium compounds in precision polymer synthesis: Controlled radical polymerization and radical coupling reactions. Bull. Chem. Soc. Jpn. 2020, 93, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Mashima, K. Redox-active α-diimine complexes of early transition metals: From bonding to catalysis. Bull. Chem. Soc. Jpn. 2020, 93, 799–820. [Google Scholar] [CrossRef]
- Hosono, N. Design of porous coordination materials with dynamic properties. Bull. Chem. Soc. Jpn. 2021, 94, 60–69. [Google Scholar] [CrossRef]
- Domoto, Y.; Abe, M.; Yamamoto, K.; Kikuchi, T.; Fujita, M. “Eggs in egg cartons”: Co-crystallization to embed molecular cages into crystalline lattices. Chem. Sci. 2020, 11, 10457–10460. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M. Next generation multifunctional nano-science of advanced metal complexes with quantum effect and nonlinearity. Bull. Chem. Soc. Jpn. 2021, 94, 209–264. [Google Scholar] [CrossRef]
- Fukunaga, K.; Tsutsumi, H.; Mihara, H. Self-assembling peptides as building blocks of functional materials for biomedical applications. Bull. Chem. Soc. Jpn. 2019, 92, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.; Govindaraju, T. Amino acids and peptides as functional components in arylenediimide-based molecular architectonics. Bull. Chem. Soc. Jpn. 2019, 92, 1883–1901. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Kato, Y.; Higashiharaguchi, S.; Aratsu, K.; Isobe, A.; Saito, T.; Prabhu, D.D.; Kitamoto, Y.; Hollamby, M.J.; Smith, A.J.; et al. Self-assembled poly-catenanes from supramolecular toroidal building blocks. Nature 2020, 583, 400–405. [Google Scholar] [CrossRef]
- Percec, V.; Xiao, Q. Helical self-organizations and emerging functions in architectures, biological and synthetic macromolecules. Bull. Chem. Soc. Jpn. 2021, 94, 900–928. [Google Scholar] [CrossRef]
- Wang, X.-B.; Jiang, X.-F.; Bando, Y. Blowing route towards advanced inorganic foams. Bull. Chem. Soc. Jpn. 2019, 92, 245–263. [Google Scholar] [CrossRef]
- Pileni, M.P. Au supracrystal growth processes: Unexpected morphologies. Bull. Chem. Soc. Jpn. 2019, 92, 312–329. [Google Scholar] [CrossRef] [Green Version]
- Kanao, E.; Kubo, T.; Otsuka, K. Carbon-based nanomaterials for separation media. Bull. Chem. Soc. Jpn. 2020, 93, 482–489. [Google Scholar] [CrossRef]
- Li, M.-T.; Liu, M.; Yu, Y.-H.; Li, A.-W.; Sun, H.-B. Laser-structured graphene/reduced graphene oxide films towards bio-inspired superhydrophobic surfaces. Bull. Chem. Soc. Jpn. 2019, 92, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Kise, R.; Fukumi, A.; Shioya, N.; Shimoaka, T.; Sonoyama, M.; Amii, H.; Takagi, T.; Kanamori, T.; Eda, K.; Hasegawa, T. Fluorous property of a short perfluoroalkyl-containing compound realized by self-assembled monolayer technique on a silicon substrate. Bull. Chem. Soc. Jpn. 2019, 92, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, T. Development of fullerene thin-film assemblies and fullerene-diamine adducts towards practical nanocarbon-based electronic materials. Bull. Chem. Soc. Jpn. 2019, 92, 1181–1199. [Google Scholar] [CrossRef]
