Tracking the Amide I and ?COO? Terminal ?(C=O) Raman Bands in a Family of l-Glutamic Acid-Containing Peptide Fragments: A Raman and DFT Study
Abstract
:1. Introduction
2. Methods
2.1. Experimental Methods
2.2. Theoretical Methods
3. Results and Discussion
3.1. Summed Simulated Spectra to Experiment
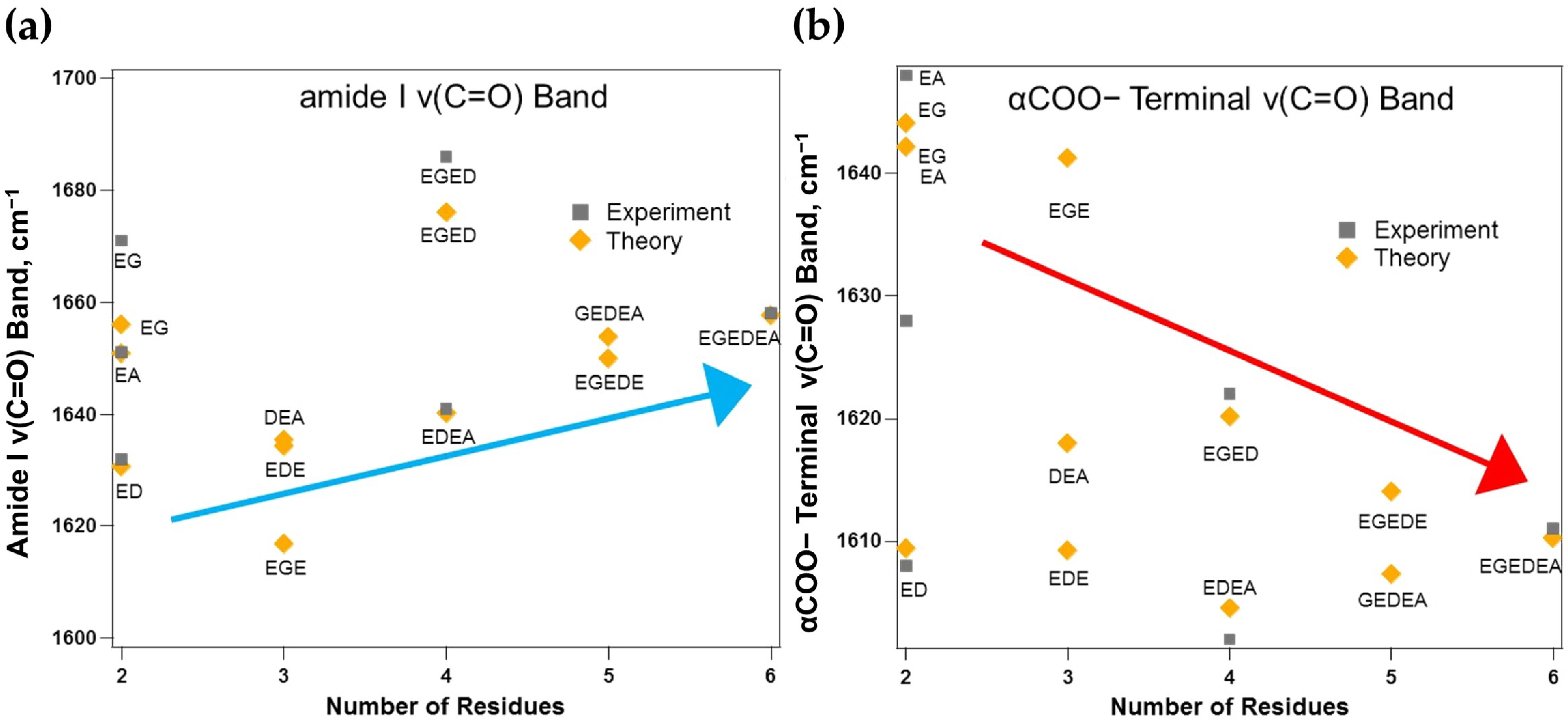
3.2. Tracking the Amide I and αCOO− Terminal ν(C=O) Bands
3.2.1. Amide I and αCOO− Vibrations Depend on Peptide Chain Length and Side-Chain Polarity
3.2.2. Intramolecular Charge Transfer Disparately Influences Amide I and αCOO− Vibrations
3.3. Experimental and Computational Vibrational Analyses Predict EGEDEA Secondary Structure
3.4. Local Structure within E-Hooks May Play Pivotal Roles in Protein Recruitment and Retention
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anfinsen, C.B. Principles that Govern the Folding of Protein Chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, P.J.; Rose, G.D. Do all backbone polar groups in proteins form hydrogen bonds? Protein Sci. 2005, 14, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L.; Corey, R.B.; Branson, H.R. The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Mikshiev, V.Y.; Pozharskii, A.F.; Filarowski, A.; Novikov, A.S.; Antonov, A.S.; Tolstoy, P.M.; Vovk, M.A.; Khoroshilova, O.V. How Strong is Hydrogen Bonding to Amide Nitrogen? Chemphyschem 2020, 21, 651–658. [Google Scholar] [CrossRef]
- Cox, C.; Wack, H.; Lectka, T. Strong Hydrogen Bonding to the Amide Nitrogen Atom in an “Amide Proton Sponge”: Consequences for Structure and Reactivity. Angew. Chem. Int. Ed. 1999, 38, 798–800. [Google Scholar] [CrossRef]
- Giubertoni, G.; Sofronov, O.O.; Bakker, H.J. Effect of intramolecular hydrogen-bond formation on the molecular conformation of amino acids. Commun. Chem. 2020, 3, 84. [Google Scholar] [CrossRef]
- Nevskaya, N.A.; Chirgadze, Y.N. Infrared spectra and resonance interactions of amide-I and II vibrations of α-helix. Biopolymers 1976, 15, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Mandal, I.; Paul, S.; Venkatramani, R. Optical backbone-sidechain charge transfer transitions in proteins sensitive to secondary structure and modifications. Faraday Discuss. 2018, 207, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Myshakina, N.S.; Ahmed, Z.; Asher, S.A. Dependence of Amide Vibrations on Hydrogen Bonding. J. Phys. Chem. B 2008, 112, 11873–11877. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.E.; Davis, J.E.; Reynolds, J.E.; Fortenberry, R.C.; Hammer, N.I.; Reinemann, D.N. Determination of Vibrational Band Positions in the E-Hook of β-Tubulin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 244, 118895. [Google Scholar] [CrossRef]
- Rybka, K.; Toal, S.E.; Verbaro, D.J.; Mathieu, D.; Schwalbe, H.; Schweitzer-Stenner, R. Disorder and order in unfolded and disordered peptides and proteins: A view derived from tripeptide conformational analysis. II. Tripeptides with short side chains populating asx and β-type like turn conformations. Proteins Struct. Funct. Bioinform. 2013, 81, 968–983. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Cruz, V.L. Conformational analysis of short polar side-chain amino-acids through umbrella sampling and DFT calculations. J. Mol. Modeling 2016, 22, 273. [Google Scholar] [CrossRef] [PubMed]
- Pogostin, B.H.; Malmendal, A.; Londergan, C.H.; Åkerfeldt, K.S. pKa Determination of a Histidine Residue in a Short Peptide Using Raman Spectroscopy. Molecules 2019, 24, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryall, J.P.; Dines, T.J.; Chowdhry, B.Z.; Leharne, S.A.; Withnall, R. Vibrational spectra and structures of urazole and 4-methylurazole: DFT calculations of the normal modes in aqueous solution and in the solid state, and the influence of hydrogen bonding. Chem. Phys. 2010, 373, 219–227. [Google Scholar] [CrossRef]
- Huang, S.R.; Liu, Y.; Tureček, F. Non-covalent complexes of the peptide fragment Gly-Asn-Asn-Gln-Gln-Asn-Tyr in the gas-phase. Photodissociative cross-linking, Born–Oppenheimer molecular dynamics, and ab initio computational binding study. Phys. Chem. Chem. Phys. 2019, 21, 2046–2056. [Google Scholar] [CrossRef]
