Electro-Persulfate Processes for the Treatment of Complex Wastewater Matrices: Present and Future
Abstract
:1. Introduction
2. Fundamentals of the Electro-Persulfate Processes
3. Treatment of Complex Wastewater Matrices by Electro-Persulfate Processes
3.1. Single Electrolytic Activation of Persulfate
3.2. Combined Electrolytic and Metal Activation of Persulfate
3.2.1. Persulfate Electro-Activation through Sacrificial Anodes
3.2.2. Persulfate Electro-Activation with Metal Ions Addition
3.2.3. Persulfate Electro-Activation Using Metal-Based Catalysts
3.3. Electro-Persulfate Processes Involving Co-Activation by Irradiation or Heat
4. Major Challenges and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrivastava, P.; Naoghare, P.K.; Gandhi, D.; Devi, S.S.; Krishnamurthi, K.; Bafana, A.; Kashyap, S.M.; Chakrabarti, T. Application of cell-based assays for toxicity characterization of complex wastewater matrices: Possible applications in wastewater recycle and reuse. Ecotoxicol. Environ. Saf. 2017, 142, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Zhi, D.; Lin, Y.; Jiang, L.; Zhou, Y.; Huang, A.; Yang, J.; Luo, L. Remediation of persistent organic pollutants in aqueous systems by electrochemical activation of persulfates: A review. J. Environ. Manag. 2020, 260, 110125. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Q.; Cui, Y.-H.; Liu, Y.-Y.; Liu, Z.-Q.; Li, X.-Y. Electrochemical generation of persulfate and its performance on 4-bromophenol treatment. Sep. Purif. Technol. 2018, 207, 461–469. [Google Scholar] [CrossRef]
- Karim, A.V.; Jiao, Y.; Zhou, M.; Nidheesh, P.V. Iron-based persulfate activation process for environmental decontamination in water and soil. Chemosphere 2021, 265, 129057. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; El-Din, M.G. Insight into in-situ radical and non-radical oxidative degradation of organic compounds in complex real matrix during electrooxidation with boron doped diamond electrode: A case study of oil sands process water treatment. Appl. Catal. B 2020, 279, 119366. [Google Scholar] [CrossRef]
- Matzek, L.W.; Tipton, M.J.; Farmer, A.T.; Steen, A.D.; Carter, K.E. Understanding electrochemically activated persulfate and its application to ciprofloxacin abatement. Environ. Sci. Technol. 2018, 52, 5875–5883. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Guvenc, S.; Varank, G.; Demir, A.; Guvenk, E. Energy consumption and efficiency improvement of electro-activated persulfate processes: Optimization by CCD for TOC Removal from leachate concentrate. Sigma J. Eng. Nat. Sci. 2020, 38, 1791–1810. [Google Scholar]