- Kuodis, Z.; Matulaitienė, I.; Špandyreva, M.; Labanauskas, L.; Stončius, S.; Eicher-Lorka, O.; Sadzevičienė, R.; Niaura, G. Reflection absorption iInfrared spectroscopy characterization of SAM formation from 8-mercapto-N-(phenethyl)octanamide thiols with Phe ring and amide groups. Molecules 2020, 25, 5633. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Li, J. Langmuir nanoarchitectonics from basic to frontier. Langmuir 2019, 35, 3585–3599. [Google Scholar] [CrossRef]
- Ariga, K. Don’t forget Langmuir–Blodgett films 2020: Interfacial nanoarchitectonics with molecules, materials, and living objects. Langmuir 2020, 36, 7158–7180. [Google Scholar] [CrossRef]
- Rydzek, G.; Ji, Q.; Li, M.; Schaaf, P.; Hill, J.P.; Boulmedais, F.; Ariga, K. Electrochemical nanoarchitectonics and layer-by-layer assembly: From basics to future. Nano Today 2015, 10, 138–167. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Li, J. Molecular assembly of rotary and linear motor proteins. Acc. Chem. Res. 2019, 52, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Irisawa, T.; Hatori, H.; Inagaki, M. Yarns of carbon nanotubes and reduced graphene oxides. Carbon 2020, 165, 358–377. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, Y.; Hu, W.; Ye, W. Carbon dots-modified nanoporous membrane and Fe3O4@Au magnet nanocomposites-based FRET assay for ultrasensitive histamine detection. Molecules 2019, 24, 3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.; Shiraki, T.; Fujigaya, T. Thermal conversion of triazine-based covalent organic frameworks to nitrogen-doped nanoporous carbons and their capacitor performance. Bull. Chem. Soc. Jpn. 2020, 93, 414–420. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Suzuki, N.; Terashima, C.; Fujishima, A. Recent improvements in the production of solar fuels: From CO2 reduction to water splitting and artificial photosynthesis. Bull. Chem. Soc. Jpn. 2019, 92, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y. Concentrated battery electrolytes: Developing new functions by manipulating the coordination states. Bull. Chem. Soc. Jpn. 2020, 93, 109–118. [Google Scholar] [CrossRef]
- Li, Y.; Henzie, J.; Park, T.; Wang, J.; Young, C.; Xie, H.; Yi, J.W.; Li, J.; Kim, M.; Kim, J.; et al. Fabrication of flexible microsupercapacitors with binder-free ZIF-8 derived carbon films via electrophoretic deposition. Bull. Chem. Soc. Jpn. 2020, 93, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Sai-Anand, G.; Sivanesan, A.; Benzigar, M.R.; Singh, G.; Gopalan, A.-I.; Baskar, A.V.; Ilbeygi, H.; Ramadass, K.; Kambala, V.; Vinu, A. Recent progress on the sensing of pathogenic bacteria using advanced nanostructures. Bull. Chem. Soc. Jpn. 2019, 92, 216–244. [Google Scholar] [CrossRef] [Green Version]
- Pang, P.; Lai, Y.; Zhang, Y.; Wang, H.; Conlan, X.A.; Barrow, C.J.; Yang, W. Recent advancement of biosensor technology for the detection of microcystin-LR. Bull. Chem. Soc. Jpn. 2020, 93, 637–646. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, C.; Yan, H.; Li, L.; He, H.; Wang, D.; Li, Y. Single-atom site catalysts for environmental catalysis. Nano Res. 2020, 13, 3165–3182. [Google Scholar] [CrossRef]