- Silva, C.B.; da Silva Filho, J.G.; Pinheiro, G.S.; Teixeira, A.M.R.; de Sousa, F.F.; Freire, P.T.C. High-pressure studies on l,l-dileucine crystals by Raman spectroscopy and synchrotron X-ray diffraction combined with DFT calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117899. [Google Scholar] [CrossRef]
- Kecel-Gunduz, S.; Bicak, B.; Celik, S.; Akyuz, S.; Ozel, A.E. Structural and spectroscopic investigation on antioxidant dipeptide, l-Methionyl-l-Serine: A combined experimental and DFT study. J. Mol. Struct. 2017, 1137, 756–770. [Google Scholar] [CrossRef]
- Derbel, N.; Hernández, B.; Pflüger, F.; Liquier, J.; Geinguenaud, F.; Jaïdane, N.; Ben Lakhdar, Z.; Ghomi, M. Vibrational Analysis of Amino Acids and Short Peptides in Hydrated Media. I. l-glycine and l-leucine. J. Phys. Chem. B 2007, 111, 1470–1477. [Google Scholar] [CrossRef]
- Buchanan, E.G.; James, W.H.; Choi, S.H.; Guo, L.; Gellman, S.H.; Müller, C.W.; Zwier, T.S. Single-conformation infrared spectra of model peptides in the amide I and amide II regions: Experiment-based determination of local mode frequencies and inter-mode coupling. J. Chem. Phys. 2012, 137, 094301. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Srivastava, S.K.; Materny, A.; Ojha, A.K. A vibrational and conformational characterization of arginine at different pH values investigated using Raman spectroscopy combined with DFT calculations. J. Raman Spectrosc. 2016, 47, 1073–1085. [Google Scholar] [CrossRef]
- Eker, F.; Cao, X.; Nafie, L.; Schweitzer-Stenner, R. Tripeptides Adopt Stable Structures in Water. A Combined Polarized Visible Raman, FTIR, and VCD Spectroscopy Study. J. Am. Chem. Soc. 2002, 124, 14330–14341. [Google Scholar] [CrossRef]
- Walsh, P.S.; Dean, J.C.; McBurney, C.; Kang, H.; Gellman, S.H.; Zwier, T.S. Conformation-specific spectroscopy of capped glutamine-containing peptides: Role of a single glutamine residue on peptide backbone preferences. Phys. Chem. Chem. Phys. 2016, 18, 11306–11322. [Google Scholar] [CrossRef]
- Habka, S.; Sohn, W.Y.; Vaquero-Vara, V.; Géléoc, M.; Tardivel, B.; Brenner, V.; Gloaguen, E.; Mons, M. On the turn-inducing properties of asparagine: The structuring role of the amide side chain, from isolated model peptides to crystallized proteins. Phys. Chem. Chem. Phys. 2018, 20, 3411–3423. [Google Scholar] [CrossRef]
- Martial, B.; Lefèvre, T.; Auger, M. Understanding amyloid fibril formation using protein fragments: Structural investigations via vibrational spectroscopy and solid-state NMR. Biophys. Rev. 2018, 10, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, K.J.; Suhai, S. N-Acetyl-l-alanine N′-methylamide: A density functional analysis of the vibrational absorption and vibrational circular dichroism spectra. Chem. Phys. 1996, 208, 81–116. [Google Scholar] [CrossRef]
- Jalkanen, K.J.; Elstner, M.; Suhai, S. Amino acids and small peptides as building blocks for proteins: Comparative theoretical and spectroscopic studies. J. Mol. Struct. THEOCHEM 2004, 675, 61–77. [Google Scholar] [CrossRef]
- Correia, C.F.; Balaj, P.O.; Scuderi, D.; Maitre, P.; Ohanessian, G. Vibrational Signatures of Protonated, Phosphorylated Amino Acids in the Gas Phase. J. Am. Chem. Soc. 2008, 130, 3359–3370. [Google Scholar] [CrossRef] [PubMed]
- Bour, P.; Kubelka, J.; Keiderling, T.A. Ab initio quantum mechanical models of peptide helices and their vibrational spectra. Biopolymers 2002, 65, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Woutersen, S.; Pfister, R.; Hamm, P.; Mu, Y.; Kosov, D.S.; Stock, G. Peptide conformational heterogeneity revealed from nonlinear vibrational spectroscopy and molecular-dynamics simulations. J. Chem. Phys. 2002, 117, 6833–6840. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-K.; Oh, K.-I.; Lee, H.; Joo, C.; Han, H.; Cho, M. Dipeptide Structure Determination by Vibrational Circular Dichroism Combined with Quantum Chemistry Calculations. Chemphyschem 2007, 8, 2218–2226. [Google Scholar] [CrossRef]