- Jaafarzadeh, N.; Ghanbari, F.; Alvandi, M. Integration of coagulation and electro-activated HSO5−to treat pulp and paper wastewater. Sustain. Environ. Res. 2017, 27, 223–229. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ghanbari, F. Optimizing COD removal from greywater by photoelectro-persulfate process using Box-Behnken Design: Assessment of effluent quality and electrical energy consumption. Environ. Sci. Pollut. Res. 2016, 2, 19350–19361. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liao, P.; Alshawabkeh, A.N. Electrolytic manipulation of persulfate reactivity by iron electrodes for trichloroethylene degradation in groundwater. Environ. Sci. Technol. 2014, 48, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Varank, G.; Guvenc, S.; Demir, A.; Kavan, N.; Donmez, N.; Onen, Z. Modeling and optimizing electro-persulfate processes using Fe and Al electrodes for paper industry wastewater treatment. Water Sci. Tech. 2020, 81, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, M.; Wang, D.; Yan, M.; Liu, Z. Different activation methods in sulfate radical-based oxidation for organic pollutants degradation: Catalytic mechanism and toxicity assessment of degradation intermediates. Sci. Total Environ. 2021, 772, 145522. [Google Scholar] [CrossRef]
- Liu, H.; Bruton, T.A.; Doyle, F.M.; Sedlak, D.L. In situ chemical oxidation of contaminated groundwater by persulfate: Decomposition by Fe(III)- and Mn(IV)-containing oxides and aquifer materials. Environ. Sci. Technol. 2014, 48, 10330–10336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, W.D. Activation of Peroxymonosulfate by Heterogeneous Catalysts for the Removal of Organic Pollutants in Water. Ph.D. Thesis, Nanyang Technological University, Singapore, 2016. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Kang, S.-G.; Chiu, P.C. Degradation of 2,4-dinitrotoluene by persulfate activated with zero-valent iron. Sci. Total Environ. 2010, 408, 3464–3468. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.E.; Zazo, J.A.; Pliego, G.; Casas, J.A. Landfill leachate treatment by sequential combination of activated persulfate and Fenton oxidation. Waste Manag. 2018, 81, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Darsinou, B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Sono-activated persulfate oxidation of bisphenol A: Kinetics, pathways and the controversial role of temperature. Chem. Eng. J. 2015, 280, 623–633. [Google Scholar] [CrossRef]
- Yousefi, N.; Pourfadakari, S.; Esmaeili, S.; Babaei, A.A. Mineralization of high saline petrochemical wastewater using Sonoelectro-activated persulfate: Degradation mechanisms and reaction kinetics. Microchem. J. 2019, 147, 1075–1082. [Google Scholar] [CrossRef]
- Xue, W.; Cui, Y.; Liu, Z.; Yang, S.; Li, J.; Guo, X. Treatment of landfill leachate nanofiltration concentrate after ultrafiltration by electrochemically assisted heat activation of peroxydisulfate. Sep. Purif. Technol. 2020, 231, 115928. [Google Scholar] [CrossRef]
- Chanikya, P.; Nidheesh, P.V.; Babu, D.S.; Gopinath, A.; Kumar, M.S. Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes. Sep. Purif. Technol. 2021, 254, 117570. [Google Scholar] [CrossRef]
- Chen, W.-S.; Jhou, Y.-C.; Huang, C.-P. Mineralization of dinitrotoluenes in industrial wastewater by electro-activated persulfate oxidation. Chem. Eng. J. 2014, 252, 166–172. [Google Scholar] [CrossRef]