- Singh, B.; Na, J.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional mesoporous silica nanomaterials for catalysis and environmental applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Kobayashi, J.; Okano, T. Design of temperature-responsive polymer-grafted surfaces for cell sheet preparation and manipulation. Bull. Chem. Soc. Jpn. 2019, 92, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.I.; Vallet-Regí, M. Ultrasound-activated nanomaterials for therapeutics. Bull. Chem. Soc. Jpn. 2020, 93, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Kankala, R.K.; Han, Y.; Na, J.; Lee, C.; Sun, Z.; Wang, S.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.; et al. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Arora, H.; Ramesh, M.; Rajasekhar, K.; Govindaraju, T. Molecular tools to detect alloforms of Aβ and Tau: Implications for multiplexing and multimodal diagnosis of Alzheimer’s disease. Bull. Chem. Soc. Jpn. 2020, 93, 507–546. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnology. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Feynman, R.P. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Roukes, M. Plenty of room, indeed. Sci. Am. 2001, 285, 48–51. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Hill, J.P.; Bando, Y.; Aono, M. Forming nanomaterials as layered functional structures toward materials nanoarchitectonics. NPG Asia Mater. 2012, 4, e17. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K. Nanoarchitectonics revolution and evolution: From small science to big technology. Small Sci. 2021, 1, 2000032. [Google Scholar] [CrossRef]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for dynamic functional materials from atomic-/molecular-level manipulation to macroscopic action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K. Nanoarchitectonics: What’s coming next after nanotechnology? Nanoscale Horiz. 2021, 6, 364–378. [Google Scholar] [CrossRef]
- Aono, M.; Ariga, K. The way to nanoarchitectonics and the way of nanoarchitectonics. Adv. Mater. 2015, 28, 989–992. [Google Scholar] [CrossRef]
- Ariga, K.; Jia, X.; Song, J.; Hill, J.P.; Leong, D.T.; Jia, Y.; Li, J. Nanoarchitectonics beyond self-assembly: Challenges to create bio-like hierarchic organization. Angew. Chem. Int. Ed. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Azhar, A.; Zakaria, M.B.; Kim, J.; Na, J.; Kaneti, Y.V.; Fatehmulla, A.; Aldhafiri, A.M.; Farooq, W.A.; Bando, Y.; Yamauchi, Y.; et al. Single crystal growth of two-dimensional cyano-bridged coordination polymer of Co(H2O)2Ni(CN)4·4H2O using trisodium citrate dihydrate. Bull. Chem. Soc. Jpn. 2019, 92, 1263–1267. [Google Scholar] [CrossRef]
- Ariga, K.; Shionoya, M. Nanoarchitectonics for coordination asymmetry and related chemistry. Bull. Chem. Soc. Jpn. 2021, 94, 839–859. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, J.; Hsieh, P.-H.; Hsu, Y.-S.; Kaneti, Y.V.; Shieh, F.-K.; Yamauchi, Y.; Wu, K.C.-W. Nanoarchitectonics of biofunctionalized metal-organic frameworks with biological macromolecules and living cells. Small Methods 2019, 3, 1900213. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Kitao, T.; Uemura, T. Supramolecular chiral nanoarchitectonics. Adv. Mater. 2020, 32, 1905657. [Google Scholar] [CrossRef]