- Kolev, T.; Spiteller, M.; Koleva, B. Spectroscopic and structural elucidation of amino acid derivatives and small peptides: Experimental and theoretical tools. Amino Acids 2010, 38, 45–50. [Google Scholar] [CrossRef]
- Kobko, N.; Dannenberg, J.J. Cooperativity in Amide Hydrogen Bonding Chains. A Comparison between Vibrational Coupling through Hydrogen Bonds and Covalent Bonds. Implications for Peptide Vibrational Spectra. J. Phys. Chem. A 2003, 107, 6688–6697. [Google Scholar] [CrossRef]
- Pires, D.A.T.; Arake, L.M.R.; Silva, L.P.; Lopez-Castillo, A.; Prates, M.V.; Nascimento, C.J.; Bloch, C. A previously undescribed hexapeptide His-Arg-Phe-Leu-Arg-His-NH2 from amphibian skin secretion shows CO2 and metal biding affinities. Peptides 2018, 106, 37–44. [Google Scholar] [CrossRef]
- Furić, K.; Mohaček Grošev, V.; Bonifacic, M.; Štefanić, I. Raman spectroscopic study of H2O and D2O water solutions of glycine. J. Mol. Struct. 1992, 267, 39–44. [Google Scholar] [CrossRef]
- Navarrete, J.T.L.; Hernández, V.; Ramírez, F.J. Vibrational study of aspartic acid and glutamic acid dipeptides. J. Mol. Struct. 1995, 348, 249–252. [Google Scholar] [CrossRef]
- Parameswari, A.; Premkumar, S.; Premkumar, R.; Milton Franklin Benial, A. Surface enhanced Raman spectroscopy and quantum chemical studies on glycine single crystal. J. Mol. Struct. 2016, 1116, 180–187. [Google Scholar] [CrossRef]
- Mendham, A.P.; Dines, T.J.; Snowden, M.J.; Chowdhry, B.Z.; Withnall, R. Vibrational spectroscopy and DFT calculations of di-amino acid cyclic peptides. Part I: Cyclo(Gly-Gly), cyclo(L-Ala-l-Ala) and cyclo(L-Ala-Gly) in the solid state and in aqueous solution. J. Raman Spectrosc. 2009, 40, 1478–1497. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef]
- Barron, L.D.; Gargaro, A.R.; Hecht, L.; Polavarapu, P.L. Experimental and ab initio theoretical vibrational Raman optical activity of alanine. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 1001–1016. [Google Scholar] [CrossRef]
- Rožman, M. Aspartic Acid Side Chain Effect—Experimental and Theoretical Insight. J. Am. Soc. Mass Spectrom. 2007, 18, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kausar, N.; Dines, T.J.; Chowdhry, B.Z.; Alexander, B.D. Vibrational spectroscopy and DFT calculations of the di-amino acid peptide l-aspartyl-l-glutamic acid in the zwitterionic state. Phys. Chem. Chem. Phys. 2009, 11, 6389–6400. [Google Scholar] [CrossRef]
- Peica, N.; Lehene, C.; Leopold, N.; Schlücker, S.; Kiefer, W. Monosodium glutamate in its anhydrous and monohydrate form: Differentiation by Raman spectroscopies and density functional calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 66, 604–615. [Google Scholar] [CrossRef]
- Navarrete, J.T.L.; Hernández, V.; Ramírez, F.J. Vibrational spectra of [15N]glutamic acid and [2H4]glutamic acid. J. Raman Spectrosc. 1994, 25, 861–867. [Google Scholar] [CrossRef]
- Bouř, P.; Sopková, J.; Bednárová, L.; Maloň, P.; Keiderling, T.A. Transfer of molecular property tensors in cartesian coordinates: A new algorithm for simulation of vibrational spectra. J. Comput. Chem. 1997, 18, 646–659. [Google Scholar] [CrossRef]
- Bouř, P.; Keiderling, T.A. Partial optimization of molecular geometry in normal coordinates and use as a tool for simulation of vibrational spectra. J. Chem. Phys. 2002, 117, 4126–4132. [Google Scholar] [CrossRef] [Green Version]
- Jacob, C.R.; Reiher, M. Localizing normal modes in large molecules. J. Chem. Phys. 2009, 130, 084106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, C.; Neugebauer, J.; Reiher, M. Finding a needle in a haystack: Direct determination of vibrational signatures in complex systems. New J. Chem. 2007, 31, 818–831. [Google Scholar] [CrossRef]
- Herrmann, C.; Ruud, K.; Reiher, M. Importance of backbone angles versus amino acid configurations in peptide vibrational Raman optical activity spectra. Chem. Phys. 2008, 343, 200–209. [Google Scholar] [CrossRef]