- Ghanbari, F.; Martínez-Huitle, C.A. Electrochemical advanced oxidation processes coupled with peroxymonosulfate for the treatment of real washing machine effluent: A comparative study. J. Electroanal. Chem. 2019, 847, 113182. [Google Scholar] [CrossRef]
- Yang, W.; Liu, G.; Chen, Y.; Miao, D.; Wei, Q.; Li, H.; Ma, L.; Zhou, K.; Liu, L.; Yu, Z. Persulfate enhanced electrochemical oxidation of highly toxic cyanide-containing organic wastewater using boron-doped diamond anode. Chemosphere 2020, 252, 126499. [Google Scholar] [CrossRef] [PubMed]
- Onn, S.; Bashir, M.; Sethupathi, S.; Amr, S.; Nguyen, T. Colour and COD removal from mature landfill leachate using electro-persulphate oxidation process. Mater. Today Proc. 2020, 31, 69–74. [Google Scholar] [CrossRef]
- Varank, G.; Guvenc, S.; Dincer, K.; Demir, A. Concentrated leachate treatment by electro-Fenton and electro-persulfate processes using Central Composite Design. Int. J. Environ. Res. 2020, 14, 439–461. [Google Scholar] [CrossRef]
- Varank, G.; Guvenc, S.; Demir, A. Electro-activated peroxymonosulfate and peroxydisulfate oxidation of leachate nanofiltration concentrate: Multiple-response optimization. Int. J. Environ. Sci. Tech. 2020, 17, 2707–2720. [Google Scholar] [CrossRef]
- Guvenc, S.; Varank, G. Box-Behnken Design optimization of electro-Fenton/-persulfate processes following the acidification for TSS removal from biodiesel wastewater. Sigma J. Eng. Nat. Sci. 2020, 38, 1767–1780. [Google Scholar]
- Guvenc, S.; Varank, G.; Cebi, A.; Ozkaya, B. Electro-activated persulfate oxidation of biodiesel wastewater following acidification phase: Optimization of process parameters using Box–Behnken Design. Water Air Soil Pollut. 2021, 232, 10. [Google Scholar] [CrossRef]
- Bashir, M.; Wei, C.; Aun, N.; Amr, S. Electro persulphate oxidation for polishing of biologically treated palm oil mill effluent (POME). J. Environ. Manag. 2017, 193, 458–469. [Google Scholar] [CrossRef]
- Durna, E.; Genç, N. Application of a multiple criteria analysis for the selection of appropriate radical based processes in treatment of car wash wastewater. Environ. Eng. Res. 2021, 26, 200115. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, C.; Guo, Y.; Shan, N.; Meng, C.; Sun, L. Removal of COD from landfill leachate by an electro/Fe2+/peroxydisulfate process. Chem. Eng. J. 2014, 250, 76–82. [Google Scholar] [CrossRef]
- Cui, Y.H.; Xue, W.J.; Yang, S.Q.; Tu, J.L.; Guo, X.L.; Liu, Z.Q. Electrochemical/peroxydisulfate/Fe3+ treatment of landfill leachate nanofiltration concentrate after ultrafiltration. Chem. Eng. J. 2018, 353, 208–217. [Google Scholar] [CrossRef]
- Popat, A.; Nidheesh, P.V.; Singh, T.A.; Kumar, M.S. Mixed industrial wastewater treatment by combined electrochemical advanced oxidation and biological processes. Chemosphere 2019, 237. [Google Scholar] [CrossRef]