- Deka, R.C.; Deka, A.; Deka, P.; Saikia, S.; Baruah, J.; Sarma, P.J. Recent advances in nanoarchitectonics of SnO₂ clusters and their applications in catalysis. J. Nanosci. Nanotechnol. 2020, 20, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.K.R.; Madakannu, I.; Prasanthi, I. Hetero atom doped graphene nanoarchitectonics as electrocatalysts towards the oxygen reduction and evolution reactions in acidic medium. J. Inorg. Organomet. Polym. 2021, 31, 1859–1876. [Google Scholar]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Mori, T.; Ariga, K. Molecular imprinting: Materials nanoarchitectonics with molecular information. Bull. Chem. Soc. Jpn. 2018, 91, 1075–1111. [Google Scholar] [CrossRef]
- Giussi, J.M.; Cortez, M.L.; Marmisollé, W.A.; Azzaroni, O. Practical use of polymer brushes in sustainable energy applications: Interfacial nanoarchitectonics for high-efficiency devices. Chem. Soc. Rev. 2019, 48, 814–849. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Atom/molecular nanoarchitectonics for devices and related applications. Nano Today 2019, 28. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J.P. Enzyme nanoarchitectonics: Organization and device application. Chem. Soc. Rev. 2013, 42, 6322–6345. [Google Scholar] [CrossRef] [PubMed]
- Stulz, E. Nanoarchitectonics with porphyrin functionalized DNA. Acc. Chem. Res. 2017, 50, 823–831. [Google Scholar] [CrossRef]
- Liang, X.; Li, L.; Tang, J.; Komiyama, M.; Ariga, K. Dynamism of supramolecular DNA/RNA nanoarchitectonics: From interlocked structures to molecular machines. Bull. Chem. Soc. Jpn. 2020, 93, 581–603. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, Q.; Yan, X. Self-assembling peptide-based nanoarchitectonics. Bull. Chem. Soc. Jpn. 2019, 92, 70–79. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, J. Solid lipid matrix mediated nanoarchitectonics for improved oral bioavailability of drug. Expert Opin. Drug Metab. Toxicol. 2019, 15, 499–515. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Dang, Q.; Chen, W.; Tang, L.; Hu, M. Recent advances in rechargeable batteries with prussian blue analogs nanoarchitectonics. J. Inorg. Organomet. Polym. 2021, 31, 1877–1893. [Google Scholar] [CrossRef]
- Pandeeswar, M.; Senanayak, S.P.; Govindaraju, T. Nanoarchitectonics of small molecule and DNA for ultrasensitive detection of mercury. ACS Appl. Mater. Interfaces 2016, 8, 30362–30371. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Darder, M.; Boutahala, M.; Aranda, P.; Ruiz-Hitzky, E. Composite nanoarchitectonics: Alginate beads encapsulating sepiolite/magnetite/prussian blue for removal of cesium ions from water. Bull. Chem. Soc. Jpn. 2021, 94, 122–132. [Google Scholar] [CrossRef]
- Imaoka, T.; Yamamoto, K. Wet-chemical strategy for atom-precise metal cluster catalysts. Bull. Chem. Soc. Jpn. 2019, 92, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Nagaura, T.; Park, T.; Lim, H.; Lin, J.; Iqbal, M.; AlShehri, S.M.; Ahamad, T.; Kaneti, Y.V.; Yi, J.W.; Kim, Y.; et al. Controlled synthesis of mesoporous Pt, Pt-Pd and Pt-Pd-Rh nanoparticles in aqueous nonionic surfactant solution. Bull. Chem. Soc. Jpn. 2020, 93, 455–460. [Google Scholar] [CrossRef]