- Jacob, C.R.; Luber, S.; Reiher, M. Analysis of Secondary Structure Effects on the IR and Raman Spectra of Polypeptides in Terms of Localized Vibrations. J. Phys. Chem. B 2009, 113, 6558–6573. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, K.K.; Singh, S.K.; Borish, K.; Dey, S.; Sarkar, B.; Das, A. Observation of a weak intra-residue C5 hydrogen-bond in a dipeptide containing Gly-Pro sequence. J. Chem. Phys. 2019, 151, 104309. [Google Scholar] [CrossRef]
- Gorbitz, C. Structures of dipeptides: The head-to-tail story. Acta Crystallogr. Sect. B 2010, 66, 84–93. [Google Scholar] [CrossRef]
- Hanyu, M.; Ninomiya, D.; Yanagihara, R.; Murashima, T.; Miyazawa, T.; Yamada, T. Studies on intramolecular hydrogen bonding between the pyridine nitrogen and the amide hydrogen of the peptide: Synthesis and conformational analysis of tripeptides containing novel amino acids with a pyridine ring. J. Pept. Sci. 2005, 11, 491–498. [Google Scholar] [CrossRef]
- Torii, H.; Tasumi, M. Ab Initio Molecular Orbital Study of the Amide I Vibrational Interactions between the Peptide Groups in Di- and Tripeptides and Considerations on the Conformation of the Extended Helix. J. Raman Spectrosc. 1998, 29, 81–86. [Google Scholar] [CrossRef]
- Barth, A.; Zscherp, C. What vibrations tell about proteins. Q. Rev. Biophys. 2002, 35, 369–430. [Google Scholar] [CrossRef]
- Wieczorek, R.; Dannenberg, J.J. Hydrogen-Bond Cooperativity, Vibrational Coupling, and Dependence of Helix Stability on Changes in Amino Acid Sequence in Small 310-Helical Peptides. A Density Functional Theory Study. J. Am. Chem. Soc. 2003, 125, 14065–14071. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Yu, Z.-Y.; Wu, J.; Liu, C.-B. Electron Delocalization and Charge Transfer in Polypeptide Chains. J. Phys. Chem. A 2009, 113, 10521–10526. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Fomin, D.P.; Tsirelson, V.G. DFT study of H-bonds in the peptide secondary structures: The backbone–side-chain and polar side-chains interactions. J. Mol. Struct. 2010, 972, 11–15. [Google Scholar] [CrossRef]
- Prell, J.S.; O’Brien, J.T.; Steill, J.D.; Oomens, J.; Williams, E.R. Structures of Protonated Dipeptides: The Role of Arginine in Stabilizing Salt Bridges. J. Am. Chem. Soc. 2009, 131, 11442–11449. [Google Scholar] [CrossRef]
- Laurin, Y.; Eyer, J.; Robert, C.H.; Prevost, C.; Sacquin-Mora, S. Mobility and Core-Protein Binding Patterns of Disordered C-Terminal Tails in β-Tubulin Isotypes. Biochemistry 2017, 56, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Hirokawa, N. Mechanism of the single-headed processivity: Diffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proc. Natl. Acad. Sci. USA 2000, 97, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinemann, D.N.; Norris, S.R.; Ohi, R.; Lang, M.J. Processive Kinesin-14 HSET Exhibits Directional Flexibility Depending on Motor Traffic. Curr. Biol. 2018, 28, 2356–2362.e2355. [Google Scholar] [CrossRef] [Green Version]
- Reinemann, D.N.; Sturgill, E.G.; Das, D.K.; Degen, M.S.; Vörös, Z.; Hwang, W.; Ohi, R.; Lang, M.J. Collective Force Regulation in Anti-parallel Microtubule Gliding by Dimeric Kif15 Kinesin Motors. Curr. Biol. 2017, 27, 2810–2820.e2816. [Google Scholar] [CrossRef] [Green Version]
- Thorn, K.S.; Ubersax, J.A.; Vale, R.D. Engineering the Processive Run Length of the Kinesin Motor. J. Cell Biol. 2000, 151, 1093–1100. [Google Scholar] [CrossRef]
- Wang, Z.; Sheetz, M.P. The C-terminus of tubulin increases cytoplasmic dynein and kinesin processivity. Biophys. J. 2000, 78, 1955–1964. [Google Scholar] [CrossRef] [Green Version]
- Woehlke, G.; Ruby, A.K.; Hart, C.L.; Ly, B.; Hom-Booher, N.; Vale, R.D. Microtubule Interaction Site of the Kinesin Motor. Cell 1997, 90, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Dixit, R.; Ross, J.L.; Goldman, Y.E.; Holzbaur, E.L.F. Differential Regulation of Dynein and Kinesin Motor Proteins by Tau. Science 2008, 319, 1086–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, H.; Luchko, T.; Luduena, R.F.; Tuszynski, J.A. Molecular Dynamics Modeling of Tubulin C-Terminal Tail Interactions with the Microtubule Surface. Proteins Struct. Funct. Bioinform. 2011, 79, 2968–2982. [Google Scholar] [CrossRef] [PubMed]