- Görmez, F.; Görmez, Ö.; Yabalak, E.; Gözmen, B. Application of the central composite design to mineralization of olive mill wastewater by the electro/FeII/persulfate oxidation method. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Cui, J.; Li, X.; Zhang, H.; Pei, Y. Electrochemical treatment of organic pollutants in landfill leachate using a three-dimensional electrode system. Chemosphere 2020, 243, 125438. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yang, Q.; Wang, D.; Wu, Y.; Zhu, X.; Wei, J.; Liu, Y.; Hou, L.; Chen, C. Pretreatment of landfill leachate in near-neutral pH condition by persulfate activated Fe-C micro-electrolysis system. Chemosphere 2019, 216, 749–756. [Google Scholar] [CrossRef]
- Ahmadi, M.; Haghighifard, N.J.; Soltani, R.D.C.; Tobeishi, M.; Jorfi, S. Treatment of a saline petrochemical wastewater containing recalcitrant organics using electro-Fenton process: Persulfate and ultrasonic intensification. Desalin. Water Treat. 2019, 169, 241–250. [Google Scholar] [CrossRef]
- Johin, J.; Nidheesh, P.V.; Sivasankar, T. Sono-electro-chemical treatment of Reactive Black 5 dye and real textile effluent using MnSO4/Na2S2O8 electrolytes. Arab. J. Sci. Eng. 2019, 44, 9987–9996. [Google Scholar] [CrossRef]
- Jorfi, S.; Ghaedrahmat, Z. Evaluating the efficiency of advanced oxidation processes for textile wastewater treatment: Electro-kinetic, sonochemical and persulfate. Environ. Prog. Sustain. Energy 2020, 40, 1–7. [Google Scholar] [CrossRef]
- Trellu, C.; Chaplin, B.P.; Coetsier, C.; Esmilaire, R.; Cerneaux, S.; Causserand, C.; Cretin, M. Electro-oxidation of organic pollutants by reactive electrochemical membranes. Chemosphere 2018, 208, 159–175. [Google Scholar] [CrossRef] [Green Version]
- Chaplin, B.P. The prospect of electrochemical technologies advancing worldwide water treatment. Acc. Chem. Res. 2019, 52, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Chuah, C.Y.; Lee, J.; Bae, T.-H. Graphene-based membranes for H2 separation: Recent progress and future perspective. Membranes 2020, 10, 336. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Toxicity changes of wastewater during various advanced oxidation processes treatment: An overview. J. Clean Prod. 2021, 315. [Google Scholar] [CrossRef]
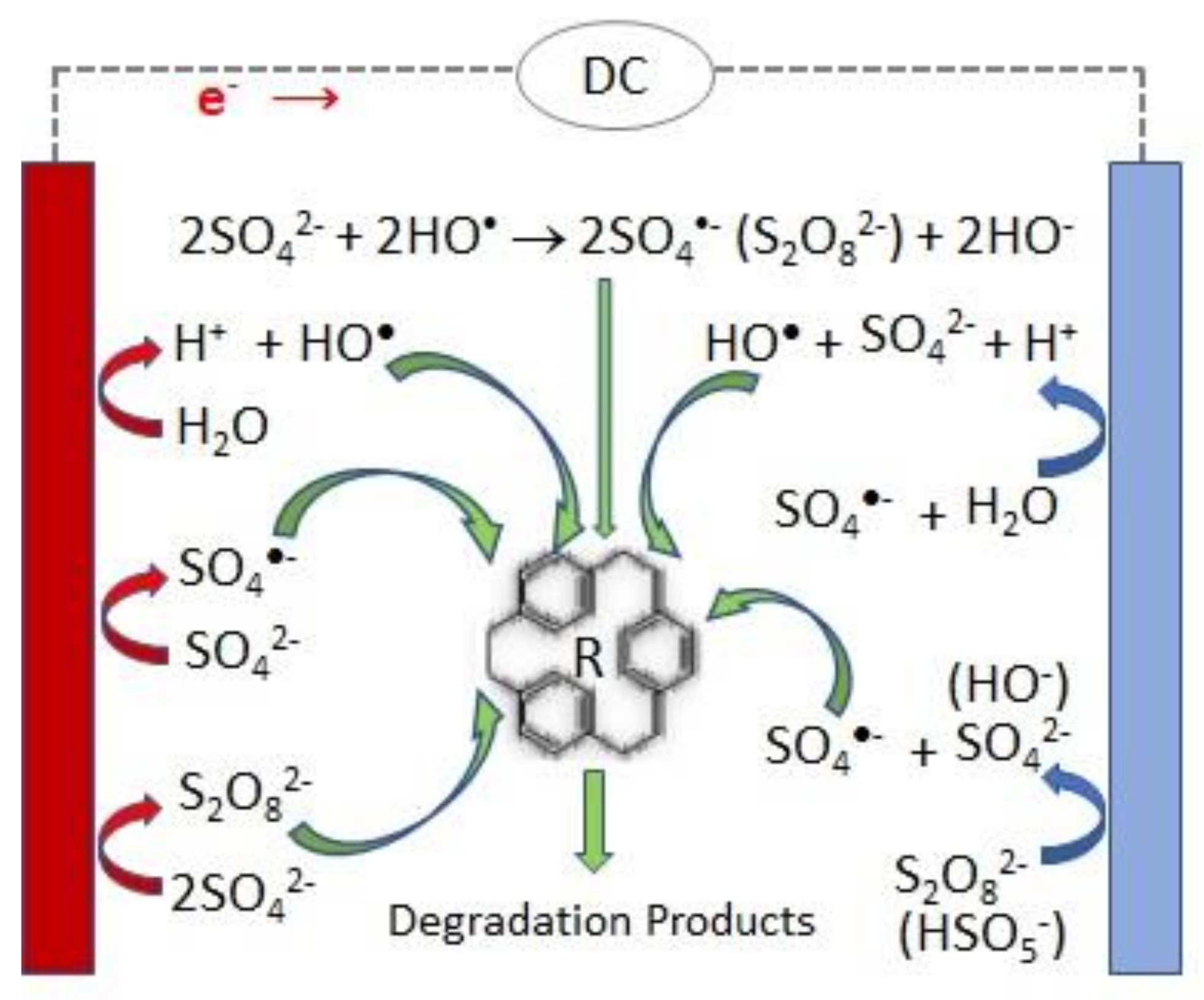
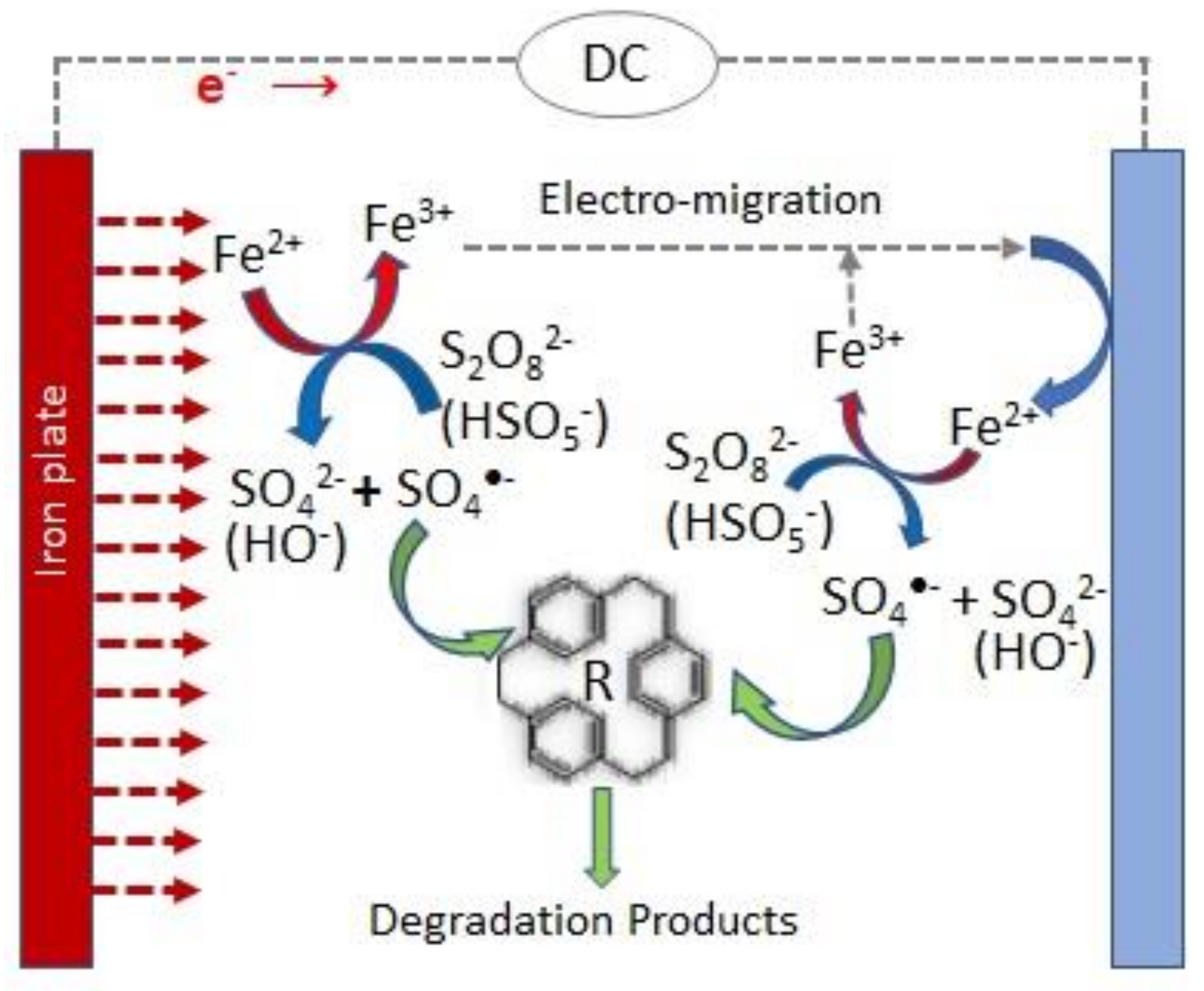
| Type of Effluent | Anode/ Cathode | PS Source | [PS]added | Applied Current | T/°C | Time/h | Treated Volume/L | pH0 | [Organic Load]0/mg L−1 | Organic Load Removal/% | Energy Consumption/kWh m−3 Order−1 | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD | TOC | COD | TOC | |||||||||||
| Dyeing wastewater | (Pt-Ti)/ Fe | PDS | 0 g L−1 | 3 V | NS | 1 | 1 | 6 | 237 | NS | 56 | NS | NS | [24] |
| 0.5 g L−1 | 76 | |||||||||||||
| 1 g L−1 | 72 | |||||||||||||
| Industrial effluent containing DNTs | Pt/ Pt | PDS | 1 wt% | 3 V | 30 | 8 | 0.45 | 0.5 | NS | 300 | NS | 20 | NS | [25] |
| 4 V | 35 | |||||||||||||
| 5 V | 48 | |||||||||||||
| 6 V | 70 | |||||||||||||
| 35 | 73 | |||||||||||||
| 40 | 75 | |||||||||||||
| 45 | 79 | |||||||||||||
| 0.7 wt% | 70 | |||||||||||||
| 1.3 wt% | 84 | |||||||||||||
| 1.7 wt% | 95 | |||||||||||||
| 1 wt% | 1 | 74 | ||||||||||||
| 1 wt% | 2 | 70 | ||||||||||||
| 1 wt% | 3 | 66 | ||||||||||||
| Washing machine effluent | Pt/ Graphite felt | PMS | 2 mM | 30 mA cm−2 | NS | 3 | 0.2 | 6.7 | NS | 202 | NS | 32.7 | NS | [26] |