- Yoshida, H.; Kawakami, Y.; Tokuzumi, W.; Shimokawa, Y.; Hirakawa, T.; Ohyama, J.; Machida, M. Low-temperature NO reduction over Fe-Ni alloy nanoparticles using synergistic effects of Fe and Ni in a catalytic NO-CO-C3H6-O2 reaction. Bull. Chem. Soc. Jpn. 2020, 93, 1050–1055. [Google Scholar] [CrossRef]
- Glotov, A.; Stavitskaya, A.; Chudakov, Y.; Ivanov, E.; Huang, W.; Vinokurov, V.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Mesoporous metal catalysts templated on clay nanotubes. Bull. Chem. Soc. Jpn. 2019, 92, 61–69. [Google Scholar] [CrossRef]
- Fujigaya, T. Development of thermoelectric conversion materials using carbon nanotube sheets. Bull. Chem. Soc. Jpn. 2019, 92, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Toshimitsu, F.; Ishimaru, W.; Nakashima, N. Individual solubilization behavior of single-walled carbon nanotubes by riboflavin (vitamin B2) in water and its analyses using regression approach and computational simulations. Bull. Chem. Soc. Jpn. 2019, 92, 1679–1683. [Google Scholar] [CrossRef]
- Mizuno, A.; Shuku, Y.; Awaga, K. Recent developments in molecular spin gyroid research. Bull. Chem. Soc. Jpn. 2019, 92, 1068–1093. [Google Scholar] [CrossRef] [Green Version]
- Hippler, M.; Blasco, E.; Qu, J.; Tanaka, M.; Barner-Kowollik, C.; Wegener, M.; Bastmeyer, M. Controlling the shape of 3D microstructures by temperature and light. Nat. Commun. 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, H.; Yajima, T.; Tsujimoto, Y.; Yamamoto, T.; Tassel, C.; Kobayashi, Y. Exploring structures and properties through anion chemistry. Bull. Chem. Soc. Jpn. 2019, 92, 1349–1357. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Liang, Z.; Gao, S.; Yang, C.; Zhu, B.; Zhao, J.; Qu, C.; Zou, R.; Xu, Q. Puffing up energetic metal-organic frameworks to large carbon networks with hierarchical porosity and atomically dispersed metal sites. Angew. Chem. Int. Ed. 2019, 58, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Aiba, Y.; Ariyasu, S.; Abe, S. Molecular design and regulation of metalloenzyme activities through two novel approaches: Ferritin and P450s. Bull. Chem. Soc. Jpn. 2020, 93, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.M.; Pumera, M. Two-dimensional materials on the rocks: Positive and negative role of dopants and impurities in electrochemistry. ACS Nano 2019, 13, 2681–2728. [Google Scholar] [CrossRef]
- Maeda, K.; Mallouk, T.E. Two-dimensional metal oxide nanosheets as building blocks for artificial photosynthetic assemblies. Bull. Chem. Soc. Jpn. 2019, 92, 38–54. [Google Scholar] [CrossRef]
- Eom, S.; Choi, G.; Nakamura, H.; Choy, J.-H. 2-Dimensional nanomaterials with imaging and diagnostic functions for nanomedicine; a review. Bull. Chem. Soc. Jpn. 2020, 93, 1–12. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Pramoda, K. Borocarbonitrides, BxCyNz, 2D nanocomposites with novel properties. Bull. Chem. Soc. Jpn. 2019, 92, 441–468. [Google Scholar] [CrossRef] [Green Version]
- Ruan, D.; Fujitsuka, M.; Majima, T. Exfoliated Mo2C nanosheets hybridized on CdS with fast electron transfer for efficient photocatalytic H2 production under visible light irradiation. Appl. Catal. B Environ. 2020, 264, 118541. [Google Scholar] [CrossRef]