- Gennerich, A.; Vale, R.D. Walking the Walk: How Kinesin and Dynein Coordinate Their Steps. Curr. Opin. Cell Biol. 2009, 21, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, L.S.B. Kinesin molecular motors: Transport pathways, receptors, and human disease. Proc. Natl. Acad. Sci. USA 2001, 98, 6999–7003. [Google Scholar] [CrossRef] [Green Version]
- Heald, R.; Khodjakov, A. Thirty years of search and capture: The complex simplicity of mitotic spindle assembly. J. Cell Biol. 2015, 211, 1103–1111. [Google Scholar] [CrossRef]
- Janke, C.; Bulinski, J.C. Post-Translational Regulation of the Microtubule Cytoskeleton: Mechanisms and Functions. Nat. Rev. Mol. Cell Biol. 2011, 12, 773–786. [Google Scholar] [CrossRef]
- Karsenti, E.; Vernos, I. The Mitotic Spindle: A Self-Made Machine. Science 2001, 294, 543–547. [Google Scholar] [CrossRef]
- Lakämper, S.; Meyhöfer, E. The E-Hook of Tubulin Interacts with Kinesin’s Head to Increase Processivity and Speed. Biophys. J. 2005, 89, 3223–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansky, Z.; Braun, M.; Lüdecke, A.; Schlierf, M.; ten Wolde, P.R.; Janson, M.E.; Diez, S. Diffusible Crosslinkers Generate Directed Forces in Microtubule Networks. Cell 2015, 160, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Luchko, T.; Huzil, J.T.; Stepanova, M.; Tuszynski, J. Conformational Analysis of the Carboxy-Terminal Tails of Human β-Tubulin Isotypes. Biophys. J. 2008, 94, 1971–1982. [Google Scholar] [CrossRef] [Green Version]
- Nogales, E. Structural Insights into Microtubule Function. Annu. Rev. Biochem. 2000, 30, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Otter, A.; Kotovych, G. The Solution Conformation of the Synthetic Tubulin Fragment Ac-Tubulin-α (430–441)-Amide Based on Two-Dimensional ROESY Experiments. Can. J. Chem. 1988, 66, 1814–1820. [Google Scholar] [CrossRef]
- Panneerselvam, M.; Muthu, K.; Jayaraman, M.; Sridharan, U.; Jenardhanan, P.; Ramadas, K. Molecular Dynamic Simulations of the Tubulin-Human Gamma Synuclein Complex: Structural Insight into the Regulatory Mechanism Involved in Inducing Resistance against Taxol. Mol. Biosyst. 2013, 9, 1470–1488. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.; de la Torre, J.; Maccioni, R.B.; Avila, J. Involvement of the carboxyl-terminal domain of tubulin in the regulation of its assembly. Proc. Natl. Acad. Sci. USA 1984, 81, 5989. [Google Scholar] [CrossRef] [Green Version]
- Sirajuddin, M.; Rice, L.M.; Vale, R.D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 2014, 16, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Wall, K.P.; Pagratis, M.; Armstrong, G.; Balsbaugh, J.L.; Verbeke, E.; Pearson, C.G.; Hough, L.E. Molecular Determinants of Tubulin’s C-Terminal Tail Conformational Ensemble. ACS Chem. Biol. 2016, 11, 2891–2990. [Google Scholar] [CrossRef] [Green Version]
- Westermann, S.; Weber, K. Post-Translational Modifications Regulate Microtubule Function. Nat. Rev. Mol. Cell Biol. 2003, 4, 938–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wordeman, L. How kinesin motor proteins drive mitotic spindle function: Lessons from molecular assays. Semin. Cell Dev. Biol. 2010, 21, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz, A.; Tomishige, M.; Vale, R.D.; Selvin, P.R. Kinesin Walks Hand-Over-Hand. Science 2004, 303, 676–678. [Google Scholar] [CrossRef] [Green Version]
- Sahu, N.; Gadre, S.R. Vibrational infrared and Raman spectra of polypeptides: Fragments-in-fragments within molecular tailoring approach. J. Chem. Phys. 2016, 144, 114113. [Google Scholar] [CrossRef]
- Yamamoto, S.; Bouř, P. Calculation of Vibrational Spectra of Large Molecules from Their Fragments. In Frontiers of Quantum Chemistry; Wójcik, M.J., Nakatsuji, H., Kirtman, B., Ozaki, Y., Eds.; Springer: Singapore, 2018; pp. 181–197. [Google Scholar] [CrossRef]