| Oil sands process water | BDD/Stainless steel | NA | NA | 5 mA cm−2 | 23 | 6 | 0.45 | 8.6 | NS | 73.8 | NS | 95 1 | NS | [8] |
| 10 mA cm−2 | 100 1 | |||||||||||||
| 20 mA cm−2 | 100 1 | |||||||||||||
| 30 mA cm−2 | 100 1 | |||||||||||||
| Cyanide-containing wastewater | BDD/ Stainless steel | PDS | 0.1 M | 10 mA cm−2 | 20 | 24 | 0.5 | 5.6 | 11290 | 4456 | 88.8 | 82.8 | 60.6 | [27] |
| 20 mA cm−2 | 99.5 | 87 (16 h) | 62.5 | |||||||||||
| 30 mA cm−2 | 99.8 | 98.2 (16 h) | 84.8 | |||||||||||
| 10 mA cm−2 | 2.1 | 90.1 | 86.3 | 56.7 | ||||||||||
| 11.8 | 73 | 61 | 122.5 | |||||||||||
| 40 | 5.6 | 95.8 | 87.8 | 41.6 | ||||||||||
| 50 | 97 | 90 | 30.1 | |||||||||||
| Type of Effluent | Anode/Cathode | PS Source | [PS]added | Applied Current | Electrolysis Time/Min | Treated Volume/L | pH0 | [Organic Load]0/mg L−1 | Organic Load Removal/% | Energy Consumption | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD | TOC | COD | TOC | ||||||||||
| Landfill leachate | Al/Al | PDS | 0.5 g L−1 | 10 mA cm−2 | 80 | NS | 4 | 492 | NS | 31.52 | NS | NS | [28] |
| 60 mA cm−2 | 36.39 | NS | NS | ||||||||||
| 3 g L−1 | 10 mA cm−2 | 17.27 | NS | NS | |||||||||
| 60 mA cm−2 | 28.01 | NS | NS | ||||||||||
| 0.88 g L−1 | 44.66 mA cm−2 | 68.3 | 45.70 | NS | NS | ||||||||
| Landfill leachate concentrate | Fe/Fe | PDS | PDS/COD ratio 1.72 | 1.26 A | 34.8 | 0.5 | 5 | 5250 | NS | 72.6 | NS | NS | [29] |
| Landfill leachate concentrate | Fe/Fe | PMS | PS/COD ratio 2.5 | 1.8 A | 35.9 | 1 | 6.4 | 6200 | NS | 84.2 | NS | NS | [30] |
| PDS | PS/COD ratio 1.9 | 2.1 A | 32.3 | 5.1 | 79.6 | NS | NS | ||||||
| Landfill leachate concentrate | Fe/Fe | PMS | PS/COD ratio 2 | 1 A | 15 | NS | 5.64 | 5250 | NS | NS | 56.91 | 1.87 kWh m−3 | [11] |
| PDS | 33.8 | 4.55 | NS | 58.43 | 5.81 kWh m−3 | ||||||||
| Biodiesel wastewater | Fe/Fe | PDS | PDS/COD ratio 1 | 1 A | 15.6 | 0.5 | 2 | 95000 | 1300 (TSS) | NS | 90.6 (TSS) | NS | [31] |
| Biodiesel wastewater | Fe/Fe | PDS | PDS/COD ratio 4.4 | 4 A | 15 | 0.5 | 2 | 95488 | NS | 49.0 | NS | NS | [32] |
| Pulp and paper wastewater | Fe/Fe | PMS | 0 mM | 0.5 mA cm−2 | 60 | 0.5 | 4.9 | 585 1 | NS | 6 | NS | NS | [12] |
| 2 mM | 0.5 mA cm−2 | 18 | NS | NS | |||||||||
| 4 mM | 0.5 mA cm−2 | 32 | NS | NS | |||||||||
| 6 mM | 0 mA cm−2 | 20 | NS | NS | |||||||||
| 0.25 mA cm−2 | 32 | NS | NS | ||||||||||
| 0.5 mA cm−2 | 41 | NS | NS | ||||||||||
| 0.75 mA cm−2 | 53 | NS | NS | ||||||||||
| 1 mA cm−2 | 51 | NS | NS | ||||||||||
| 8 mM | 0.5 mA cm−2 | 40 | NS | NS | |||||||||
| Paper industry wastewater | Fe/Fe | PDS | PDS/COD ratio 1.25 | 4.1 A | 5 | 0.5 | 6.0 | 11700 | NS | 63.5 | NS | NS | [15] |
| Al/Al | PDS/COD ratio 0.5 | 4.25 A | 25 | 7.25 | 72.8 | NS | NS | ||||||
| Greywater | Fe/Graphite | PDS | 0 mM | 2 mA cm−2 | 60 | 0.4 | 6.9 | 530 | NS | 50 1 | NS | 1.81 kWh m−3 | [13] |
| 8.8 mM | 0 mA cm−2 | 10 1 | NS | NS | |||||||||
| 2 mA cm−2 | 68 1 | NS | 1.49 kWh m−3 | ||||||||||