- Kahn, E.; Liu, M.; Zhang, T.; Liu, H.; Fujisawa, K.; Bepete, G.; Ajayan, P.M.; Terrones, M. Functional hetero-interfaces in atomically thin materials. Mater. Today 2020, 37, 74–92. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Cheng, J.; Chen, M.-S.; Fu, W.; Liu, B.; Zhang, M.; Shen, Z. Intercalation of carbon nanosheet into layered TiO2 grain for highly interfacial lithium storage. ACS Appl. Mater. Interfaces 2020, 12, 21709–21719. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.M.; Pourrahimi, A.M.; Sofer, Z.; Pumera, M. Atomically thin 2D-arsenene by liquid-phased exfoliation: Toward selective vapor sensing. Adv. Funct. Mater. 2019, 29, 1807004. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Imai, H.; Oaki, Y. Redox-mediated high-yield exfoliation of layered composites into nanosheets. Bull. Chem. Soc. Jpn. 2019, 92, 779–784. [Google Scholar] [CrossRef]
- Shree, S.; George, A.; Lehnert, T.; Neumann, C.; Benelajla, M.; Robert, C.; Marie, X.; Watanabe, K.; Taniguchi, T.; Kaiser, U.; et al. High optical quality of MoS2 monolayers grown by chemical vapor deposition. 2D Mater. 2020, 7, 015011. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical vapour deposition of graphene-synthesis, characterisation, and applications: A review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Xu, X.; Müllen, K.; Narita, A. Syntheses and characterizations of functional polycyclic aromatic hydrocarbons and graphene nanoribbons. Bull. Chem. Soc. Jpn. 2020, 93, 490–506. [Google Scholar] [CrossRef]
- Chen, Z.; Narita, A.; Müllen, K. Graphene nanoribbons: On-surface synthesis and integration into electronic devices. Adv. Mater. 2020, 32, 2001893. [Google Scholar] [CrossRef]
- Mori, T.; Tanaka, H.; Dalui, A.; Mitoma, N.; Suzuki, K.; Matsumoto, M.; Aggarwal, N.; Patnaik, A.; Acharya, S.; Shrestha, L.K.; et al. Carbon nanosheets by morphology-retained carbonization of two-dimensional assembled anisotropic carbon nanorings. Angew. Chem. Int. Ed. 2018, 57, 9679–9683. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Ji, Q.; Mori, T.; Miyazawa, K.; Yamauchi, Y.; Hill, J.P.; Ariga, K. Fullerene nanoarchitectonics: From zero to higher dimensions. Chem. Asian J. 2013, 8, 1662–1679. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Shrestha, L.K. Zero-to-one (or more) nanoarchitectonics: How to produce functional materials from zero-dimensional single-element unit, fullerene. Mater. Adv. 2021, 2, 582–597. [Google Scholar] [CrossRef]
- Minato, J.; Miyazawa, K. Solvated structure of C60 nanowhiskers. Carbon 2005, 43, 2837–2841. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.P.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous carbon tubes from fullerene crystals as the π-electron carbon source. Angew. Chem. Int. Ed. 2015, 54, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.K.; Sathish, M.; Hill, J.P.; Miyazawa, K.; Tsuruoka, T.; Sanchez-Ballester, N.M.; Honma, I.; Ji, Q.; Ariga, K. Alcohol-induced decomposition of Olmstead’s crystalline Ag(I)-fullerene heteronanostructure yields ‘bucky cubes’. J. Mater. Chem. C 2013, 1, 1174–1181. [Google Scholar] [CrossRef]