- Yamamoto, S. Conformational analyses of peptides and proteins by vibrational Raman optical activity. Anal. Bioanal. Chem. 2012, 403, 2203–2212. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, R.B.J.S.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO Version 3.1; ScienceOpen: Burlington, MA, USA, 2001. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Huang, K. Introduction to Statistical Physics; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Tien, C.L.L.; John, H. Statistical Thermodynamics; Hemisphere Publishing Corporation: Lonndon, UK, 1985. [Google Scholar]
- Andersson, M.P.; Uvdal, P. New Scale Factors for Harmonic Vibrational Frequencies Using the B3LYP Density Functional Method with the Triple-ζ Basis Set 6-311+G(d,p). J. Phys. Chem. A 2005, 109, 2937–2941. [Google Scholar] [CrossRef] [PubMed]
- Baldauf, C.; Günther, R.; Hofmann, H.-J. δ-Peptides and δ-Amino Acids as Tools for Peptide Structure DesignA Theoretical Study. J. Org. Chem. 2004, 69, 6214–6220. [Google Scholar] [CrossRef] [PubMed]
- Echenique, P.; Chass, G.A. Efficient model chemistries for peptides. II. Basis set convergence in the B3LYP method. arXiv 2008, arXiv:0811.1271. [Google Scholar]
- Jiménez-Hoyos, C.A.; Janesko, B.G.; Scuseria, G.E. Evaluation of range-separated hybrid density functionals for the prediction of vibrational frequencies, infrared intensities, and Raman activities. Phys. Chem. Chem. Phys. 2008, 10, 6621–6629. [Google Scholar] [CrossRef] [Green Version]
- Sjöberg, B.; Foley, S.; Cardey, B.; Enescu, M. An experimental and theoretical study of the amino acid side chain Raman bands in proteins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 128, 300–311. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP Density Functional Methods for a Large Set of Organic Molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef]
- Lelimousin, M.; Limongelli, V.; Sansom, M.S.P. Conformational Changes in the Epidermal Growth Factor Receptor: Role of the Transmembrane Domain Investigated by Coarse-Grained MetaDynamics Free Energy Calculations. J. Am. Chem. Soc. 2016, 138, 10611–10622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdés, H.; Řeha, D.; Hobza, P. Structure of isolated tryptophyl-glycine dipeptide and tryptophyl-glycyl- glycine tripeptide: Ab initio SCC-DFTB-D molecular dynamics simulations and high-level correlated ab initio quantum chemical calculations. J. Phys. Chem. B 2006, 110, 6385–6396. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, P.N.; Wales, D.J. Energy landscapes, global optimization and dynamics of the polyalanine Ac(ala)8NHMe. J. Chem. Phys. 2001, 114, 6443–6454. [Google Scholar] [CrossRef] [Green Version]
- Papaleo, E.; Mereghetti, P.; Fantucci, P.; Grandori, R.; De Gioia, L. Free-energy landscape, principal component analysis, and structural clustering to identify representative conformations from molecular dynamics simulations: The myoglobin case. J. Mol. Graph. Model. 2009, 27, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; Van Gunsteren, W.F. On searching in, sampling of, and dynamically moving through conformational space of biomolecular systems: A review. J. Comput. Chem. 2008, 29, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Maximova, T.; Moffatt, R.; Ma, B.; Nussinov, R.; Shehu, A. Principles and Overview of Sampling Methods for Modeling Macromolecular Structure and Dynamics. PLoS Comput. Biol. 2016, 12, e1004619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.; Xi, W.; Nussinov, R.; Ma, B. Protein Ensembles: How Does Nature Harness Thermodynamic Fluctuations for Life? the Diverse Functional Roles of Conformational Ensembles in the Cell. Chem. Rev. 2016, 116, 6516–6551. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, L.; Nguyen, P.H.; Stock, G. Construction of the Free Energy Landscape of Peptide Aggregation from Molecular Dynamics Simulations. J. Chem. Theory Comput. 2012, 8, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Wenzel, W. A free-energy approach for all-atom protein simulation. Biophys. J. 2009, 96, 3483–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippert, J.L.; Tyminski, D.; Desmeules, P.J. Determination of the Secondary Structure of Proteins by Laser Raman Spectroscopy. J. Am. Chem. Soc. 1976, 98, 7075–7080. [Google Scholar] [CrossRef]