| Palm oil mill effluent | Al/Al | PDS | 1 g L−1 1 | 20 mA cm−2 | 60 | 0.5 | 3 | 2420 | NS | 76.35 | NS | NS | [33] |
| 5 | 75.79 | NS | NS | ||||||||||
| 50 mA cm−2 | 3 | 79.58 | NS | NS | |||||||||
| 5 | 75.86 | NS | NS | ||||||||||
| 8 g L−1 1 | 20 mA cm−2 | 3 | 72.66 | NS | NS | ||||||||
| 5 | 68.95 | NS | NS | ||||||||||
| 50 mA cm−2 | 3 | 74.07 | NS | NS | |||||||||
| 5 | 73.24 | NS | NS | ||||||||||
| 1.784 g L−1 1 | 45 mA cm−2 | 45 | 4 | 77.7 | NS | 12.76 kWh m−3 | |||||||
| Type of Effluent | Anode/Cathode | [PDS]added | Iron Source | [Fen+] | Applied Current | Electrolysis Time/h | Treated Volume/L | pH0 | COD0/mg L−1 | COD Removal/% | Energy Consumption/kWh kg−1 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Landfill leachate | (Ti/IrO2-RuO2-TiO2)/ Ti | 0 mM | FeSO4·7H2O | 0 mM | 13.89 mA cm−2 | 4 | 1 | 3 | 1900 | 28.1 | NS | [35] |
| 15.6 mM | 15.6 mM | 55 1 | NS | |||||||||
| 31.3 mM | 62 1 | NS | ||||||||||
| 62.5 mM | 67.7 | NS | ||||||||||
| 6 | 62 1 | NS | ||||||||||
| 9 | 47.0 | NS | ||||||||||
| 0 mA cm−2 | 3 | 44.8 | NS | |||||||||
| 6.94 mA cm−2 | 55 1 | NS | ||||||||||
| 27.78 mA cm−2 | 62 1 | NS | ||||||||||
| 7.81 mM | 13.89 mA cm−2 | 55 1 | NS | |||||||||
| 31.2 mM | 60 1 | NS | ||||||||||
| 15.6 mM | 1 | 62.2 | 5.7 | |||||||||
| Landfill leachate concentrate | (Ti/IrO2)/Ti | 0 mM | Fe2(SO4)3 | 0 mM | 80 mA | 1 | 0.15 | 7.6 | 1281 | 10.9 | NS | [36] |
| 0 mM | 15 mM | 0 mA | 26 | NS | ||||||||
| 18.75 mM | 80 mA | 41 1 | NS | |||||||||
| 37.5 mM | 0 mM | 0 mA | 11.9 | NS | ||||||||
| 3.75 mM | 80 mA | 22 1 | NS | |||||||||
| 5 mM | 30 1 | NS | ||||||||||
| 7.5 mM | 37 1 | NS | ||||||||||
| 15 mM | 0 mA | 38 | NS | |||||||||
| 40 mA | 39 1 | NS | ||||||||||
| 80 mA | 55 1 | 4.42 | ||||||||||
| 120 mA | 50 1 | NS | ||||||||||
| 160 mA | 47 1 | NS | ||||||||||
| 56.25 mM | 80 mA | 48 1 | NS | |||||||||
| 75 mM | 80 mA | 45 1 | NS | |||||||||
| Mixed industrial wastewater | (Ti/Pt)/Graphite felt | 0 mg L−1 | FeSO4·7H2O | 20 mg L−1 | 10 V | 1 | 1 | 3 | 1152 | 31 1 | NS | [37] |
| 100 mg L−1 | 31 | NS | ||||||||||
| 200 mg L−1 | 10 mg L−1 | 60 | NS | |||||||||
| 20 mg L−1 | 60 1 | NS | ||||||||||
| 300 mg L−1 | 46 1 | NS | ||||||||||
| Olive mill wastewater | Pt/Graphite | 200 mM | FeSO4·7H2O | 20 mM | 200 mA | 6 | 0.2 | 5 | 6265 | 63.4 | 5.63 | [38] |
| 250 mM | 25 mM | 71.2 | 4.50 | |||||||||
| Dyeing wastewater | (Pt/Ti)/ Fe | 500 mg L−1 | FeSO4·7H2O | 0 mg L−1 | 3 V | 1 | 1 | 6 | 1024 | 76 1 | NS | [24] |
| 100 mg L−1 | 80 | 0.11 | ||||||||||
| 3 | 83 1 | NS | ||||||||||
| 8.2 | 76 1 | NS | ||||||||||
| 12 | 78 1 | NS | ||||||||||
| 5 V | 6 | 82 1 | 1.28 | |||||||||
| 9 V | 89.4 | 8.45 | ||||||||||
| 250 mg L−1 | 3 V | 70 1 | NS |
| Type of Effluent | Anode/Cathode | PS Source | [PS]added/mM | Catalyst | [Catalyst]/g L−1 | Applied Current | Electrolysis Time/h | Treated Volume/L | pH0 | COD0/ mg L−1 | COD Removal/% | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Landfill leachate | NA | PDS | 85 | Fe-C | 40 | NA | 2 | 0.1 | 7 | 9514 | 62.91 | [40] |