- Peng, Z.; Hu, Y.; Wang, J.; Liu, S.; Li, C.; Jiang, Q.; Lu, J.; Zeng, X.; Peng, P.; Li, F.-F. Fullerene-based in situ doping of N and Fe into a 3D cross-like hierarchical carbon composite for high-performance supercapacitors. Adv. Energy Mater. 2019, 9, 1802928. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Nakanishi, W.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Hierarchically structured fullerene C70 cube for sensing volatile aromatic solvent vapors. ACS Nano 2016, 10, 6631–6637. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Intentional closing/opening of “hole-in-cube” fullerene crystals with microscopic recognition properties. ACS Nano 2017, 11, 7790–7796. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.; Miyazawa, K. Size-tunable hexagonal fullerene (C60) nanosheets at the liquid-liquid interface. J. Am. Chem. Soc. 2007, 129, 13816–13817. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K.; Hill, J.P.; Ariga, K. Solvent engineering for shape-shifter pure fullerene (C60). J. Am. Chem. Soc. 2009, 131, 6372–6373. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Yamauchi, Y.; Hill, J.P.; Miyazawa, K.; Ariga, K. Fullerene crystals with bimodal pore architectures consisting of macropores and mesopores. J. Am. Chem. Soc. 2013, 135, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wang, S.; Lai, Z.; Yao, X.; Zhao, Y.; Zhang, H.; Chen, H. Two-dimensional C60 nano-meshes via crystal transformation. Nanoscale 2019, 11, 8692–8698. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Yu, J.; Paley, D.W.; Trinh, M.T.; Paley, M.V.; Karch, J.M.; Crowther, A.C.; Lee, C.-H.; Lalancette, R.A.; Zhu, X.; et al. van der Waals solids from self-assembled nanoscale building blocks. Nano Lett. 2016, 16, 1445–1449. [Google Scholar] [CrossRef]
- Lee, K.; Choi, B.; Plante, I.J.-L.; Paley, M.V.; Zhong, X.; Crowther, A.C.; Jonathan, S.; Owen, J.S.; Zhu, X.; Roy, X. Two-dimensional fullerene assembly from an exfoliated van der Waals template. Angew. Chem. Int. Ed. 2018, 57, 6125–6129. [Google Scholar] [CrossRef] [PubMed]
- Wakahara, T.; Sathish, M.; Miyazawa, K.; Hu, C.; Tateyama, Y.; Nemoto, Y.; Sasaki, T.; Ito, O. Preparation and optical properties of fullerene/ferrocene hybrid hexagonal nanosheets and large-scale production of fullerene hexagonal nanosheets. J. Am. Chem. Soc. 2009, 131, 9940–9944. [Google Scholar] [CrossRef]
- Wakahara, T.; D’Angelo, P.; Miyazawa, K.; Nemoto, Y.; Ito, O.; Tanigaki, N.; Bradley, D.D.C.; Anthopoulos, T.D. Fullerene/cobalt porphyrin hybrid nanosheets with ambipolar charge transporting characteristics. J. Am. Chem. Soc. 2012, 134, 7204–7206. [Google Scholar] [CrossRef]
- Li, M.; Deng, K.; Lei, S.-B.; Yang, Y.-L.; Wang, T.-S.; Shen, Y.-T.; Wang, C.-R.; Zeng, Q.-D.; Wang, C. Site-selective fabrication of two-dimensional fullerene arrays by using a supramolecular template at the liquid-solid interface. Angew. Chem. Int. Ed. 2008, 47, 6717–6721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, X.; Zhu, G.; Wang, Y.; Ding, H.; Lin, H.; Li, Y.; Li, Q.; Gao, J.; Pan, M.; et al. Two-dimensional van der Waals supramolecular frameworks from Co-hosted molecular assembly and C60 dimerization. J. Phys. Chem. C 2020, 124, 12589–12595. [Google Scholar] [CrossRef]