- Choi, J.-H.; Ham, S.; Cho, M. Local Amide I Mode Frequencies and Coupling Constants in Polypeptides. J. Phys. Chem. B 2003, 107, 9132–9138. [Google Scholar] [CrossRef]
- Jansen, T.l.C.; Dijkstra, A.G.; Watson, T.M.; Hirst, J.D.; Knoester, J. Modeling the amide I bands of small peptides. J. Chem. Phys. 2006, 125, 044312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Roll-Mecak, A. Intrinsically disordered tubulin tails: Complex tuners of microtubule functions? Semin. Cell Dev. Biol. 2015, 37, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Verhey, K.J.; Gaertig, J. The Tubulin Code. Cell Cycle 2007, 6, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are not available from the authors. |
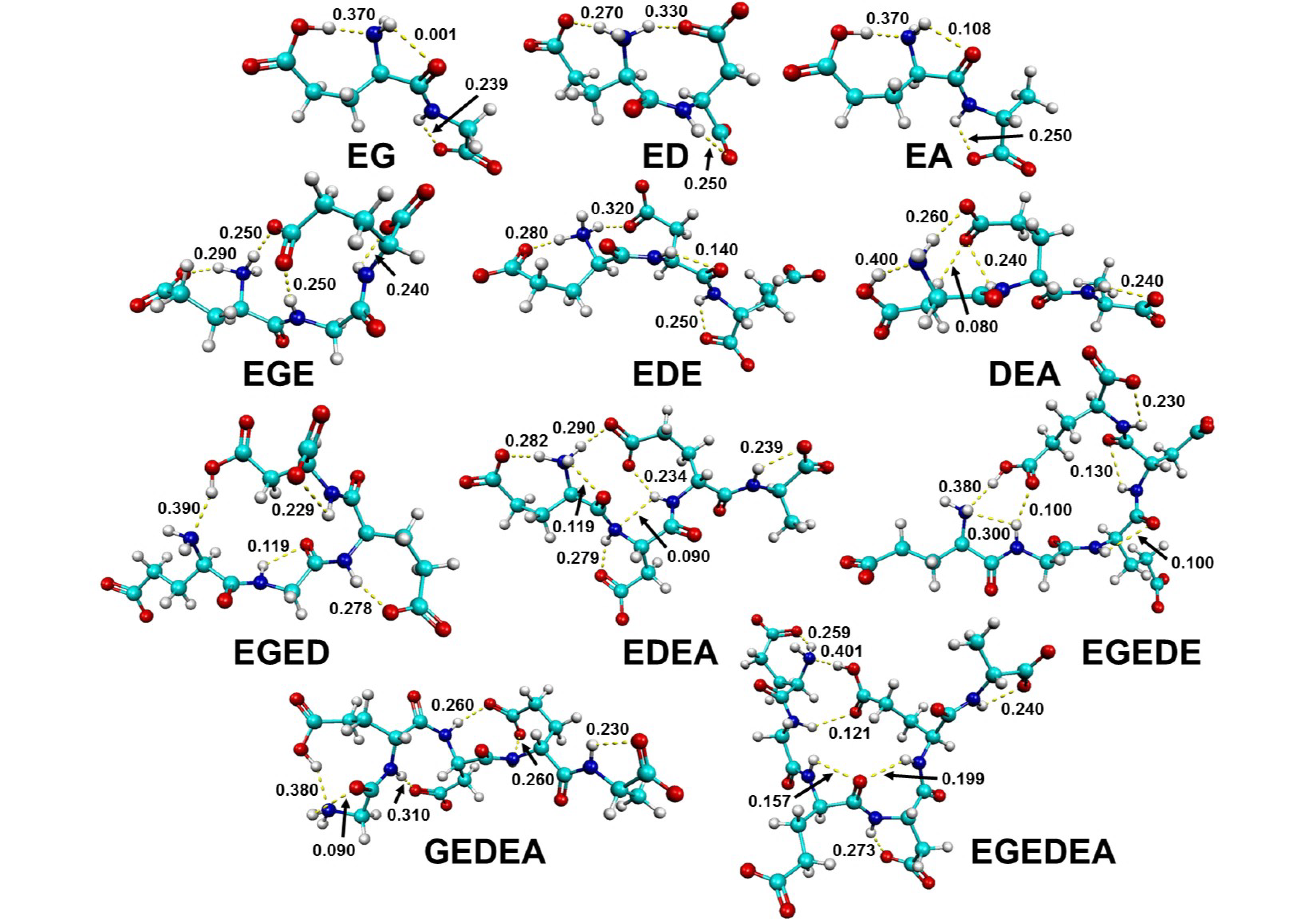



| Structure | ΔE | Ni | HBs | αNH3+ H Bond, Δq, e− | αCOO− H Bond, Δq, e− | qT, e− | Amide I ν(C=O) Band, cm−1 | αCOO− Terminal ν(C=O) Band, cm−1 |
|---|---|---|---|---|---|---|---|---|
| EG-A | 0.00 | 9.74 × 10−1 | 2 | 0.360 | 0.234 | 5.94 × 10−1 | 1655 | 1645 |
| EG-B | 2.39 | 1.73 × 10−2 | 2 | 0.006 | 0.003 | 9.70 × 10−3 | −4 | 0 |
| EG-C | 3.03 | 5.94 × 10−3 | 3 | 0.002 | 0.002 | 5.28 × 10−3 | +29 | −6 |
| EG-D | 3.65 | 2.06 × 10−3 | 3 | 0.001 | 0.001 | 1.85 × 10−3 | +26 | −13 |
| EG-E | 4.04 | 1.07 × 10−3 | 2 | 0.000 | 0.000 | 6.71 × 10−4 | −3 | −1 |
| EG-F | 8.91 | 2.83 × 10−7 | 2 | 0.000 | 0.000 | 1.98 × 10−7 | +43 | +81 |
| ED-A | 0.00 | 9.99 × 10−1 | 3 | 0.270 | 0.250 | 8.49 × 10−1 | 1631 | 1609 |
| ED-B | 4.06 | 1.04 × 10−3 | 3 | 0.000 | 0.000 | 7.60 × 10−4 | +34 | +138 |
| ED-C | 4.95 | 2.33 × 10−4 | 4 | 0.000 | 0.000 | 1.72 × 10−4 | +38 | +146 |
| ED-D | 7.03 | 6.94 × 10−6 | 3 | 0.000 | 0.000 | 5.83 × 10−6 | +21 | −1 |
| ED-E | 9.45 | 1.16 × 10−7 | 3 | 0.000 | 0.000 | 1.03 × 10−7 | +37 | +4 |
| ED-F | 9.69 | 7.81 × 10−8 | 3 | 0.000 | 0.000 | 6.64 × 10−8 | +39 | +3 |
| EA-A | 0.00 | 9.71 × 10−1 | 3 | 0.359 | 0.243 | 7.09 × 10−1 | 1651 | 1642 |
| EA-B | 2.13 | 2.64 × 10−2 | 2 | 0.010 | 0.007 | 1.63 × 10−2 | −2 | 0 |
| EA-C | 3.49 | 2.66 × 10−3 | 3 | 0.001 | 0.001 | 2.34 × 10−3 | +28 | −14 |
| EA-D | 7.08 | 6.24 × 10−6 | 3 | 0.000 | 0.000 | 3.68 × 10−6 | +2 | +116 |
| EA-E | 12.27 | 9.63 × 10−10 | 3 | 0.000 | 0.000 | 8.09 × 10−10 | +29 | +125 |
| EA-F | 24.01 | 2.35 × 10−18 | 1 | 0.000 | --- | 9.15 × 10−19 | +61 | +92 |
| EGE-A | 0.00 | 1.00 | 4 | 0.290 | 0.240 | 1.03 | 1617 | 1641 |
| EGE-B | 10.13 | 3.70 × 10−8 | 2 | 0.000 | 0.000 | 2.30 × 10−8 | +9 | +44 |
| EGE-C | 16.59 | 6.73 × 10−13 | 4 | 0.000 | 0.000 | 6.19 × 10−13 | +47 | −13 |
| EGE-D | 17.14 | 2.67 × 10−13 | 4 | 0.000 | 0.000 | 2.43 × 10−13 | +38 | −15 |
| EGE-E | 19.73 | 3.33 × 10−15 | 4 | 0.000 | 0.000 | 2.99 × 10−15 | +50 | −18 |
| EGE-F | 24.09 | 2.10 × 10−18 | 4 | 0.000 | 0.000 | 2.23 × 10−18 | +85 | −11 |
| EDE-A | 0.00 | 9.97 × 10−1 | 4 | 0.279 | 0.249 | 9.87 × 10−1 | 1634 | 1609 |
| EDE-B | 3.64 | 2.14 × 10−3 | 4 | 0.001 | 0.001 | 2.25 × 10−3 | −9 | +4 |
| EDE-C | 4.46 | 5.31 × 10−4 | 5 | 0.000 | 0.000 | 6.68 × 10−4 | +4 | −2 |
| EDE-D | 5.03 | 2.02 × 10−4 | 5 | 0.000 | 0.000 | 2.36 × 10−4 | +3 | −2 |
| EDE-E | 7.89 | 1.62 × 10−6 | 3 | 0.000 | 0.000 | 1.47 × 10−6 | +24 | −2 |
| EDE-F | 12.00 | 1.57 × 10−9 | 3 | 0.000 | 0.000 | 1.45 × 10−9 | +1 | −2 |
| DEA-A | 0.00 | 9.99 × 10−1 | 5 | 0.399 | 0.240 | 1.22 | 1635 | 1618 |
| DEA-B | 4.16 | 8.79 × 10−4 | 3 | 0.000 | 0.000 | 8.27 × 10−4 | +8 | +4 |
| DEA-C | 4.54 | 4.69 × 10−4 | 4 | 0.000 | 0.000 | 4.60 × 10−4 | −2 | +10 |
| DEA-D | 6.60 | 1.43 × 10−5 | 4 | 0.000 | 0.000 | 1.54 × 10−5 | −21 | +6 |
| DEA-E | 7.89 | 1.62 × 10−6 | 3 | 0.000 | 0.000 | 1.15 × 10−6 | +28 | +8 |
| DEA-F | 13.89 | 6.39 × 10−11 | 3 | 0.000 | 0.000 | 5.75 × 10−11 | −14 | −1 |
| EGED-A | 0.00 | 9.88 × 10−1 | 4 | 0.385 | 0.227 | 1.01 | 1676 | 1620 |
| EGED-B | 3.02 | 6.04 × 10−3 | 3 | 0.002 | 0.001 | 3.51 × 10−3 | −1 | +30 |
| EGED-C | 3.44 | 2.93 × 10−3 | 4 | 0.001 | 0.001 | 2.49 v 10−3 | −16 | +11 |