| Landfill leachate | Iron filings/Hydrothermal carbonization biochar | PDS | 0 | Fe/C granules | 0 | 5 V | 2 | 0.06 | 7.81 | 1041.38 | 46.9 | [39] |
| 16.(6) 1 | 50.4 | |||||||||||
| 7 | 47.4 | |||||||||||
| 14 | 55 1 | |||||||||||
| 28 | 0 | 53.7 | ||||||||||
| 8.(3) 1 | 58.7 | |||||||||||
| 12.5 1 | 66.2 | |||||||||||
| 16.(6) 1 | 0 V | 4 | ||||||||||
| 1 V | 36.9 | |||||||||||
| 3 V | 43.1 | |||||||||||
| 5 V | 72.9 | |||||||||||
| 7.5 V | 73.4 | |||||||||||
| 33.(3) 1 | 5 V | 73.3 | ||||||||||
| 56 | 16.(6) 1 | 75.6 | ||||||||||
| Landfill leachate concentrate | Stainless steel/Stainless steel | PMS | 0 | NrGO-MnFe2O4 | 0 | 20 mA cm−2 | 2 | 0.5 | 5.0 | 1250 | 30.12 | [16] |
| 0 | 1.00 | 0 mA cm−2 | 9.21 | |||||||||
| 0.5 | 20 mA cm−2 | 47.59 | ||||||||||
| 1.0 | 54 1 | |||||||||||
| 1.5 | 65 1 | |||||||||||
| 2.00 | 0 | 0 mA cm−2 | 12.17 | |||||||||
| 0 | 20 mA cm−2 | 42.33 | ||||||||||
| 0.25 | 48 1 | |||||||||||
| 0.50 | 60 1 | |||||||||||
| 0.75 | 66 1 | |||||||||||
| 1.00 | 0 mA cm−2 | 32 1 | ||||||||||
| 5 mA cm−2 | 42 1 | |||||||||||
| 10 mA cm−2 | 48 1 | |||||||||||
| 15 mA cm−2 | 60 1 | |||||||||||
| 20 mA cm−2 | 72.89 | |||||||||||
| 3.0 | 60 1 | |||||||||||
| 4.0 | 65.53 | |||||||||||
| 6.0 | 46 1 | |||||||||||
| 7.0 | 33.01 | |||||||||||
| 10.0 | 41.79 | |||||||||||
| 25 mA cm−2 | 74 1 | |||||||||||
| 30 mA cm−2 | 65 1 | |||||||||||
| 1.25 | 20 mA cm−2 | 5.0 | 68 1 | |||||||||
| 2.5 | 74.39 | |||||||||||
| Washing machine effluent | Pt/ Graphite felt | PMS | 0 | Fe3O4 | 0 | 30 mA cm−2 | 3 | 0.2 | 3 | 480 | 20 | [26] |
| 0.1 | 47 | |||||||||||
| 0.5 | 5 | 50.9 | ||||||||||
| 1 | 5 | 64.3 | ||||||||||
| 2 | 0 | 3 | 32.7 | |||||||||
| 0.025 | 5 | 44 1 | ||||||||||
| 0.05 | 5 | 60 1 | ||||||||||
| 0.1 | 0 mA cm−2 | 3 | 30 | |||||||||
| 10 mA cm−2 | 5 | 38 1 | ||||||||||
| 20 mA cm−2 | 5 | 52 1 | ||||||||||
| 30 mA cm−2 | 3 | 70 1 | ||||||||||
| 5 | 74.4 | |||||||||||
| 7 | 58 1 | |||||||||||
| 9 | 57 1 | |||||||||||
| 40 mA cm−2 | 5 | 75 1 | ||||||||||
| 0.15 | 30 mA cm−2 | 84 1 | ||||||||||
| 0.2 | 84 1 | |||||||||||
| 3 | 0.1 | 85.4 | ||||||||||
| 4 | 80 1 |
| Type of Effluent | Anode/Cathode | PS Source | [PS]added | Iron Source | Irradiation | Applied Current | T/°C | Electrolysis Time/h | Treated Volume/L | pH0 | [COD]0 [TOC]0/mg L−1 | COD TOC Removal/% | Energy Consumption | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline petrochemical wastewater | Fe/Graphite | PDS | 0 mM | Fe anode | US: 300 W | 1.2 V | 21–25 | 2 | 0.3 | 5 | COD0: 850 | COD: 80.2 | NS | [41] |
| 3.5 | COD: 94.1 | 11 kWh m−3 | ||||||||||||
| 0.25 mM | 2 | COD: 85 1 | NS | |||||||||||
| 0.5 mM | COD: 88 1 | NS | ||||||||||||
| 0.75 mM | COD: 91.7 | 4.2 kWh m−3 | ||||||||||||
| 1 mM | COD: 88 1 | NS | ||||||||||||
| Saline petrochemical wastewater | Pt / Pt | PDS | 20 mM | NA | US: 100 W (35 kHz) | 10 V | 20 | 2 | 0.8 | 3 | COD0: 750 | COD: 64.8 | NS | [22] |
| US: 200 W (35 kHz) | COD: 67 1 | |||||||||||||
| US: 300 W (35 kHz) | COD: 73.9 | |||||||||||||
| US: 0 W | COD: 69.0 | |||||||||||||
| US: 300 W (130 kHz) | COD: 82.3 | |||||||||||||
| 60 | COD: 91.2 | |||||||||||||