- Nakanishi, T.; Schmitt, W.; Michinobu, T.; Kurth, D.G.; Ariga, K. Hierarchical supramolecular fullerene architectures with controlled dimensionality. Chem. Commun. 2005, 5982–5984. [Google Scholar] [CrossRef]
- Zhang, X.; Hsu, C.-H.; Ren, X.; Gu, Y.; Song, B.; Sun, H.-J.; Yang, S.; Chen, E.; Tu, Y.; Li, X.; et al. Supramolecular [60]fullerene liquid crystals formed by self-organized two-dimensional crystals. Angew. Chem. Int. Ed. 2015, 54, 114–117. [Google Scholar] [CrossRef] [PubMed]
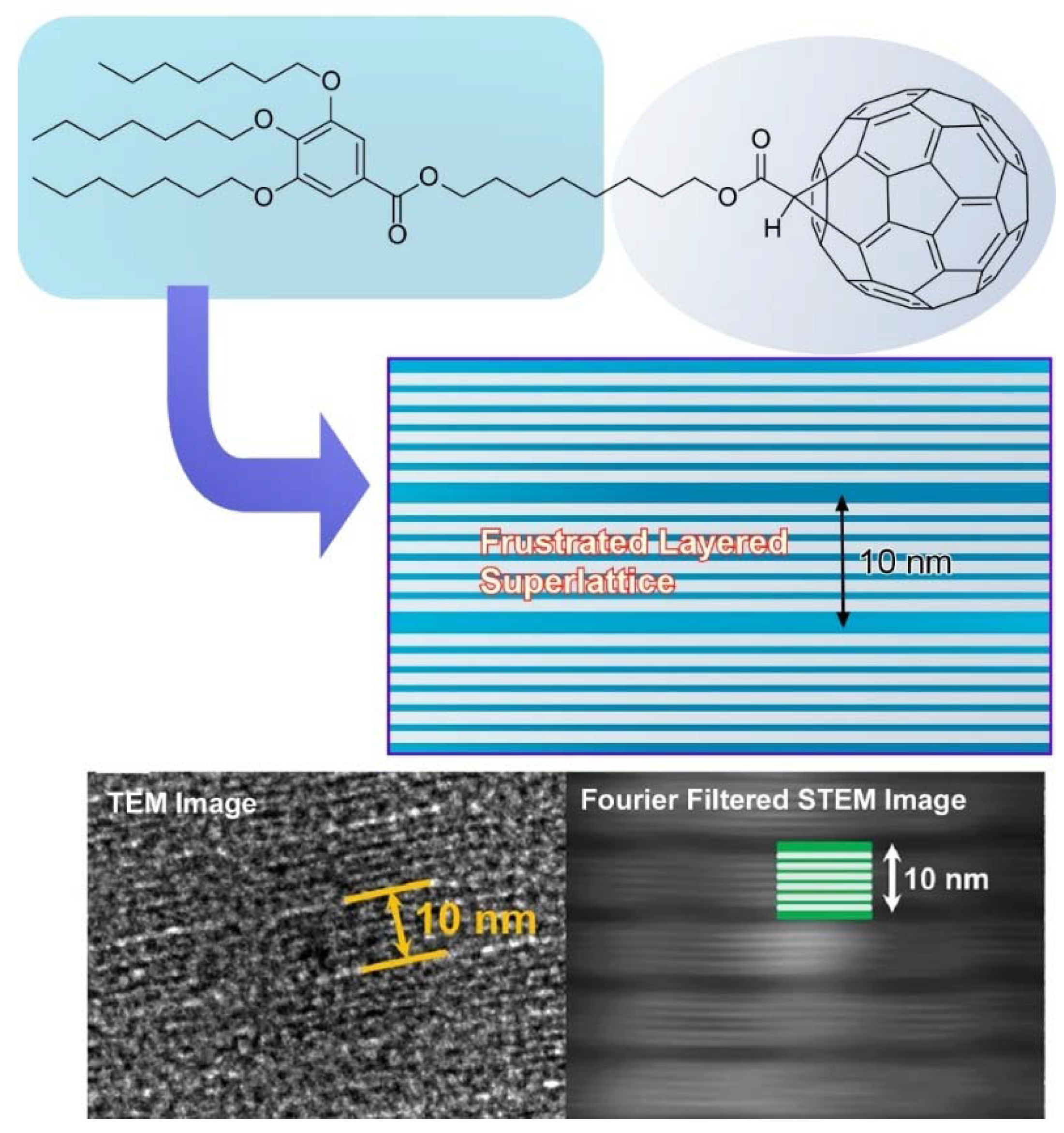
- Lu, H.; Zhang, X.; Sakurai, T.; Li, X.; Tu, Y.; Guo, J.; Seki, S.; Li, C.Y.; Ungar, G.; Cheng, S.Z.D. Frustrated layered self-assembly induced superlattice from two-dimensional nanosheets. Nano Lett. 2020, 20, 8647–8653. [Google Scholar] [CrossRef] [PubMed]
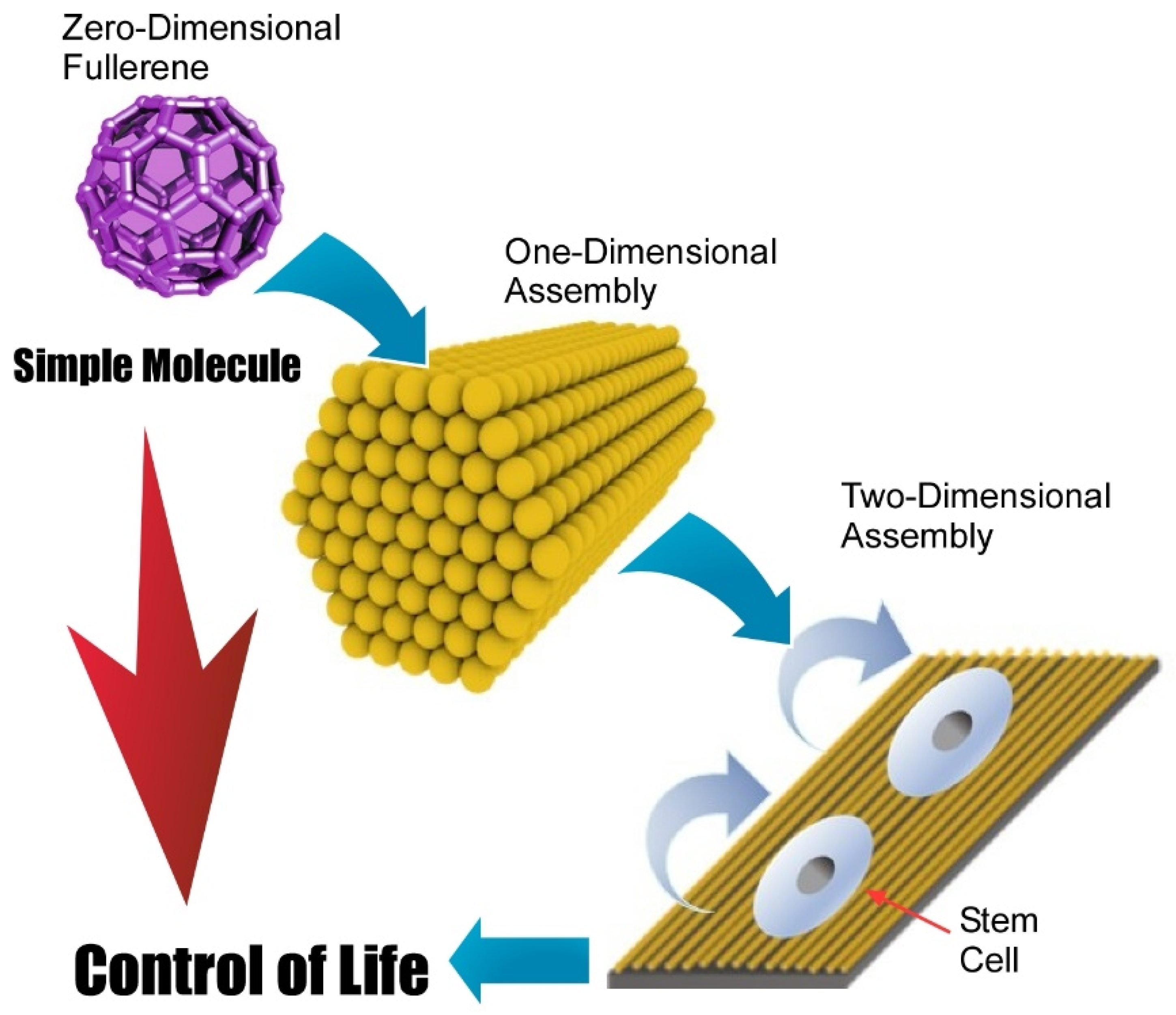
- Minami, K.; Kasuya, Y.; Yamazaki, T.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Sakai, H.; Ariga, K. Highly ordered 1D fullerene crystals for concurrent control of macroscopic cellular orientation and differentiation toward large-scale tissue engineering. Adv. Mater. 2015, 27, 4020–4026. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Kasuya, Y.; Ji, Q.; Sathish, M.; Shrestha, L.K.; Ishihara, S.; Minami, K.; Morita, H.; Yamazaki, T.; Hanagata, N.; et al. Vortex-aligned fullerene nanowhiskers as a scaffold for orienting cell growth. ACS Appl. Mater. Interfaces 2015, 7, 15667–15673. [Google Scholar] [CrossRef]
- Song, J.; Jia, X.; Minami, K.; Hill, J.P.; Nakanishi, J.; Shrestha, L.K.; Ariga, K. Large-area aligned fullerene nanocrystal scaffolds as culture substrates for enhancing mesenchymal stem cell self-renewal and multipotency. ACS Appl. Nano Mater. 2020, 3, 6497–6506. [Google Scholar] [CrossRef]
- Makiura, R.; Motoyama, S.; Umemura, Y.; Yamanaka, H.; Sakata, O.; Kitagawa, H. Surface nano-architecture of a metal–organic framework. Nat. Mater. 2010, 9, 565–571. [Google Scholar] [CrossRef]
- Bai, B.; Wang, D.; Wan, L.-J. Synthesis of covalent organic framework films at interfaces. Bull. Chem. Soc. Jpn. 2021, 94, 1090–1098. [Google Scholar] [CrossRef]
- Ariga, K. The evolution of molecular machines through interfacial nanoarchitectonics: From toys to tools. Chem. Sci. 2020, 11, 10594–10604. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Shrestha, L.K.; Ariga, K. Zero-to-Two Nanoarchitectonics: Fabrication of Two-Dimensional Materials from Zero-Dimensional Fullerene. Molecules 2021, 26, 4636. https://doi.org/10.3390/molecules26154636
Chen G, Shrestha LK, Ariga K. Zero-to-Two Nanoarchitectonics: Fabrication of Two-Dimensional Materials from Zero-Dimensional Fullerene. Molecules. 2021; 26(15):4636. https://doi.org/10.3390/molecules26154636
Chicago/Turabian StyleChen, Guoping, Lok Kumar Shrestha, and Katsuhiko Ariga. 2021. "Zero-to-Two Nanoarchitectonics: Fabrication of Two-Dimensional Materials from Zero-Dimensional Fullerene" Molecules 26, no. 15: 4636. https://doi.org/10.3390/molecules26154636
APA StyleChen, G., Shrestha, L. K., & Ariga, K. (2021). Zero-to-Two Nanoarchitectonics: Fabrication of Two-Dimensional Materials from Zero-Dimensional Fullerene. Molecules, 26(15), 4636. https://doi.org/10.3390/molecules26154636