| EGED-D | 3.71 | 1.87 × 10−3 | 4 | 0.001 | 0.000 | 1.48 × 10−3 | +4 | +13 |
| EGED-E | 4.29 | 7.09 × 10−4 | 4 | 0.000 | 0.000 | 6.31 × 10−4 | −7 | +13 |
| EGED-F | 4.68 | 3.66 × 10−4 | 2 | 0.000 | --- | 2.20 × 10−4 | +1 | +107 |
| EDEA-A | 0.00 | 9.29 × 10−1 | 7 | 0.260 | 0.223 | 1.34 | 1639 | 1604 |
| EDEA-B | 1.53 | 7.05 × 10−2 | 6 | 0.022 | 0.016 | 1.05 × 10−1 | +13 | +2 |
| EDEA-C | 7.87 | 1.55 × 10−6 | 5 | 0.000 | 0.000 | 1.91 × 10−6 | +3 | +8 |
| EDEA-D | 16.11 | 1.41 × 10−12 | 5 | 0.000 | 0.000 | 1.79 × 10−12 | +32 | −3 |
| EDEA-E | 34.60 | 3.84 × 10−26 | 6 | 0.000 | 0.000 | 5.42 × 10−26 | +69 | +11 |
| EDEA-F | 47.14 | 2.40 × 10−35 | 5 | 0.000 | --- | 2.96 × 10−35 | +100 | +5 |
| EGEDE-A | 0.00 | 1.00 | 6 | 0.380 | 0.230 | 1.24 | 1650 | 1614 |
| EGEDE-B | 4.52 | 4.83 × 10−4 | 5 | 0.000 | 0.000 | 4.49 × 10−4 | +9 | +11 |
| EGEDE-C | 8.65 | 4.51 × 10−7 | 5 | 0.000 | 0.000 | 4.29 × 10−7 | +32 | −5 |
| EGEDE-D | 11.67 | 2.72 × 10−9 | 6 | 0.000 | 0.000 | 3.40 × 10−9 | +2 | +83 |
| EGEDE-E | 11.70 | 2.62 × 10−9 | 6 | 0.000 | 0.000 | 3.77 × 10−9 | +8 | −21 |
| EGEDE-F | 32.43 | 1.59 × 10−24 | 6 | 0.000 | 0.000 | 2.28 × 10−24 | +48 | −9 |
| GEDEA-A | 0.00 | 1.00 | 6 | 0.380 | 0.230 | 1.53 | 1654 | 1607 |
| GEDEA-B | 5.92 | 4.55 × 10−5 | 4 | 0.000 | 0.000 | 5.60 × 10−5 | +7 | +3 |
| GEDEA-C | 11.41 | 4.23 × 10−9 | 5 | 0.000 | 0.000 | 4.40 × 10−9 | +19 | +11 |
| GEDEA-D | 11.47 | 3.86 × 10−9 | 6 | 0.000 | 0.000 | 4.86 × 10−9 | +18 | −2 |
| GEDEA-E | 21.81 | 9.90 × 10−17 | 4 | 0.000 | --- | 9.11 × 10−17 | +15 | +106 |
| GEDEA-F | 27.11 | 1.28 × 10−20 | 6 | 0.000 | 0.000 | 1.42 × 10−20 | +52 | +29 |
| EGEDEA-A | 0.00 | 9.20 × 10−1 | 7 | 0.368 | 0.221 | 1.51 | 1658 | 1610 |
| EGEDEA-B | 1.44 | 8.02 × 10−2 | 7 | 0.033 | 0.019 | 1.41 × 10−1 | −1 | +1 |
| EGEDEA-C | 11.19 | 5.70 × 10−9 | 6 | 0.000 | 0.000 | 8.84 × 10−9 | +16 | −6 |
| EGEDEA-D | 11.35 | 4.34 × 10−9 | 6 | 0.000 | 0.000 | 6.25 × 10−9 | +7 | −8 |
| EGEDEA-E | 11.73 | 2.29 × 10−9 | 6 | 0.000 | 0.000 | 3.55 × 10−9 | −5 | −6 |
| EGEDEA-F | 17.75 | 8.72 × 10−14 | 6 | 0.000 | 0.000 | 1.19 × 10−13 | +7 | −4 |
| Structure | qBT | Boltzmann Sum of the Amide I ν(C=O) Band, cm−1 | Experimental Amide I ν(C=O) Band, cm−1 [113] | Boltzmann Sum of the αCOO− Terminal ν(C=O) Band, cm−1 | Experimental αCOO− Terminal ν(C=O) Band, cm−1 [113] |
|---|---|---|---|---|---|
| EG | 0.611 | 1656 | 1671 | 1644 | 1648 |
| ED | 0.850 | 1631 | 1632 | 1609 | 1608 |
| EA | 0.727 | 1651 | 1651 | 1642 | 1628 |
| EGE | 1.030 | 1617 | --- | 1641 | --- |
| EDE | 0.990 | 1634 | --- | 1609 | --- |
| DEA | 1.220 | 1635 | --- | 1618 | --- |
| EGED | 1.016 | 1676 | 1686 | 1620 | 1622 |
| EDEA | 1.444 | 1640 | 1641 | 1605 | 1602 |
| EGEDE | 1.240 | 1650 | --- | 1614 | --- |
| GEDEA | 1.530 | 1654 | --- | 1607 | --- |
| EGEDEA | 1.650 | 1658 | 1658 | 1610 | 1611 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, A.E.; Hammer, N.I.; Fortenberry, R.C.; Reinemann, D.N. Tracking the Amide I and ?COO? Terminal ?(C=O) Raman Bands in a Family of l-Glutamic Acid-Containing Peptide Fragments: A Raman and DFT Study. Molecules 2021, 26, 4790. https://doi.org/10.3390/molecules26164790
Williams AE, Hammer NI, Fortenberry RC, Reinemann DN. Tracking the Amide I and ?COO? Terminal ?(C=O) Raman Bands in a Family of l-Glutamic Acid-Containing Peptide Fragments: A Raman and DFT Study. Molecules. 2021; 26(16):4790. https://doi.org/10.3390/molecules26164790
Chicago/Turabian StyleWilliams, Ashley E., Nathan I. Hammer, Ryan C. Fortenberry, and Dana N. Reinemann. 2021. "Tracking the Amide I and ?COO? Terminal ?(C=O) Raman Bands in a Family of l-Glutamic Acid-Containing Peptide Fragments: A Raman and DFT Study" Molecules 26, no. 16: 4790. https://doi.org/10.3390/molecules26164790
APA StyleWilliams, A. E., Hammer, N. I., Fortenberry, R. C., & Reinemann, D. N. (2021). Tracking the Amide I and ?COO? Terminal ?(C=O) Raman Bands in a Family of l-Glutamic Acid-Containing Peptide Fragments: A Raman and DFT Study. Molecules, 26(16), 4790. https://doi.org/10.3390/molecules26164790