| Textile effluent | Stainless Steel/Graphite | PDS | 0.42 mM 1 | NA | US: 0 W | 8 V | NS | 1 | 0.4 | 8.1 | TOC0: 723 | TOC: 85 1 | NS | [42] |
| US: 44 W | TOC: 87 1 | |||||||||||||
| 20 V | TOC: 90 | |||||||||||||
| Textile effluent | Fe/Graphite | PDS | 0.5 mM | Fe anode | US: 0 W | 0.5 V | NS | 1.5 | NS | 5 | COD0: 1250 | COD: 78 | NS | [43] |
| US: 100 W | COD: 92 1 | |||||||||||||
| US: 200 W | COD: 94 1 | |||||||||||||
| US: 300 W | COD: 96 | |||||||||||||
| Washing machine effluent | Pt/Graphite-felt | PMS | 2 mM | Fe3O4 0.1 g L−1 | UV 1.02 mW cm−2 | 30 mA cm−2 | NS | 3 | 0.2 | 5 | COD0: 480 TOC0: 202 | COD: 99.5 TOC: 97.1 | NS | [26] |
| Sanitary landfill leachate | Ti/IrO2-TaO2 / Ti | PDS | 0 mM | FeTiO3 1 g L−1 | UV-LED 30 W | 100 mA cm−2 | 25 | 5 | 0.3 | 8.5 | TOC0: 5600 | TOC: 12 | 300 kWh kg−1 | [20] |
| 234 mM | TOC: 39 1 | 87 kWh kg−1 1 | ||||||||||||
| 50 mA cm−2 | TOC: 23 | 54 kWh kg−1 1 | ||||||||||||
| 200 mA cm−2 | TOC: 53 | 234 kWh kg−1 | ||||||||||||
| Greywater | Fe / Graphite-sheet | PDS | 3 mM | Fe anode | UVC 12 W | 2 mA cm−2 | NS | 0.8(3) 1 | 0.4 | 7 | COD0: 530 | COD: 60.3 | NS | [13] |
| 6 mM | 1 mA cm−2 | COD: 66.9 | NS | |||||||||||
| 2 mA cm−2 | 5 | COD: 71.9 | NS | |||||||||||
| 9 | COD: 68.9 | NS | ||||||||||||
| 3 mA cm−2 | 7 | COD: 72.3 | NS | |||||||||||
| 9 mM | 2 mA cm−2 | COD: 79.6 | NS | |||||||||||
| 0 mM | 1 | 6.9 | COD: 53 1 | 28.48 kWh m−3 | ||||||||||
| 8.8 mM | COD: 77 | 28.16 kWh m−3 | ||||||||||||
| Sanitary landfill leachate | Ti/IrO2/Ti | PDS | 75 mM | Fe2(SO4)3 15 mM | NA | 80 mA | 60 | 2 | 0.15 | 2 | COD0: 1281 | COD: 50 1 | NS | [23] |
| 70 | COD: 58 1 | NS | ||||||||||||
| 80 | COD: 87 | 91.9 kWh kg−1 | ||||||||||||
| 90 | COD: 87 1 | NS |
| Activation Method Besides Electrochemical | Strengths | Weaknesses |
|---|---|---|
| Metal sacrificial anodes |
|
|
| Metal catalysts |
|
|
| UV or visible radiation |
|
|
| Ultrasound |
|
|
| Heat |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.; Nunes, M.J.; Rodrigues, A.S.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Electro-Persulfate Processes for the Treatment of Complex Wastewater Matrices: Present and Future. Molecules 2021, 26, 4821. https://doi.org/10.3390/molecules26164821
Fernandes A, Nunes MJ, Rodrigues AS, Pacheco MJ, Ciríaco L, Lopes A. Electro-Persulfate Processes for the Treatment of Complex Wastewater Matrices: Present and Future. Molecules. 2021; 26(16):4821. https://doi.org/10.3390/molecules26164821
Chicago/Turabian StyleFernandes, Annabel, Maria João Nunes, Ana Sofia Rodrigues, Maria José Pacheco, Lurdes Ciríaco, and Ana Lopes. 2021. "Electro-Persulfate Processes for the Treatment of Complex Wastewater Matrices: Present and Future" Molecules 26, no. 16: 4821. https://doi.org/10.3390/molecules26164821
APA StyleFernandes, A., Nunes, M. J., Rodrigues, A. S., Pacheco, M. J., Ciríaco, L., & Lopes, A. (2021). Electro-Persulfate Processes for the Treatment of Complex Wastewater Matrices: Present and Future. Molecules, 26(16), 4821. https://doi.org/10.3390/molecules26164821







